Introduction
The use of botanicals for the prevention of various
diseases has been a subject of interest, and phytochemicals have
been indicated to be useful as protective agents against certain
types of cancer, but the investigation of proanthocyanidins has
been limited (1).
Proanthocyanidins, also termed condensed tannins,
are a group of oligomers or polymers of flavan-3-ols. They are
ubiquitous and represent the second most abundant group of natural
phenolics after lignin (2). Oligomers
and polymers of proanthocyanidins can be widely found in the plant
kingdom in fruits, berries, seeds, flowers and leaves (3). The proanthocyanidins that exclusively
consist of epicatechin units are termed procyanidins and are the
most abundant type of proanthocyanidins in plants. The less common
proanthocyanidins that contain epigallocatechin subunits are termed
prodelphinidins (1).
Chinese bayberry (Myrica rubra) is a
subtropical evergreen fruit tree that is widely grown in southern
China (4,5). Chinese bayberry leaves are rich in
prodelphinidins (6,7). Few studies have been conducted to assess
the anticancer effects of prodelphinidins extracted from Chinese
bayberry leaves (PCBLs), and the molecular mechanisms underlying
the anticancer effect of PCBLs has been minimally investigated in
human cancer cells. As it is difficult to separate prodelphinidins
according to their degree of polymerization and isolate the pure
standard from mixtures including other polyphenols, the association
between the degree of polymerization and anti-carcinogenic activity
remains ambiguous (8).
Apoptosis is triggered through two major pathways:
The intrinsic pathway and the extrinsic pathway (9). Apoptosis serves a major role in
establishing a natural balance between cell death and renewal by
destroying excess, damaged or abnormal cells (3). As cancer may result from uncontrolled
cell proliferation and dysregulation of apoptosis, the induction of
apoptosis is a highly desirable goal in developing preventive
strategies for cancer control (10).
The present study aimed to isolate and characterize
PCBLs, and investigate the mechanism of their anticancer effect.
The study focused on caspase-dependent apoptosis, particularly the
two major apoptotic pathways (intrinsic and extrinsic pathway) and
p53-associated apoptosis, to determine whether PCBLs exhibited
anticancer properties in OVCAR-3 cells.
Materials and methods
Preparation of PCBLs, oligomeric
proanthocyanidins (OPAs) and polymeric proanthocyanidins
(PPAs)
PCBLs, OPAs and PPAs, which are all proanthocyanidin
extracts, were isolated from Chinese bayberry leaves according to
our previous study (6). The finely
ground powder of the leaves was extracted with aqueous acetone
[acetone:water, 80:20 (v/v)] containing 0.1% (w/v) ascorbic acid at
room temperature for 12 h, and was then subjected to
rotary-evaporation to remove the acetone. The aqueous phase was
recovered and washed with hexane to remove nonpolar material, and
the organic solvents were subsequently evaporated and lyophilized
to obtain the bayberry leaf extracts.
To further purify the sample, a solution of the
extracts, re-dissolved in 50% methanol, was loaded onto a column of
Sephadex LH-20 and eluted stepwise with 50% methanol to remove
pigments and sugars, 90% methanol to remove the majority of
flavonoids, 50% acetone to elute the majority of proanthocyanidins
and 70% acetone to clean the column. The fraction that was eluted
by 50% acetone was used for subsequent analyses and was considered
to consist of PCBLs.
Further separation of PCBLs according to
polymerization degree was conducted using high-performance liquid
chromatography (HPLC) on a Luna silica preparative column
(Phenomenex, Torrance, CA, USA; 21.2 mm inner diameter ×250 mm)
with a 5-µm particle size at 37°C using a hexane/methanol/ethyl
acetate solution as the mobile phase. A Shimadzu preparative HPLC
system (Shimadzu Corporation, Kyoto, Japan) equipped with a CBM-20A
module, an SIL-10AP autosampler, an SPD-20A ultraviolet-visible
detector and two LC-8A pumps were used. On each run, 1 ml (200
mg/ml) PCBL was applied (6). The
fractions were collected manually when the target peak was visible
on the screen and evaporated under a vacuum. According to our
previous results (6), fractions
containing dimers and trimers (N2-N7) were considered to be OPAs,
which made up almost 23.9% of the PCBLs, whereas fractions
containing tetramers and higher polymerizations of
proanthocyanidins (N8-N10) were considered to be PPAs and composed
almost 47.8% of the PCBLs. The remaining ~30% consisted of
epigallocatechin gallate (EGCG), myricetin deoxyhexoside, myricetin
deoxyhexoside-gallate, and other polyphenols not detected by
normal-phase HPLC/electrospray ionization mass spectrometry.
Cell culture
OVCAR-3 human ovarian cancer cells were provided by
Dr B. Jiang, Department of Microbiology, Immunology, and Cell
Biology, West Virginia University (Morgantown, WV, USA). Cells were
cultured in RPMI-1640 medium (Sigma-Aldrich, Merck Millipore,
Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C in a humidified incubator with 5% CO2.
Cell viability assay
OVCAR-3 cells were seeded onto 96-well plates at a
density of 1×104 cells/well (100 µl) and incubated
overnight at 37°C prior to treatment with 0 (control), 5, 10, 20,
40, 80 or 160 µg/ml of PCBL, OPA or PPA for 24 h. A stock solution
of each sample was prepared in dimethyl sulfoxide (DMSO) at 160
µg/ml and stored at −20°C. The varying concentrations of each
sample were prepared in RPMI-1640 medium for cell treatments, and
DMSO was included in the preparations to ensure equal
concentrations of DMSO in each treatment. Control cells received an
equal volume of DMSO only. Cell viability was analyzed with a
CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega
Corporation, Madison, WI, USA), following the protocol of the
manufacturer, and normalized to control wells for statistical
analysis.
