Introduction
Human epidermal growth factor receptor 2 (HER2) is
overexpressed in ~20% of primary invasive breast cancer cases,
while higher amplification has been correlated with more aggressive
behavior and poorer clinical outcomes (1,2).
Trastuzumab, a monoclonal antibody that binds to the extracellular
domain of HER2, is used as a targeted molecular therapy in
combination with chemotherapy and has been demonstrated to improve
the survival rate of patients with HER-2-positive metastatic breast
cancer (3). Furthermore, the addition
of trastuzumab to adjuvant chemotherapy has been demonstrated to
improve disease-free survival (DFS) and overall survival (OS) of
patients with HER2-positive operable breast cancer (4). Therefore, trastuzumab has become an
significant therapeutic agent for patients with HER2-positive
breast cancer.
The use of neoadjuvant chemotherapy is a recent
treatment strategy for breast cancer that offers survival benefits
equivalent to those of adjuvant chemotherapy and provides a means
to predict prognosis (5,6). Pathological complete response (pCR),
which is characterized by the disappearance of invasive tumors in
surgical specimens, is considered a useful prognostic marker, since
patients who achieve pCR exhibit significant improvement in DFS and
OS. Therefore, the identification of biomarkers to predict
pathological responses may help to maximize the benefit of
treatment and minimize the risk of adverse effects. The addition of
trastuzumab to neoadjuvant chemotherapy for patients with
HER2-positive breast cancer significantly improves the rate of pCR
for these patients (7,8); thus, it was recommended by the St.
Gallen International Expert Consensus on the Primary Therapy of
Early Breast Cancer in 2013 (9).
HER2-positive breast cancer is divided into two
subtypes according to estrogen receptor (ER) status using
immunohistochemical analysis as a surrogate for the classification
of intrinsic subtypes by molecular assays, while ER-positive tumors
are classified as either ‘HER2-positive (nonluminal)’ or ‘luminal
B-like (HER2-positive)’ subtypes, respectively (9). However, there exists a discrepancy in
the pathological responses and survival benefits of neoadjuvant
chemotherapy between these two HER2-positive subtypes (10). Therefore, new predictive markers in
combination with conventional markers, including
immunohistochemical ER and HER2 status, are required.
MicroRNAs (miRNAs) are short noncoding RNAs, ranging
in length from 20 to 25 nucleotides, which regulate protein
synthesis at the post-transcriptional level by binding to the 3′
untranslated region of mRNAs (11).
Numerous miRNAs are reported to be related to dysregulation and
various cancers. For example, miR-21, which is a well-studied
oncomiR, was reported to be associated with HER2-positive breast
cancer. Higher expression of miR-21 is associated with positive
HER2 status, and miR-21 inhibits apoptosis through targeting tumor
suppressors including phosphatase and tensin homolog (12). miRNAs play essential roles in breast
cancer pathogenesis through a complex gene regulation network, and
several have been implicated in resistance and sensitivity to
breast cancer therapeutic drugs (13). miRNAs are well-preserved in a range of
specimen types, including plasma, urine and formalin-fixed
paraffin-embedded (FFPE) tissues (13). Since miRNA expression profiles differ
among breast cancer subtypes, as determined by gene expression
profiles and immunohistochemical findings (14,15), we
speculated that miRNAs may be efficient predictive markers of the
response of neoadjuvant chemotherapy.
In the present study, we evaluated the use of
differentially expressed miRNA profiles of biopsy specimens
collected prior to neoadjuvant chemotherapy with trastuzumab for
patients with HER2-positive breast cancer between pCR and non-pCR
patient groups to predict the pathological response of neoadjuvant
chemotherapy. To the best of our knowledge, few studies have
investigated miRNA profiles associated with the pathological
response of neoadjuvant chemotherapy for patients with
HER2-positive breast cancer according to ER status. The aim of this
study was to develop a more reliable prediction of neoadjuvant
chemotherapy outcomes using differentially expressed miRNA profiles
in combination with ER and HER2 status.
Materials and methods
Patient population
The study protocol was approved by the Bioethics
Committee for Human Genome and Gene Analysis of Jichi Medical
University, Tochigi, Japan (approval no. 13–10), and written
informed consent was obtained from all participants. We
retrospectively reviewed the medical records of 47 consecutive
patients with HER2-positive breast cancer who received neoadjuvant
chemotherapy with trastuzumab at the Department of Breast Surgery,
Jichi Medical University Hospital, between January 2006 and
December 2011. All patients had pathologically confirmed
HER2-positive invasive breast cancer by ultrasound-guided core
needle biopsies prior to treatment. FFPE blocks of pretreatment
biopsy specimens were available for 40 cases (85.1%). The remaining
seven cases were excluded from this study as the biopsies were
performed in other institutions.
Treatment
Neoadjuvant chemotherapy consisted of
anthracycline-based regimens, followed by taxane-based regimens.
The following three anthracycline-based regimens were used: three
or four cycles of FEC (fluorouracil 500 mg/m2,
epirubicin 100 mg/m2, and cyclophosphamide 500
mg/m2 every 3 weeks); four cycles of AC (adriamycin 60
mg/m2 and cyclophosphamide 600 mg/m2 every 3
weeks); and four cycles of EC (epirubicin 90 mg/m2 and
cyclophosphamide 600 mg/m2 every 3 weeks). The three
taxane-based regimens were as follows: three or four cycles of
docetaxel (100 mg/m2 every 3 weeks); four cycles of
docetaxel (75 mg/m2 every 3 weeks); and 12 cycles of
paclitaxel (80 mg/m2 every weeks). All patients received
trastuzumab regimens (4 mg/kg as a loading dose and 2 mg/kg from
the second dose onward every week) concomitantly with a
taxane-based regimen. Surgery was performed 3–4 weeks after the
final dose of the neoadjuvant chemotherapy regimen.
Pathological evaluation
Expression levels of ER (SP1; Roche Diagnostics
Deutschland GmbH, Mannheim, Germany), progesterone receptor (PR;
1E2; Roche Diagnostics) and HER2 (4B5; Roche Diagnostics) in the
biopsy specimens were routinely subjected to immunohistochemical
analysis. The cut-off value for ER and PR positivity was ≥10%
positive cells. HER2 expression was scored on a scale of 0–3+, in
accordance with the American Society of Clinical Oncology/College
of American Pathologists guidelines (16). A score of 0–1+ was considered
negative, while a score of 3+ was considered positive. For 2+
cases, HER2 amplification was verified by fluorescence in
situ hybridization using a PathVysion HER-2 DNA Probe kit
(Abbott/Vysis, Des Plaines, IL, USA). Tumors were categorized into
two subtypes on the basis of ER and HER2 status of the biopsy
specimens as surrogate markers: the luminal B-like (HER2-positive)
subtype (ER- and/or PR-positive, HER2-positive) and the
HER2-positive (nonluminal) subtype (ER- and PR-negative,
HER2-positive). Following surgery, residual tumors in the resected
specimens were pathologically evaluated. The pathological responses
of neoadjuvant chemotherapy were determined according to the status
of residual invasive tumors. A pCR was defined as no pathological
evidence of residual invasive cancer in breast and axillary lymph
nodes, irrespective of the remaining intraductal components.