Apoptosis assessment by Hoechst 33342
staining
The OVCAR-3 cells were seeded in 60 mm dishes at a
concentration of 6×105 cells/dish and incubated
overnight at 37°C. The cells were treated with 0, 10, 20, 40 or 80
µg/ml of PCBLs, OPAs and PPAs for 24 h. Subsequent to treatment,
the cells were stained with 10 µg/ml Hoechst 33342 (Sigma-Aldrich;
Merck Millipore) in PBS for 10 min in the dark at 37°C. Cell
apoptosis was examined under a fluorescence microscope (Zeiss AG,
Oberkochen, Germany). Apoptotic cells were considered to exhibit
condensed or fragmented nuclei.
Flow cytometry
To quantify the induction of apoptotic death of
ovarian cancer cells by PCBLs, OPAs and PPAs, annexin V and
propidium iodide (PI) staining was performed, followed by flow
cytometry as previously described (11). The cells were plated to 60% confluence
overnight under the aforementioned conditions, and subsequently
treated with 0, 20 and 40 µg/ml PCBLs, OPAs and PPAs. After 24 h of
treatment, the adherent cells were harvested by trypsinization, and
adherent and nonadherent cells were collected using centrifugation
at 1000 × g for 5 min. Annexin V and PI staining was then performed
on the cells using a Vybrant Apoptosis Assay kit 2 (Molecular
Probes; Thermo Fisher Scientific Inc.) following the protocol
provided by the manufacturer. Subsequent to staining, a flow
cytometer (FACSCalibur™ system; BD Biosciences, San Jose, CA, USA)
was used for the quantification of the apoptotic cells.
Caspase-3/7, −8 and −9 assays
Cellular caspase-3/7, −8 and −9 activities were
measured with Caspase-Glo Assay kits (Promega Corporation). The
assays provide a proluminescent substrate that is cleaved to
aminoluciferin by caspase-3/7, −8 or −9. The released
aminoluciferin substrate reacts with luciferase, generating a
luminescent signal, which is proportional to the activity of the
caspases. To perform the assay, OVCAR-3 cells were seeded into
96-well plates at a density of 1×104 cells/well and
incubated overnight at 37°C. The cells were treated with 0, 5, 10,
20 or 40 µg/ml of PCBLs, OPAs or PPAs for 24 h, before the plates
containing the cells were removed from the incubator and allowed to
equilibrate to room temperature for 30 min. Caspase-Glo reagent
(100 µl) was then added to each well, and the plates were incubated
at room temperature for 2 h. The luminescence of each sample was
measured in a Synergy HT Multi-Mode Microplate Reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Western blot analysis
Ovarian cancer cells (6×105 cells) were
seeded in 60-mm dishes, incubated overnight at 37°C, and treated
with 0, 10 or 20 µg/ml of PCBLs, OPAs or PPAs for 24 h. The cells
were harvested and lysed with M-PER Mammalian Protein Extraction
Reagent (Pierce; Thermo Fisher Scientific, Inc.) supplemented with
Halt Protease and Phosphatase Inhibitor Single-Use Cocktail
(Pierce; Thermo Fisher Scientific, Inc.) according to the protocol
of the manufacturer. Total protein levels were assayed with a
Bicinchoninic Acid Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.). The cell lysates were separated by 10% SDS-PAGE
and blotted onto a nitrocellulose membrane with a Mini-Protean 3
System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membranes were then blocked in 5% nonfat milk in TBS containing
0.1% Tween-20 (TBST) for 1 h at room temperature and incubated with
indicated primary antibodies overnight at 4°C followed by
horseradish peroxidase (HRP)-conjugated secondary antibodies for 2
h at 37°C. Antibodies against caspase-3 (cat. no. 9662), caspase-8
(cat. no. 9746), caspase-9 (cat. no. 9508), p53 upregulated
modulator of apoptosis (PUMA; cat. no. 12450), B-cell lymphoma 2
(Bcl-2; cat. no. 3498), FAS (cat. no. 8023), Fas L (cat. no. 4273),
Fas-associated protein with death domain (FADD; cat. no. 2782), p21
(cat. no. 2947), anti-mouse IgG with HRP-conjugated secondary
antibody (cat. no. 7076) and anti-rabbit IgG with HRP-conjugated
secondary antibody (cat. no. 7074) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA) and all were used at
a dilution of 1:1,000. Antibodies against Bcl-extra large (xL; cat.
no. 136207), Bcl-2-associated X protein (Bax; cat. no. 4239),
Bcl-2-associated death promoter (Bad; cat. no. 4702), DR4 (cat. no.
65312), DR5 (cat. no. 65314), PTEN (cat. no. 7974), p53 (cat. no.
47698), MDM2 (cat. no. 812) and GAPDH (cat. no. 47724) were
purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA) and
all were used at a dilution of 1:200. Subsequent to washing with
TBST, the antigen-antibody complex was visualized with the
SuperSignal West Dura Extended Duration Substrate (Pierce; Thermo
Fisher Scientific, Inc.). The protein bands were detected and
quantitated with ChemiDoc XRS+ System and Image Lab Software
(Bio-Rad Laboratories, Inc., version 5.1) and normalized to the
corresponding GAPDH level for analysis.