Isolation of total RNA
Four serial 10-µm-thick sections of FFPE tissue
specimens were mounted onto Leica PEN-membrane slides (Leica
Mikrosysteme Vertrieb GmbH, Wetzlar, Germany) and stained with
cresyl violet (Ambion Life Technologies, Austin, TX, USA).
Neoplastic portions of each specimen were collected by laser
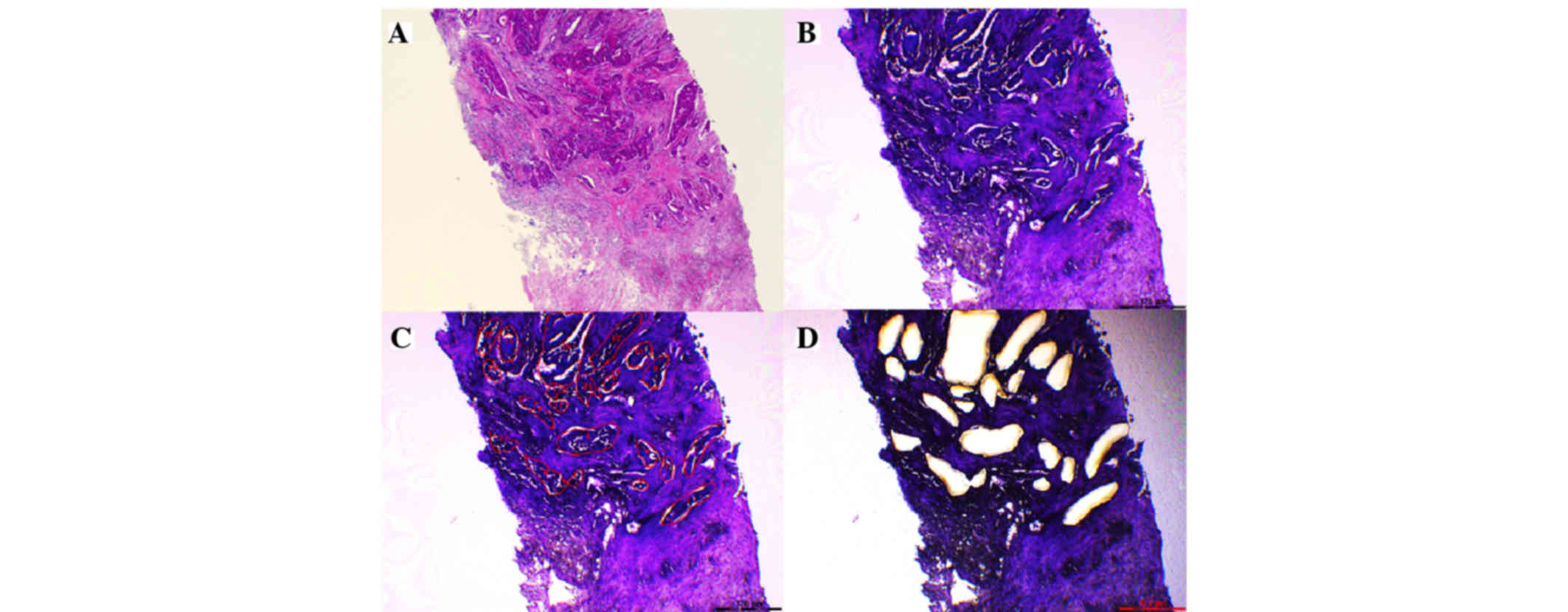
capture microdissection using a laser microdissection (LMD) system
(LMD7000; Leica Mikrosysteme Vertrieb GmbH; Fig. 1). RNA extraction was performed using
an miRNeasy FFPE kit (Qiagen, Hilden, Germany) according to the
manufacturer's instructions. Total RNA in the samples was
quantified using a Qubit RNA HS Assay kit and Qubit 2.0 fluorometer
(Life Technologies, Palo Alto, CA, USA), while RNA quality was
assessed using a NanoDrop 1000 spectrophotometer (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA) and Agilent 2100 Bioanalyzer
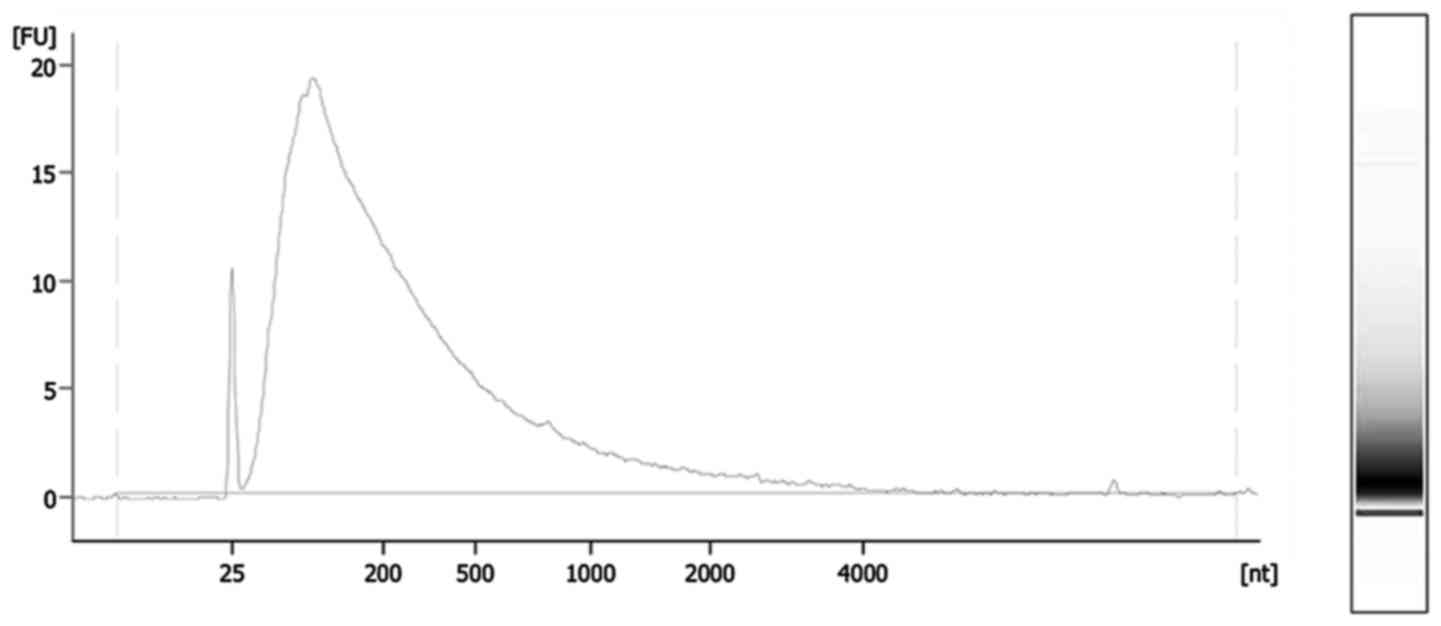
(Agilent Technologies, Inc., Santa Clara, CA, USA; Fig. 2). Total RNA was extracted from FFPE
sections of pretreatment biopsy specimens from all 40 patients. The
yield and quality of all samples were checked prior to subsequent
analyses (Table I). The median
concentration of extracted RNA was 102.1 ng/µl (range, 24.4–374.5
ng/µl). The median 260/280 and 260/230 nm ratios of absorbance were
1.96 (range, 1.70–2.06) and 1.74 (range, 1.00–1.95), respectively.
The median RNA integrity number, which indicates the degree of RNA
degradation, was 2.4 (range, 1.7–2.5).
 | Table I.Summary of yield and quality of
extracted RNA samples. |
Table I.
Summary of yield and quality of
extracted RNA samples.
| Variables | Median | Range |
|---|
| Concentration
(ng/µl) | 102.10 | 24.40 to
374.50 |
| A260/280 | 1.96 | 1.70 to 2.06 |
| A260/230 | 1.74 | 1.00 to 1.95 |
| RNA integrity
number | 2.40 | 1.70 to 2.50 |
miRNA microarray expression
analysis
Total RNA (100 ng) was labeled with cyanine 3-pCp
using the Agilent miRNA Complete Labeling and Hyb kit (Agilent
Technologies, Inc.). Labeled RNA was hybridized to an Agilent
SurePrint G3 Human miRNA microarray (8×60 K, release 19.0; Agilent
Technologies, Inc.) for 20 h at 55°C in a hybridization chamber.
Following hybridization, the array was washed and scanned with an
Agilent G2505C microarray scanner (Agilent Technologies, Inc.) at 3
µm resolution. miRNA expression data were extracted from the
scanned images with Feature Extraction software (version 10.7.3.1;
Agilent Technologies, Inc.).
Data processing and statistical
analysis
miRNA expression data were analyzed using GeneSpring
software (version 13.0; Agilent Technologies, Inc.) and normalized
using a 90th percentile normalization algorithm, followed by
preprocessing baseline to median data of all samples. miRNAs were
filtered based on signal intensity values with a lower percentile
cut-off value of 20% in more than 50% of the samples in any one
category. Unsupervised hierarchical cluster analysis was performed
on filtered data. Expression differences between objective groups
were determined using a moderated t-test. Differences of more than
two-fold with a probability P-value <0.05 were considered
statistically significant. Prediction models for pathological
responses were developed using a partial least squares
discrimination (PLSD) model with GeneSpring software, and a
prediction model for non-pCR was based on expression values of
differentially expressed miRNAs. Prediction results are presented
as t-scores computed with Prism 5 statistical software (version
5.0f; GraphPad Software, Inc., La Jolla, CA, USA). The t-scores
were plotted and compared between the pCR and non-pCR groups using
the Mann-Whitney U-test. Receiver operating characteristic (ROC)
curves were drawn and the area under the ROC curve (AUC) was
calculated. Patient characteristics were compared between the
HER2-positive (nonluminal) and luminal B-like (HER2-positive)
subtypes using Prism 5 software. Fisher's exact test was used to
compare categorical data. The Mann-Whitney U-test was used to
compare continuous data.
Results
Patient characteristics
Medical records from a total of 40 females with
stage II or III breast cancer were included for analysis (Table II). The median age at diagnosis was
55 years (range, 31–83 years). A total of 17 and 23 patients,
respectively, were diagnosed as the luminal B-like (HER2-positive)
or HER2-positive (nonluminal) subtype. Patients with HER2-enriched
tumors (ER-negative) were older than those with luminal-HER2 hybrid
tumors (ER-positive) (P=0.01). There were no significant
differences in median tumor size, clinical axillary node status,
clinical stage or neoadjuvant chemotherapy regimen between subtypes
(P=0.78, 0.37, 0.31 and 0.49, respectively). The pCR of the
HER2-positive (nonluminal) subtype was significantly greater than
that of the luminal B-like (HER2-positive) subtype (P=0.04).
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
|
Characteristics | All patients | Luminal B-like
(HER2-positive) | HER2-positive
(nonluminal) | P-value |
|---|
| Number of
patients | 40 | 17 | 23 |
|
| Median age
(range) | 55 (31–83) | 49 (33–66) | 58 (31–83) | 0.01 |
| Median tumor size
(range, cm) | 3.4 (1.2–6.8) | 3.1 (1.3–6.8) | 3.5 (1.2–6) | 0.78 |
| Clinical axillary
node status |
|
|
| 0.37 |
|
Metastasis | 34 (85.0%) | 13 (76.5%) | 21 (91.3%) |
|
| No