Transfection with small interfering
RNA (siRNA)
The ovarian cancer OVCAR-3 cells (6×105
cells) were seeded in 60-mm dishes, incubated overnight at 37°C,
and transfected with p53 siRNA (Santa Cruz Biotechnology, Inc.)
using Lipofectamine® 2000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
protocol of the manufacturer. Following a 24-h transfection period,
the cells were treated with OPAs or PPAs for 24 h. The cell lysates
were collected for western blot analysis.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Statistical significance was
determined with SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) by one-way
analysis of variance followed by the Duncan multiple range test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PCBLs, OPAs and PPAs inhibit
proliferation and induce apoptosis in OVCAR-3 human ovarian cancer
cells
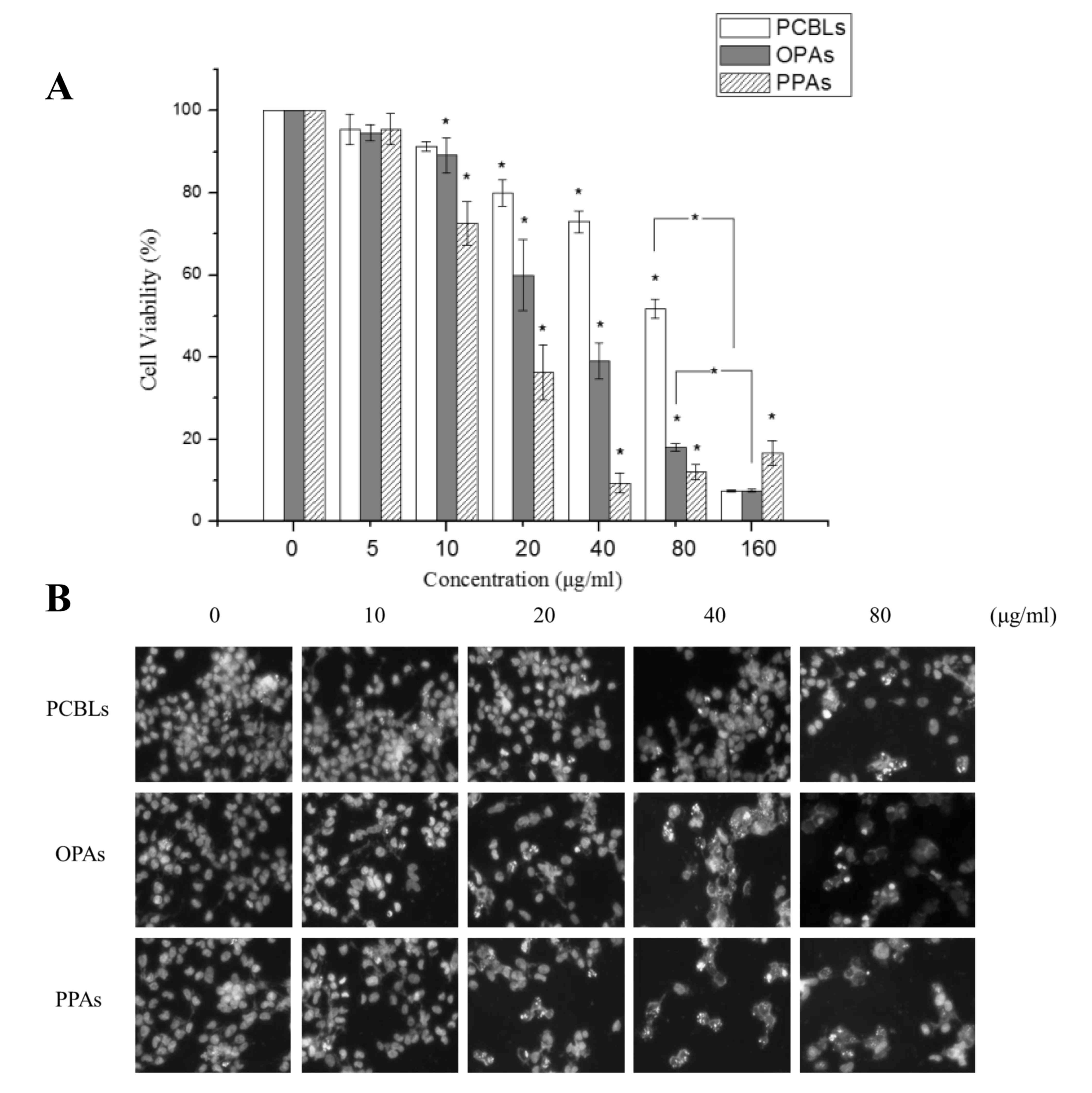
The treatment of OVCAR-3 cells with 0–100 µg/ml
PCBLs, OPAs or PPAs resulted in significant concentration-dependent
reductions in cell proliferation, with half-maximal inhibitory
concentrations (IC50) of 82.32, 34.98 and 15.11 µg/ml,
respectively (Fig. 1A). PPAs induced
a greater level of cytotoxicity in OVCAR-3 cells compared with the
OPAs.
To determine whether the decrease in cell viability
was due to apoptotic cell death, the alterations in cellular and
nuclear morphology of OVCAR-3 cells following their treatment with
PCBLs, OPAs or PPAs (0–80 µg/ml) for 24 h were assessed using
Hoechst 33342 DNA staining and fluorescence microscopy (Fig. 1B). Following each of the treatments,
numerous apoptotic cells, exhibiting condensed or fragmented
nuclei, were observed, and this effect was concentration-dependent.
As presented in Fig. 1B, OPAs and
PPAs significantly increased the percentage of apoptotic cells at
20 µg/ml (P<0.05), while an equivalent effect was observed in
cells treated with 80 µg/ml PCBLs, which was in accordance with the
cell viability results.