metastasis | 6 (15.0%) | 4 (23.5%) | 2 (8.7%) |
|
| Clinical stage |
|
|
| 0.31 |
| 2 | 26 (65.0%) | 13 (76.5%) | 13 (56.5%) |
|
| 3 | 14 (35.0%) | 4 (23.5%) | 10 (43.5%) |
|
| Regimen of
neoadjuvant chemotherapy |
|
|
| 0.49 |
|
Anthracycline followed by
taxane | 38 (95.0%) | 17 (100%) | 21 (91.3%) |
|
|
Taxane-based (without
anthracycline) | 2 (5.0%) | 0 (0%) | 2 (8.7%) |
|
| Pathological
response |
|
|
| 0.04 |
|
pCR | 15 (37.5%) | 3 (17.6%) | 12 (52.2%) |
|
|
Non-pCR | 25 (62.5%) | 14 (82.3%) | 11 (47.8%) |
|
miRNA expression profiles associated
with pathological response of neoadjuvant chemotherapy
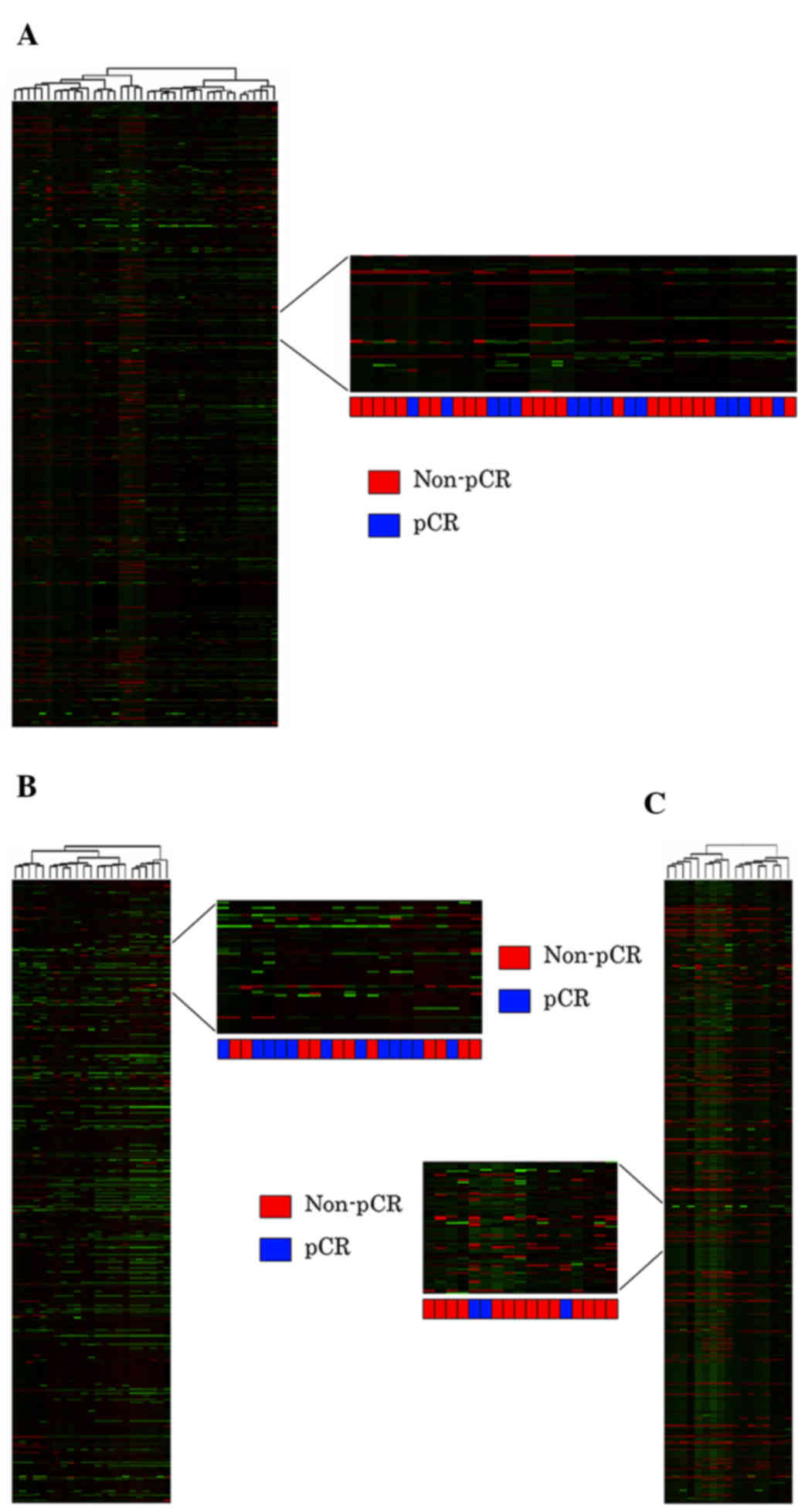
Unsupervised hierarchical clustering using the full
dataset of 2,024 miRNA expression profiles of all 40 patients was
insufficient to differentiate between the pCR and non-pCR groups
(Fig. 3A). Unsupervised cluster
analyses were also performed following categorization into
HER2-positive (nonluminal; Fig. 3B)
and luminal B-like (HER2-positive) subtypes (Fig. 3C); however, these were also
insufficient to predict the pCR group.
Twenty-one miRNAs were differentially expressed
between the non-pCR and pCR groups in the analysis of all 40
patients with HER2-positive breast cancer (Table III). Nine of these 21 miRNAs are
reported to be associated with breast cancer (17–25). The
other 12 miRNAs have no reported correlation with breast cancer.
Furthermore, differential miRNA expression was separately examined
according to the luminal B-like (HER2-positive) and HER2-positive
(nonluminal) subtypes. For the luminal B-like (HER2-positive)
subtype, 17 miRNAs were differentially expressed (Table IV), and nine of these 17 miRNAs are
reported to be correlated with breast cancer (20–22,24,26–30).
For the HER2-positive (nonluminal) subtype, 14 miRNAs were
differentially expressed (Table V),
and five of these are reported to be correlated with breast cancer
(17,23,25,31,32).
The miRNA expression profiles associated with pathological response
differed completely between the HER2-positive (nonluminal) and
luminal B-like (HER2-positive) subtypes.
 | Table III.Differential miRNA expression between
non-pCR and pCR in all 40 patients. |
Table III.
Differential miRNA expression between
non-pCR and pCR in all 40 patients.
| miRNA | P-value | Fold change | Regulation | Function correlated
with breast cancer |
|---|
| miR-106b-3p | 0.046 | 2.97 | Down | Downregulated in
bone metastasis patients with breast cancer (17) |
| miR-1180 | 0.035 | 4.64 | Down | N/A |
| miR-1238-5p | 0.046 | 3.00 | Down | N/A |
| miR-142-5p | 0.035 | 2.73 | Down | Upregulated in
human breast cancer stem cells (18) |
| miR-150-5p | 0.030 | 2.05 | Down | Overexpression
promotes growth and reduces apotosis in breast cancer cells
(19) |
| miR-181c-5p | 0.016 | 4.27 | Down | Predictive miRNA
corresponding with HER2 status in early-stage breast cancer
(20) |
| miR-182-5p | 0.021 | 3.18 | Down | N/A |
| miR-200a-5p | 0.028 | 4.85 | Down | Higher level of
circulating miR-200a in CTC-positive MBC patients (21) |
| miR-210 | 0.046 | 2.22 | Up | Higher expression
correlates with poor prognosis of patients with breast cancer
(22) |
| miR-218-5p | 0.013 | 5.99 | Down | Downregulated in
cisplatin-resistant breast cancer cell lines (23) |
| miR-31-3p | 0.043 | 2.04 | Up | Upregulated in
chemoresistant breast cancer tissues (24) |
| miR-3609 | 0.026 | 3.84 | Down | N/A |
| miR-362-5p | 0.049 | 3.33 | Down | N/A |
| miR-3620-3p | 0.046 | 3.72 | Down | N/A |
| miR-4418 | 0.030 | 4.40 | Down | N/A |
| miR-449a | 0.028 | 6.44 | Up | N/A |
| miR-449b-5p | 0.037 | 3.15 | Up | N/A |
| miR-4506 | 0.015 | 4.95 | Down | N/A |
| miR-4657 | 0.049 | 3.37 | Down | N/A |
| miR-505-3p | 0.026 | 3.44 | Down | Tumor suppressive
miRNA, which correlates inversely with drug sensitivity (25) |
| miR-505-5p | 0.012 | 3.75 | Down |
|
 | Table IV.Differential miRNA expression between
non-pCR and pCR in luminal B-like (HER2-positive) subtype. |
Table IV.