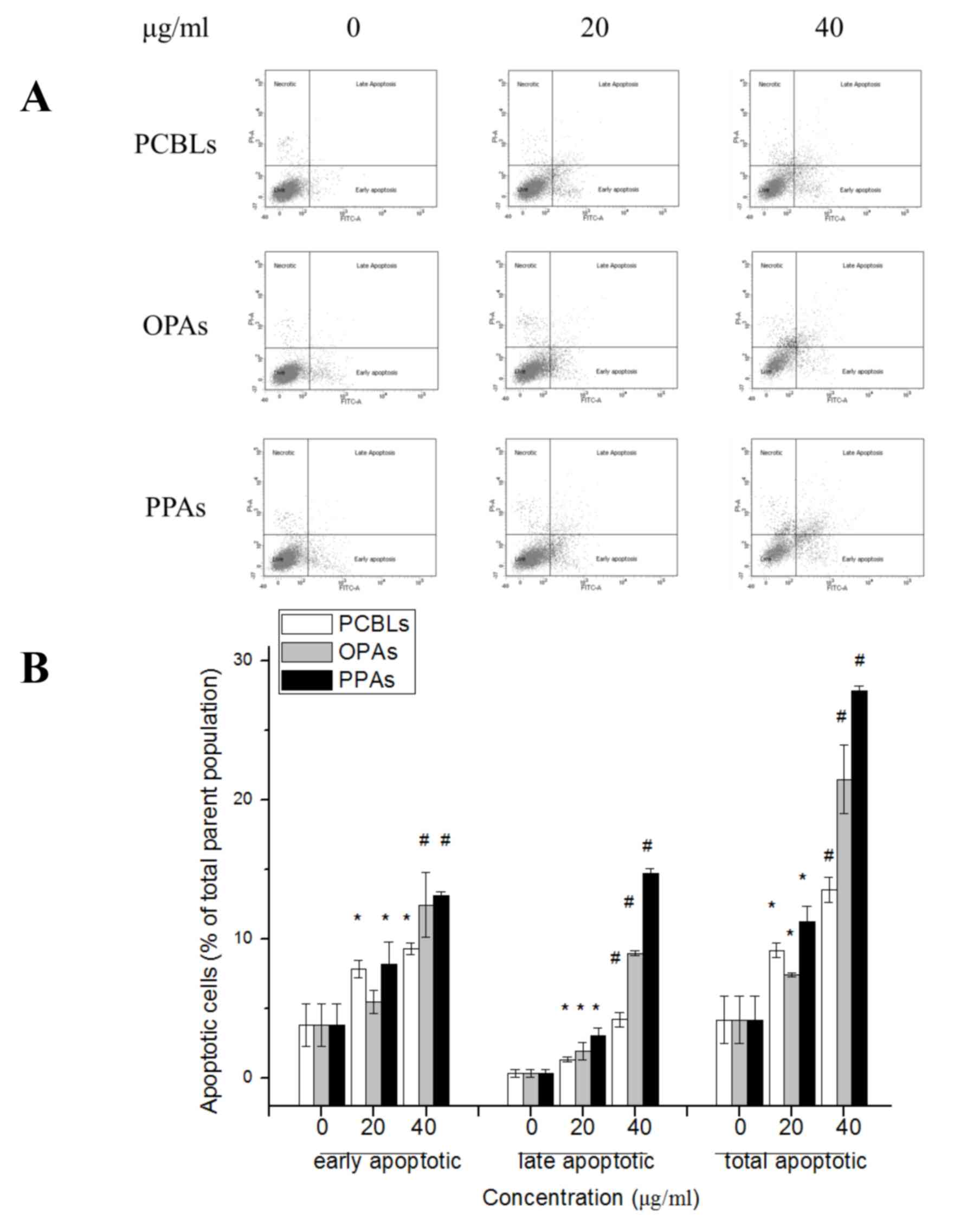
The rates of apoptosis in OVCAR-3 cells subsequent
to PCBL, OPA or PPA treatment were determined by flow cytometry.
The apoptotic cells were categorized as early or late apoptotic
cells, which are presented in the lower right and upper right
quadrants of the fluorescence-activated cell sorting histograms,
respectively (Fig. 2A). PCBLs, OPAs
and PPAs induced a significant concentration-dependent induction of
apoptosis at the early and late stages of apoptosis following 24 h
of treatment (P<0.05; Fig. 2B).
The rates of total apoptosis increased from 4.22±1.73% in untreated
control cells to 13.57±0.90, 21.47±2.44 and 27.83±0.32% in cells
treated with PCBLs, OPAs and PPAs at 40 µg/ml, respectively.