Differential miRNA expression between
non-pCR and pCR in luminal B-like (HER2-positive) subtype.
| miRNA | P-value | Fold change | Regulation | Function correlated
with breast cancer |
|---|
| miR-148b-3p | 0.041 | 5.035 | Up | Higher level of
circulating miR-148b in breast cancer patients (26) |
| miR-151a-3p | 0.043 | 4.635 | Up | N/A |
| miR-152 | 0.016 | 4.490 | Up | Upregulation
indirectly interacts with MAPK signaling pathway (27) |
| miR-203a | 0.027 | 18.271 | Up | Higher level of
circulating miR-203 in CTC-positive MBC patients (21) |
| miR-210 | 0.022 | 10.120 | Up | Higher expression
correlates with poor prognosis of patients with breast cancer
(22) |
| miR-28-5p | 0.039 | 4.214 | Up | N/A |
| miR-301b | 0.007 | 3.485 | Down | Oncogenic miRNA,
miR-301 attenuation decreases cell proliferation and invasion
(28) |
| miR-34b-5p | 0.037 | 4.754 | Up | miR-34 and p53
regulate epithelial-mesenchymal transition of cancer cells
(29) |
| miR-376c-3p | 0.019 | 21.261 | Up | Upregulated in
plasma of patients with breast cancer (30) |
| miR-377-3p | 0.039 | 11.814 | Up | Predictive miRNA
corresponding with PR status in early-stage breast cancer (20) |
| miR-3907 | 0.006 | 4.389 | Up | N/A |
| miR-429 | 0.037 | 7.079 | Up | Overexpressed in
chemoresistant breast cancer cells (24) |
| miR-4291 | 0.006 | 5.189 | Up | N/A |
| miR-4737 | 0.007 | 4.726 | Down | N/A |
| miR-487b | 0.040 | 9.634 | Up | N/A |
| miR-5684 | 0.036 | 4.578 | Up | N/A |
| miR-582-5p | 0.007 | 4.721 | Down | N/A |
 | Table V.Differential miRNA expression between
non-pCR and pCR in HER2-positive (nonluminal) subtype. |
Table V.
Differential miRNA expression between
non-pCR and pCR in HER2-positive (nonluminal) subtype.
| miRNA | P-value | Fold change | Regulation | Function correlated
with breast cancer |
|---|
| let-7a-3p | 0.048 | 2.387 | Up | Tumor suppressive
miRNA, which decreased breast cancer cell migration and invasion
(31) |
| miR-106b-3p | 0.014 | 4.186 | Down | Downregulated in
bone metastasis patients with breast cancer (17) |
| miR-1237-3p | 0.048 | 2.876 | Up | N/A |
| miR-136-5p | 0.044 | 7.102 | Down | N/A |
| miR-181a-3p | 0.028 | 7.355 | Down | N/A |
| miR-196a-5p | 0.044 | 2.222 | Down | N/A |
| miR-218-5p | 0.019 | 7.687 | Down | Downregulated in
cisplatin-resistant breast cancer cell lines (23) |
| miR-342-5p | 0.016 | 4.669 | Down | Downregulation
associates with early recurrence in patients with breast cancer
(32) |
| miR-362-5p | 0.026 | 3.357 | Down | N/A |
| miR-376a-3p | 0.027 | 3.941 | Down | N/A |
| miR-376c-3p | 0.018 | 5.352 | Down | N/A |
| miR-505-5p | 0.017 | 4.369 | Down | Tumor suppressive
miRNA, which correlates inversely with drug sensitivity (25) |
| miR-550a-5p | 0.049 | 3.647 | Up | N/A |
| miR-6515-3p | 0.010 | 6.535 | Up | N/A |
Generation of a prediction model for
pathological response to neoadjuvant chemotherapy of HER2-positive
breast cancer
After evaluating differential miRNA expression, a
prediction model for pathological response was constructed using
the 21 differentially expressed miRNAs identified by analysis of
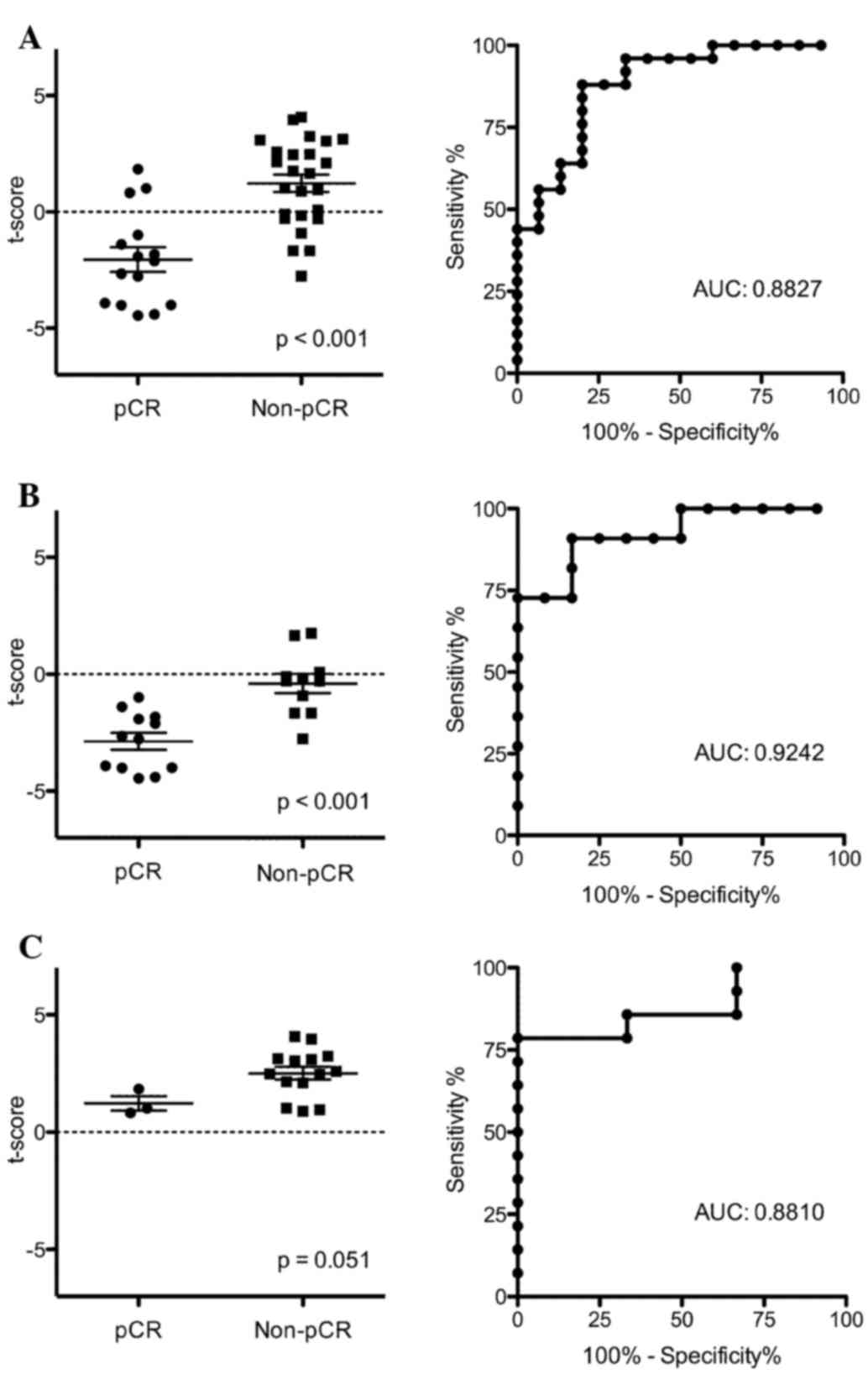
all 40 patients. The distribution of t-scores significantly
differed between the pCR and non-pCR groups (P<0.001; Fig. 4A). Performance of the prediction model
was evaluated using discriminant analysis with ROC curves. The AUC
was 0.8827, indicating moderate accuracy of this model in
predicting pathological responses. We further examined the results
of this prediction model separately for the HER2-positive
(nonluminal) and luminal B-like (HER2-positive) subtypes. There
were significant differences in the distribution of t-scores
(P<0.001) between the pCR and non-pCR groups in the
HER2-positive (nonluminal) subtype (Fig.