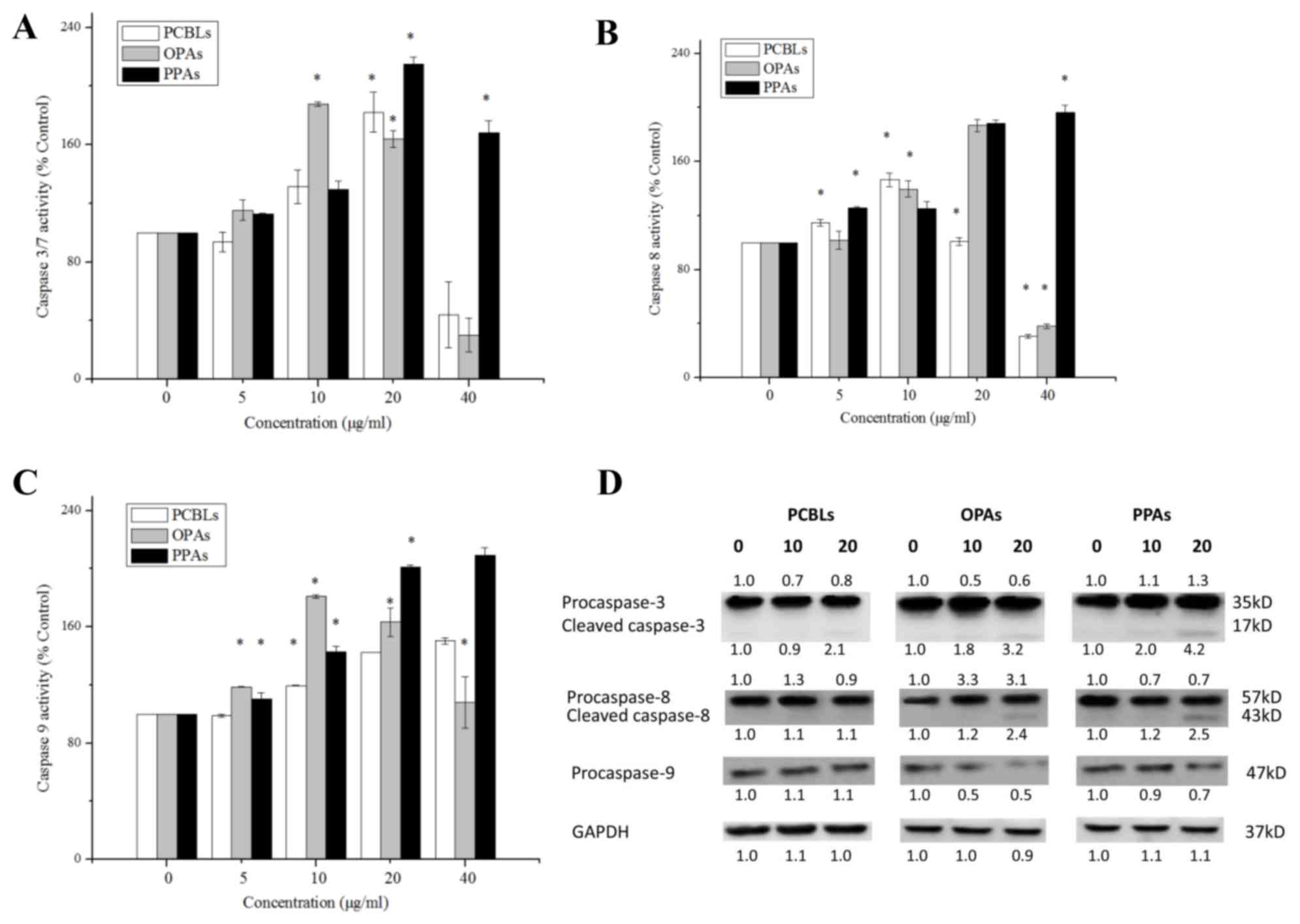
PCBL, OPA and PPA-induced apoptosis is
caspase-dependent in OVCAR-3 cells
Apoptosis may be initiated through an extrinsic
pathway, which is associated with the activation of caspase-8, or
an intrinsic pathway, which is associated with the activation of
caspase-9. These two pathways converge on the activation of
caspase-3/7 and trigger apoptosis (12). In order to investigate the induction
of apoptosis by PCBLs, OPAs and PPAs, the enzymatic activity levels
of caspase-3/7, −8 and −9 were evaluated using Caspase-Glo Assay
kits and western blot analysis in OVCAR-3 cells.
As presented in Fig.
3A, treatment with 20 µg/ml PCBLs, 10 µg/ml OPAs or 20 µg/ml
PPAs maximally increased the caspase-3/7 enzymatic activity levels
to 1.82-, 1.88- and 2.15-fold of the levels of the control group (0
µg/ml), respectively (P<0.05). With regard to the caspase-8
enzymatic activity, treatment with 10 µg/ml PCBLs, 20 µg/ml OPAs
and 40 µg/ml PPAs maximally increased the levels to 1.46-, 1.87-
and 1.96-fold of the control levels, respectively (P<0.05;
Fig. 3B). As shown in Fig. 3C, treatment with 40 µg/ml PCBLs, 10
µg/ml OPAs and 40 µg/ml PPAs, maximally increased the caspase-9
enzymatic activity to 1.50-, 1.81- and 2.09-fold of the control
levels, respectively (P<0.05).
Western blotting indicated that the PCBLs, OPAs and
PPAs decreased the expression levels of procaspase-3, procaspase-8
and procaspase-9 in a concentration-dependent manner (Fig. 3D). In addition, the level of cleaved
caspase-3 increased, which was in accordance with the
aforementioned Caspase-Glo results, and OPAs and PPAs also
increased the expression levels of cleaved caspase-8. These
findings suggest that PCBLs, OPAs and PPAs induce caspase-dependent
apoptosis.