4B), whereas no significant difference was observed for the
luminal B-like (HER2-positive) subtype (P=0.051; Fig. 4C). In discriminant analyses with the
ROC curve, the AUCs were 0.9242 for the HER2-positive (nonluminal)
subtype and 0.8810 for the luminal B-like (HER2-positive) subtype,
respectively (Fig. 4B and C). These
results demonstrate that the predictive ability for the
HER2-positive (nonluminal) subtype is better than that for the
luminal B-like (HER2-positive) subtype. A prediction model of
pathological response was also constructed using the 14 miRNAs
differentially expressed in the HER2-positive (nonluminal) subtype.
The distribution of t-scores significantly differed (P<0.001)
between the pCR and non-pCR groups, and the AUC was 0.9621
(Fig. 5). These results indicate that
the prediction model for the HER2-positive (nonluminal) subtype was
more suitable than that of the prediction model using 21
differentially expressed miRNAs in the analysis of all 40 patients.
A model for the luminal B-like (HER2-positive) subtype could not be
constructed as the data were not suitable for classification using
the PLSD algorithm.
Discussion
In the present study, we identified miRNA expression
profiles of biopsy specimens from patients with HER2-positive
breast cancer prior to neoadjuvant chemotherapy with trastuzumab.
The miRNAs associated with pathological response significantly
differed between the HER2-positive (nonluminal) and luminal B-like
(HER2-positive) subtypes. The prediction models of pathological
responses constructed using miRNAs differentially expressed between
the pCR and non-pCR groups demonstrated considerable
reliability.
Breast cancer may be classified into subtypes
according to gene expression profiles (33). Surrogate subtype classification
obtained by immunohistochemical analyses of ER, PR, Ki-67 and HER2
is often used in clinical practice (9). In previous studies, miRNA expression
profiles differed according to the breast cancer subtype (14,15).
However, several previous studies of miRNA expression profiles of
neoadjuvant chemotherapy for patients with HER2-positive breast
cancer did not classify patients according to hormone receptor
status (34,35). The pathological response and survival
benefit of neoadjuvant chemotherapy with trastuzumab reportedly
differs between two HER2-positive subtypes divided by hormone
receptor status (10). Therefore, we
classified HER2-positive breast cancer into subtypes based on ER
status and subsequently analyzed miRNA expression. The results of
our study revealed significant differences in the miRNA profiles
associated with pathological responses between the HER2-positive
(nonluminal) and luminal B-like (HER2-positive) subtypes. We
further constructed models to predict pathological responses using
differentially expressed miRNAs. The combination of breast cancer
subtypes and miRNA expression profiles offers a means to accurately
and efficiently predict pathological responses.
We identified a total of 52 differentially expressed
miRNAs in the analysis of all 40 patients: 21 according to pCR
status (Table III), 17 by analysis
of the luminal B-like (HER2-positive) subtype (Table IV), and 14 by analysis of the
HER2-positive (nonluminal) subtype (Table
V), respectively. A total of 48 miRNAs were selected due to the
overlap of four miRNAs (i.e., miR-210, miR-106b, miR-505 and
miR-218). miR-210 was upregulated in the analyses of all 40
patients and the luminal B-like (HER2-positive) subtype.
Correlations between increased miR-210 expression and poor
prognosis in various cancers, particularly in breast cancer, as
well as associations with the hypoxic pathway in a
hypoxia-inducible factor-dependent manner have been reported
elsewhere (22,36). miR-106b-3p, miR-218-5p and miR-505-5p
were downregulated in the analyses of all 40 patients and the
HER2-positive (nonluminal) subtype. Downregulation of miR-106b
involves bone metastasis of breast cancer with negative regulation
of matrix metalloproteinase 2 (17).
miR-218 was downregulated in cisplatin-resistant breast cancer cell
lines and regulated chemosensitivity by targeting BRCA1 (23). miR-505 has been reported as a
tumor-suppressive miRNA, and is inversely correlated with Akt3,
which modulates drug sensitivity (25). The remaining differentially expressed
15 miRNAs between the non-pCR and pCR groups are reportedly
involved in the progression of breast cancer (Tables III–V). To the best of our knowledge, the
involvement of the other 28 miRNAs identified in this study with
breast cancer has not been previously reported.
In a study using pretreatment biopsy specimens,
Kolacinska et al (37)
reported correlations between miRNA expression profiles and the
pathological response of neoadjuvant chemotherapy. This group
investigated the expression profiles of 19 miRNAs in frozen biopsy
specimens collected from patients with ER-negative, PR-negative and
HER2-negative (triple-negative) breast cancer, and observed that
miR-200b-3p, miR-190a and miR-512-5p were associated with a better
pathological response. However, there were no differences in the
expression levels of these three miRNAs between the non-pCR and pCR
groups in our study. Most triple-negative breast cancers belong to
different molecular subtypes of ER- and/or HER2-positive breast
cancers (38), and miRNA expression
of triple-negative breast cancers differed compared with other
subtypes (14,15). Triple-negative breast cancer tends to
behave more aggressively than other subtypes; there are currently
no therapies targeting the endocrine system or HER2 for this
subtype of breast cancer. Taken together, it appears that there are
differences in miRNA profiles associated with pathological response
according to subtype.
miRNAs associated with the pathological response of
neoadjuvant chemotherapies in patients with HER2-positive breast
cancer have been reported in several studies using circulating
miRNA. Circulating miRNAs may be exploited as noninvasive
biomarkers since miRNAs derived from tumors are stable and
detectable in serum (39,40). For example, Jung et al
(34) reported that the expression
level of circulating miR-210 was associated with the sensitivity of
neoadjuvant chemotherapy in patients with HER2-positive breast
cancer, while Müller et al (35) reported that serum levels of miR-21,
miR-210 and miR-373 were higher in patients with HER2-positive
breast cancer than in healthy females, although no associations
between circulating miRNAs with pCR were noted. The present study
revealed that miR-210 upregulation was associated with non-pCR
(Tables III and IV). Therefore, upregulation of miR-210 may
be useful to predict the pathological response of neoadjuvant
chemotherapy with trastuzumab, although these results cannot be
validated due to the differences in analyses and chemotherapy
regimens.
In this study, we determined miRNA profiles in FFPE
sections of pretreatment biopsy specimens that were obtained prior
to neoadjuvant chemotherapy. miRNA is stable and intact in FFPE,
while mRNA is fragmented in FFPE tissue compared with fresh tissue
(41). Our results also revealed
degradation of RNA, and RNA fragments extracted from FFPE tissue
were as short as 100 nt (Fig. 2).
miRNAs appear to be better preserved, possibly due to their
intrinsically shorter lengths (42).
Microarray analysis of miRNA expression profiles demonstrated good
correlations between frozen and FFPE samples (43). FFPE tissues are often the only
available tissue samples in several institutions, and have usually
been archived for long periods. This stability and availability in
the clinical setting are advantages of miRNA study using FFPE. As
another advantage of this study, an LMD system was used during the
process of RNA extraction. The LMD system enables access to
specific regions within tissue samples to collect relevant
information (44). RNA was extracted
from tumor tissue sections collected from biopsy specimens. Since
the validity of microarray analysis of microdissected tumor tissues
from FFPE samples has been reported (45), we consider that our approach was
reasonable.
There are certain limitations to this study that
should be addressed. First, the sample size was relatively small
and this retrospective study was limited to a single institution.
Second, there exists no consensus on a method to normalize miRNA
microarray data (46). Data
normalization is necessary to minimize the effects of systemic
experimental bias and technical variations. Although our data were
analyzed using 90th percentile normalization, other normalization
methods may lead to different results. Third, our results have not
yet been verified, and therefore we cannot discount the possibility
that the results were random. The candidate miRNAs should be
verified by quantitative polymerase chain reaction analysis, and
the prediction models should ideally be verified against a
validation cohort.
In conclusion, the results of our study demonstrated
differential miRNA expression profiles between pCR and non-pCR
groups, following neoadjuvant chemotherapy with trastuzumab in
patients with HER2-positive breast cancer. The combination of miRNA
profiles and ER status may improve the accuracy of prediction of
pathological responses to enable the design of personalized
treatment regimens. The results of this study may be verified in
larger and multicenter studies as this study used FFPE samples,
which are generally available in most institutions. The findings of
this study may be helpful to identify new predictive biomarkers to
monitor the response of neoadjuvant chemotherapies.
Acknowledgements
The authors thank Nobuko Nishiaki and Taeko Yatabe
of the Department of Surgery, Jichi Medical University, for their
valuable technical assistance with regard to the preparation of
pathological specimens and handling of RNA samples, as well as
Enago (www.enago.jp) for the English language
review. This study was supported by the Practical Research for
Innovative Cancer Control (grant no. 15ck0106045h0002) from the
Japan Agency for Medical Research and Development, AMED.
References
|
1
|
Ross JS, Slodkowska EA, Symmans WF,
Pusztai L, Ravdin PM and Hortobágyi GN: The HER-2 receptor and
breast cancer: ten years of targeted anti-HER-2 therapy and
personalized medicine. Oncologist. 14:320–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perez EA, Romond EH, Suman VJ, Jeong JH,
Sledge G, Geyer CE Jr, Martino S, Rastogi P, Gralow J, Swain SM, et
al: Trastuzumab plus adjuvant chemotherapy for human epidermal
growth factor receptor 2-positive breast cancer: planned joint
analysis of overall survival from NSABP B-31 and NCCTG N9831. J
Clin Oncol. 32:3744–3752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rastogi P, Anderson SJ, Bear HD, Geyer CE,
Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil
SR, et al: Preoperative chemotherapy: updates of national surgical
adjuvant breast and bowel project protocols B-18 and B-27. J Clin
Oncol. 26:778–785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kong X, Moran MS, Zhang N, Haffty B and
Yang Q: Meta-analysis confirms achieving pathological complete
response after neoadjuvant chemotherapy predicts favourable
prognosis for breast cancer patients. Eur J Cancer. 47:2084–2090.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buzdar AU: Significantly higher pathologic
complete remission rate after neoadjuvant therapy with trastuzumab,
paclitaxel, and epirubicin chemotherapy: results of a randomized
trial in human epidermal growth factor receptor 2-positive operable
breast cancer. J Clin Oncol. 23:3676–3685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gianni L, Eiermann W, Semiglazov V,
Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M,
Lichinitser M, et al: Neoadjuvant chemotherapy with trastuzumab
followed by adjuvant trastuzumab versus neoadjuvant chemotherapy
alone, in patients with HER2-positive locally advanced breast
cancer (the NOAH trial): a randomised controlled superiority trial
with a parallel HER2-negative cohort. Lancet. 375:377–384. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ: Panel members:
Personalizing the treatment of women with early breast cancer:
highlights of the St Gallen international expert consensus on the
primary therapy of early breast cancer, 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cortazar P, Zhang L, Untch M, Mehta K,
Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L,
Valagussa P, et al: Pathological complete response and long-term
clinical benefit in breast cancer: the CTNeoBC pooled analysis.
Lancet. 384:164–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mulrane L, McGee SF, Gallagher WM and
O'Connor DP: miRNA dysregulation in breast cancer. Cancer Res.
73:6554–6562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang SE and Lin RJ: MicroRNA and
HER2-overexpressing cancer. Microrna. 2:137–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Serpico D, Molino L and Di Cosimo S:
microRNAs in breast cancer development and treatment. Cancer Treat
Rev. 40:595–604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blenkiron C, Goldstein LD, Thorne NP,
Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE,
Green AR, Ellis IO, et al: MicroRNA expression profiling of human
breast cancer identifies new markers of tumor subtype. Genome Biol.
8:R2142007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai X, Chen A and Bai Z: Integrative
investigation on breast cancer in ER, PR and HER2-defined subgroups
using mRNA and miRNA expression profiling. Sci Rep. 4:65662014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolff AC, Hammond MEH, Schwartz JN,
Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna
WM, Langer A, et al: American Society of Clinical Oncology/College
of American Pathologists guideline recommendations for human
epidermal growth factor receptor 2 testing in breast cancer. J Clin
Oncol. 25:118–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ni X, Xia T, Zhao Y, Zhou W, Wu N, Liu X,
Ding Q, Zha X, Sha J and Wang S: Downregulation of miR-106b induced
breast cancer cell invasion and motility in association with
overexpression of matrix metalloproteinase 2. Cancer Sci.
105:18–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Isobe T, Hisamori S, Hogan DJ, Zabala M,
Hendrickson DG, Dalerba P, Cai S, Scheeren F, Kuo AH, Sikandar SS,
et al: miR-142 regulates the tumorigenicity of human breast cancer
stem cells through the canonical WNT signaling pathway. Elife.