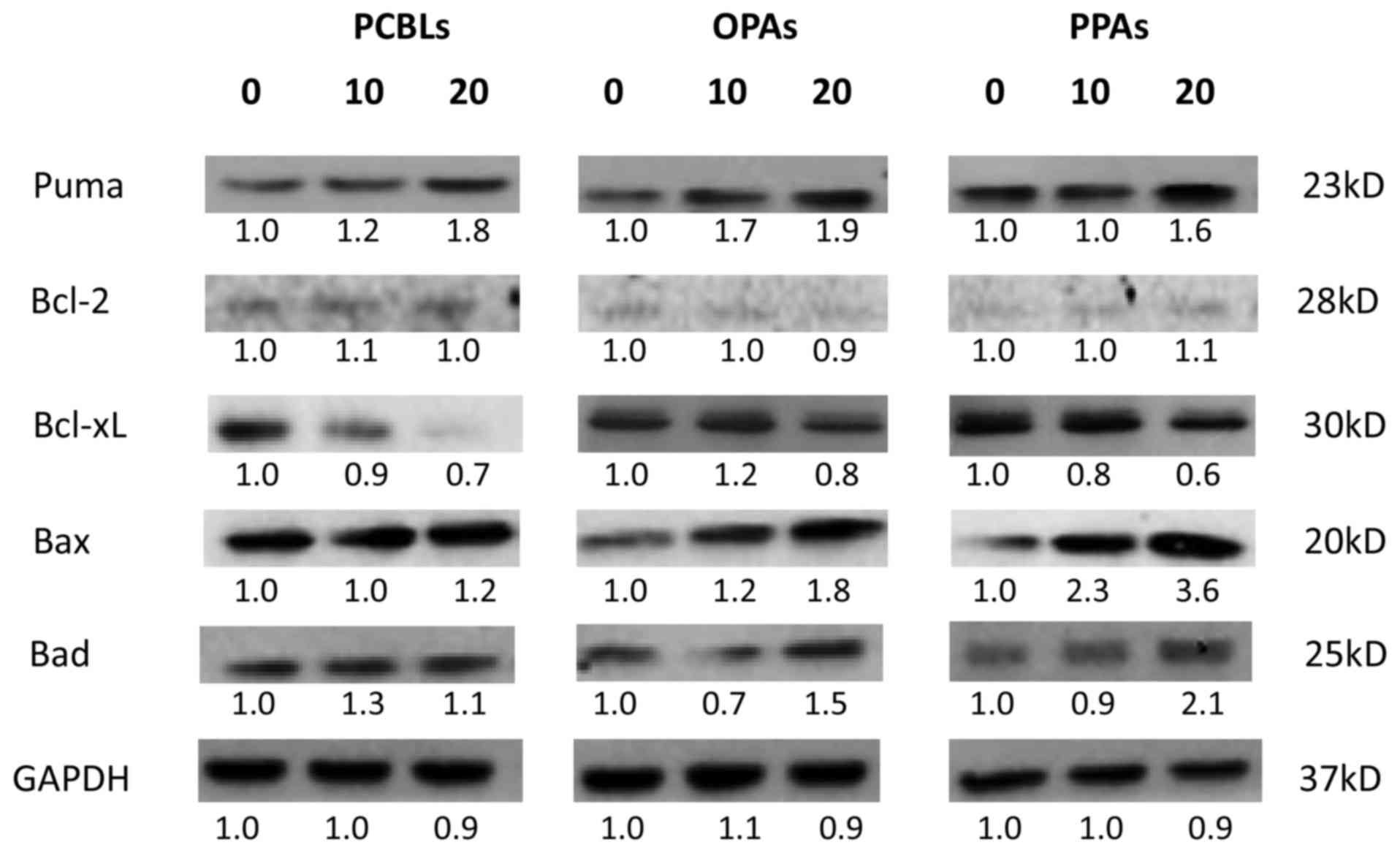
Effect of PCBLs, OPAs and PPAs on the
intrinsic apoptotic pathway
To clarify whether the intrinsic pathway was
involved in PCBL, OPA and PPA-induced apoptosis, western blot
analysis was used to examine the protein expression levels of
various proapoptotic Bcl-2 family proteins, including PUMA, Bax and
Bcl-2-associated death promoter (Bad). The antiapoptotic Bcl-2
family proteins Bcl-2 and Bcl-extra large (xL) were also assessed
(Fig. 4). PCBLs, OPAs and PPAs
increased the expression levels of PUMA, Bax and Bad. In addition,
an inhibitory effect of PCBLs, OPAs and PPAs on Bcl-xL was
observed. These findings suggest that PCBLs, OPAs and PPAs can
induce apoptosis in OVCAR-3 cells via the intrinsic apoptotic
pathway.
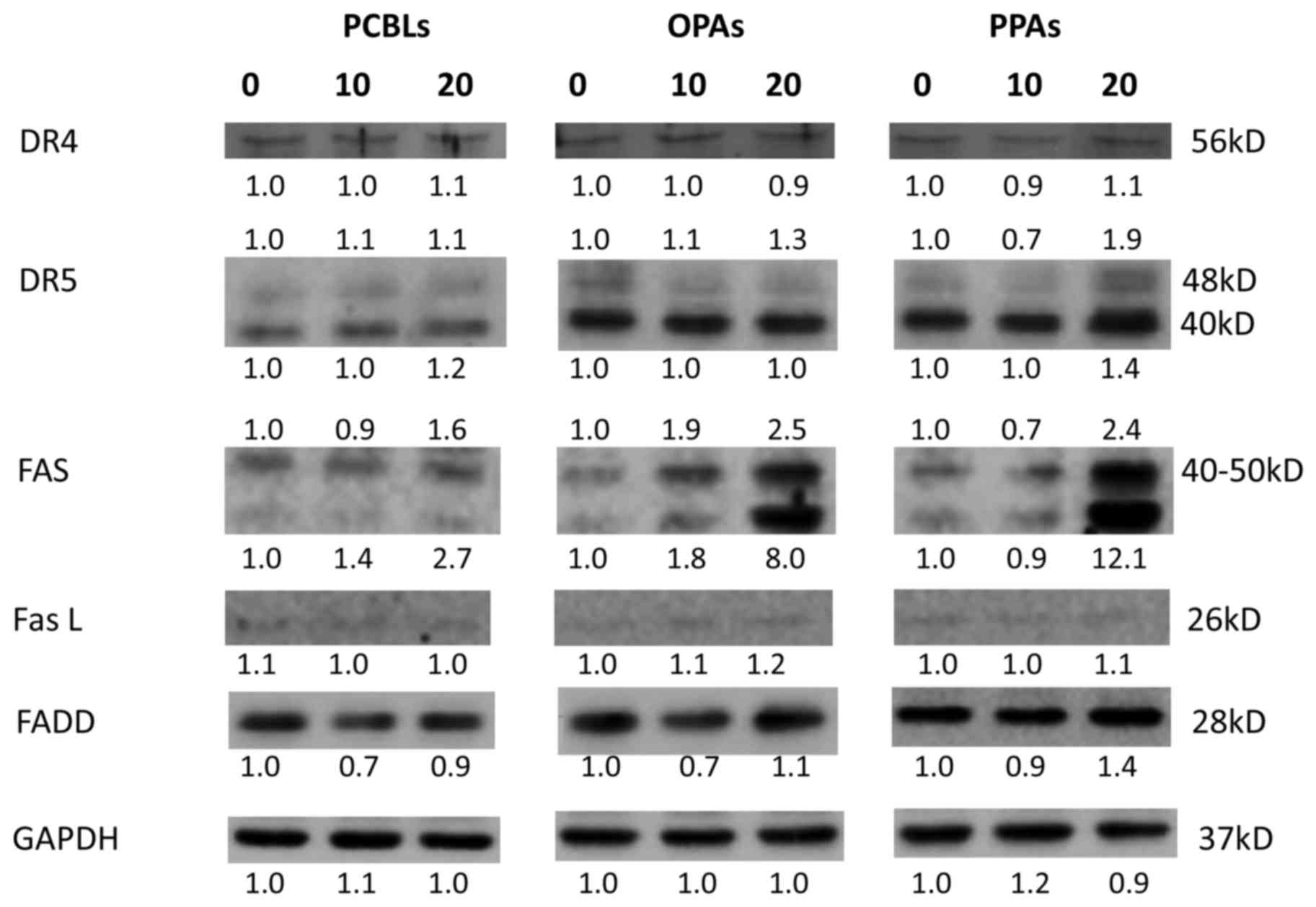
Effect of PCBLs, OPAs and PPAs on the
extrinsic apoptotic pathway
The present study subsequently investigated whether
the PCBLs, OPAs and PPAs induced apoptosis via the extrinsic
apoptotic pathway. As presented in Fig.
5, PCBLs only induced Fas expression, whereas OPAs and PPAs
increased the expression of Fas and death receptor (DR) 5 proteins
(48 kDa). PPAs also exerted a stimulatory effect on FADD protein.
PCBLs exhibited no effect on DR4, DR5, FasL or FADD expression. As
an increase in Fas expression occurred subsequent to PCBL, OPA or
PPA treatment, the results suggest that PCBLs, OPAs and PPAs may
induce apoptosis in OVCAR-3 cells through a Fas-associated
extrinsic pathway.
Role of p53 in PCBL-, OPA- and
PPA-induced apoptosis
p53 is crucial in the induction of apoptosis in
human and murine cells following DNA damage (13). Although OVCAR-3 cells harbor a point
mutation in the p53 gene, which results in single amino acid
changes, p53 still serves an important role in the apoptosis and
cell cycle arrest of OVCAR-3 cells induced by certain cytokines and
compounds (14–16). p53 levels are primarily controlled by
the proto-oncogene product mouse double minute 2 (MDM2), which
ubiquitinates p53 and facilitates the degradation of the protein by
proteasomes (17). p21 is a p53
transcription target involved in the major functions of tumor
suppressor cell cycle arrest and apoptosis (18).
As presented in Fig.
6A, the results of the current study revealed that OPAs and
PPAs stimulated p53, MDM2 and p21 expression in a
concentration-dependent manner, while PCBLs exhibited no effect on
these proteins. In order to clarify the effect of p53 on the
induction of apoptosis by OPAs and PPAs, a p53-specific siRNA was
used. The results presented in Fig.
6B indicated that the knockdown of p53 by the siRNA (50 nM)
resulted in the inhibition of p53 expression subsequent to OPA and
PPA treatment (0–20 µg/ml). The depletion of p53 led to an
associated decrease in the expression of p21, DR5, Fas, PUMA and
phosphatase and tensin homolog proteins. These observations suggest
that p53 is a critical mediator of OPA- and PPA-induced apoptosis
in OVCAR-3 cells.