3:2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang S, Chen Y, Wu W, Ouyang N, Chen J,
Li H, Liu X, Su F, Lin L and Yao Y: miR-150 promotes human breast
cancer growth and malignant behavior by targeting the pro-apoptotic
purinergic P2X7 receptor. PLoS One. 8:e807072013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lowery AJ, Miller N, Devaney A, McNeill
RE, Davoren PA, Lemetre C, Benes V, Schmidt S, Blake J, Ball G and
Kerin MJ: MicroRNA signatures predict oestrogen receptor,
progesterone receptor and HER2/neu receptor status in breast
cancer. Breast Cancer Res. 11:R272009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Madhavan D, Zucknick M, Wallwiener M, Cuk
K, Modugno C, Scharpff M, Schott S, Heil J, Turchinovich A, Yang R,
et al: Circulating miRNAs as surrogate markers for circulating
tumor cells and prognostic markers in metastatic breast cancer.
Clin Cancer Res. 18:5972–5982. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li M, Ma X, Li M, Zhang B, Huang J, Liu L
and Wei Y: Prognostic role of microRNA-210 in various carcinomas: a
systematic review and meta-analysis. Dis Markers. 2014:1061972014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He X, Xiao X, Dong L, Wan N, Zhou Z, Deng
H and Zhang X: MiR-218 regulates cisplatin chemosensitivity in
breast cancer by targeting BRCA1. Tumour Biol. 36:2065–2075. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lv J, Xia K, Xu P, Sun E, Ma J, Gao S,
Zhou Q, Zhang M, Wang F, Chen F, et al: miRNA expression patterns
in chemoresistant breast cancer tissues. Biomed Pharmacother.
68:935–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto Y, Yoshioka Y, Minoura K,
Takahashi RU, Takeshita F, Taya T, Horii R, Fukuoka Y, Kato T,
Kosaka N and Ochiya T: An integrative genomic analysis revealed the
relevance of microRNA and gene expression for drug-resistance in
human breast cancer cells. Mol Cancer. 10:1352011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen J, Hu Q, Schrauder M, Yan L, Wang D,
Medico L, Guo Y, Yao S, Zhu Q, Liu B, et al: Circulating miR-148b
and miR-133a as biomarkers for breast cancer detection. Oncotarget.
5:5284–5294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pinto R, De Summa S, Danza K, Popescu O,
Paradiso A, Micale L, Merla G, Palumbo O, Carella M and Tommasi S:
MicroRNA expression profiling in male and female familial breast
cancer. Br J Cancer. 111:2361–2368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi W, Gerster K, Alajez NM, Tsang J,
Waldron L, Pintilie M, Hui AB, Sykes J, P'ng C, Miller N, et al:
MicroRNA-301 mediates proliferation and invasion in human breast
cancer. Cancer Res. 71:2926–2937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim NH, Kim HS, Li XY, Lee I, Choi HS,
Kang SE, Cha SY, Ryu JK, Yoon D, Fearon ER, et al: A p53/miRNA-34
axis regulates Snail1-dependent cancer cell epithelial-mesenchymal
transition. J Cell Biol. 195:417–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cuk K, Zucknick M, Heil J, Madhavan D,
Schott S, Turchinovich A, Arlt D, Rath M, Sohn C, Benner A, et al:
Circulating microRNAs in plasma as early detection markers for
breast cancer. Int J Cancer. 132:1602–1612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim SJ, Shin JY, Lee KD, Bae YK, Sung KW,
Nam SJ and Chun KH: MicroRNA let-7a suppresses breast cancer cell
migration and invasion through downregulation of C-C chemokine
receptor type 7. Breast Cancer Res. 14:R142012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pérez-Rivas LG, Jerez JM, Carmona R, de
Luque V, Vicioso L, Claros MG, Viguera E, Pajares B, Sánchez A,
Ribelles N, et al: A microRNA signature associated with early
recurrence in breast cancer. PLoS One. 9:e918842014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jung EJ, Santarpia L, Kim J, Esteva FJ,
Moretti E, Buzdar AU, Di Leo A, Le XF, Bast RC Jr, Park ST, et al:
Plasma microRNA 210 levels correlate with sensitivity to
trastuzumab and tumor presence in breast cancer patients. Cancer.
118:2603–2614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Müller V, Gade S, Steinbach B, Loibl S,
von Minckwitz G, Untch M, Schwedler K, Lübbe K, Schem C, Fasching
PA, et al: Changes in serum levels of miR-21, miR-210 and miR-373
in HER2-positive breast cancer patients undergoing neoadjuvant
therapy: a translational research project within the Geparquinto
trial. Breast Cancer Res Treat. 147:61–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Camps C, Buffa FM, Colella S, Moore J,
Sotiriou C, Sheldon H, Harris AL, Gleadle JM and Ragoussis J:
hsa-miR-210 is induced by hypoxia and is an independent prognostic
factor in breast cancer. Clin Cancer Res. 14:1340–1348. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kolacinska A, Morawiec J, Fendler W,
Malachowska B, Morawiec Z, Szemraj J, Pawlowska Z, Chowdhury D,
Choi YE, Kubiak R, et al: Association of microRNAs and pathologic
response to preoperative chemotherapy in triple negative breast
cancer: preliminary report. Mol Biol Rep. 41:2851–2857. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sotiriou C and Pusztai L: Gene-expression
signatures in breast cancer. N Engl J Med. 360:790–800. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: a novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pritchard CC, Cheng HH and Tewari M:
MicroRNA profiling: approaches and considerations. Nat Rev Genet.
13:358–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hui AB, Shi W, Boutros PC, Miller N,
Pintilie M, Fyles T, McCready D, Wong D, Gerster K, Waldron L, et
al: Robust global micro-RNA profiling with formalin-fixed
paraffin-embedded breast cancer tissues. Lab Invest. 89:597–606.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xi Y, Nakajima G, Gavin E, Morris CG, Kudo
K, Hayashi K and Ju J: Systematic analysis of microRNA expression
of RNA extracted from fresh frozen and formalin-fixed
paraffin-embedded samples. RNA. 13:1668–1674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Legres LG, Janin A, Masselon C and
Bertheau P: Beyond laser microdissection technology: follow the
yellow brick road for cancer research. Am J Cancer Res. 4:1–28.
2014.PubMed/NCBI
|
|
45
|
Lassmann S, Kreutz C, Schoepflin A, Hopt
U, Timmer J and Werner M: A novel approach for reliable microarray
analysis of microdissected tumor cells from formalin-fixed and
paraffin-embedded colorectal cancer resection specimens. J Mol Med
(Berl). 87:211–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Meyer SU, Pfaffl MW and Ulbrich SE:
Normalization strategies for microRNA profiling experiments: a
‘normal’ way to a hidden layer of complexity? Biotechnol Lett.
32:1777–1788. 2010. View Article : Google Scholar : PubMed/NCBI
|