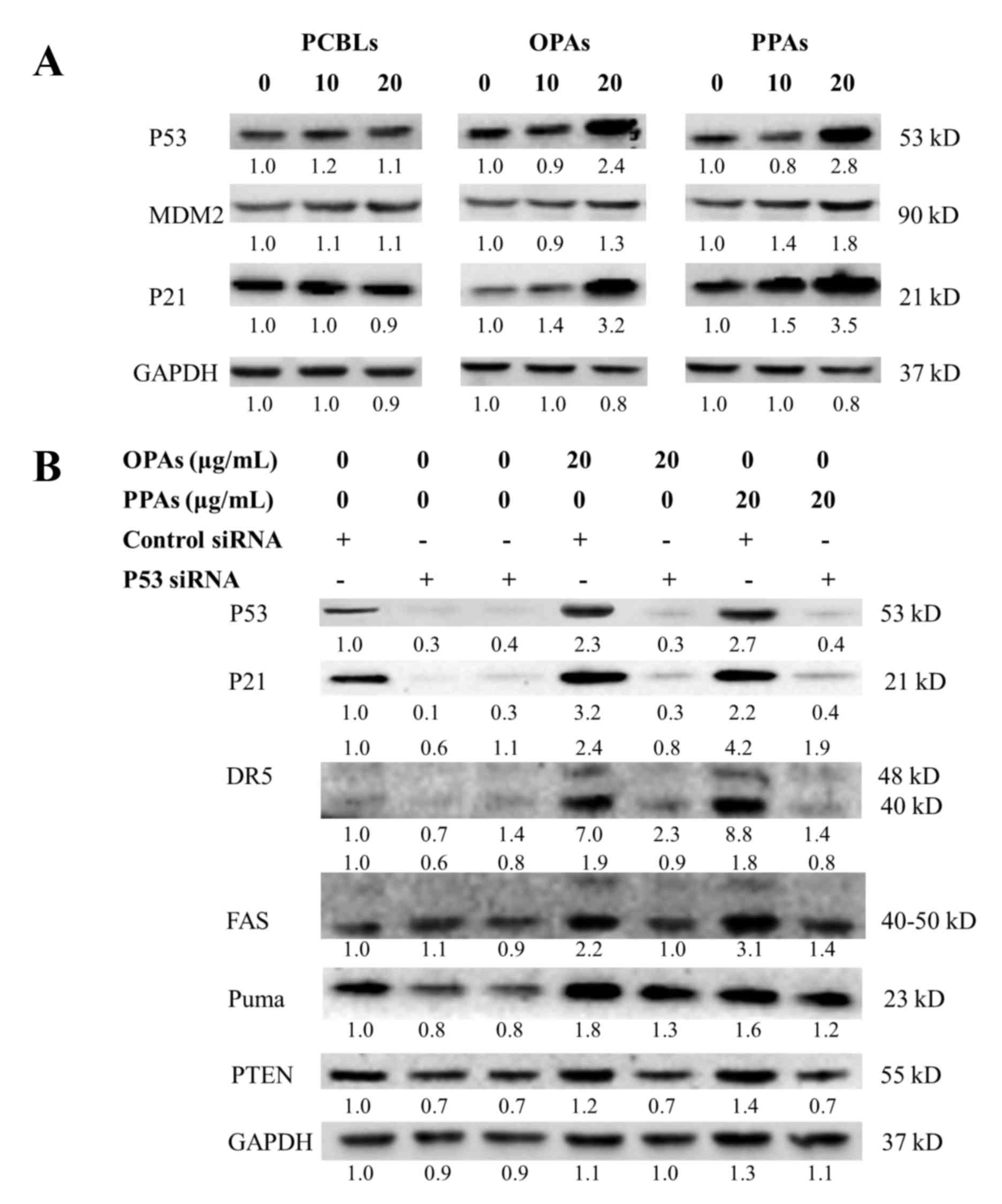 | Figure 6.p53 is associated with OPA- and
PPA-induced apoptosis in OVCAR-3 cells. (A) Effects of PCBLs, OPAs
and PPAs on the protein expression of p53, MDM2 and p21 were
determined by western blot analysis. (B) Effect of p53 siRNA (50
nM) on the protein expression of p21, DR5, Fas, PUMA and PTEN were
determined by western blot analysis. Results are representative of
≥2 independent experiments that showed similar patterns. Protein
expression levels were measured by densitometry and the values
above and below the blots indicate the relative ratios. PCBL,
Chinese bayberry leaf prodelphinidin extract; OPA, oligomeric
proanthocyanidin; PPA, polymeric proanthocyanidin; MDM2, mouse
double minute 2 homolog; PTEN, phosphate and tensin homolog; si,
small interfering; PUMA, p53-upregulated modulator of apoptosis;
DR5, death receptor 5. |
Discussion
To the best of our knowledge, the present study was
the first to examine the apoptotic effect of PCBLs. According to
our previous studies, PCBLs are almost entirely of the
galloylated-prodelphinidin type, which is unusual in the plant
kingdom as plants normally contain procyanidins and a few
prodelphins (6,7). The structural units of prodelphinidins
in Chinese bayberry leaves are epicatechin (EC), epicatechin
gallate (ECG), epigallocatechin (EGC) and EGCG (6,7). OPAs are
fractions collected from preparative HPLC, which mainly contain
dimers and trimers including EGC+ECG, EGC+EGCG, 2EGCG, 2ECG,
2EC+EGC, 2EGC+EGCG and 3EGCG. PPAs are fractions composed of
proanthocyanidin tetramers or polymers of a higher polymerization
degree, including 2EGC+2EGCG, EGC+3EGCG, 3ECG+EGCG, 4EGCG and
numerous other unidentified proanthocyanidins. OPAs constitute
~23.9% of PCBLs, whereas PPAs constitute ~47.8%.
The present study demonstrated that PCBLs, OPAs and
PPAs induced apoptosis in a concentration-dependent manner in
OVCAR-3 cells, a finding that was confirmed by Hoechst 33342 DNA
staining and annexin V and PI staining. The results revealed that
PCBLs, OPAs and PPAs led to early and late apoptosis in cells in a
concentration-dependent manner. According to the total apoptotic
cell percentage, PPAs were more effective than OPAs for inducing
apoptosis.
Based on the observed increase in apoptosis in the
treated cells, the next aim of the present study was to examine the
involvement of caspases, which have major roles in the execution of
apoptotic events. Therefore, the levels of active caspases were
investigated by Caspase-Glo assay in cell lysates. The experiments
of the present study revealed that treatment of cells with 20 or 40
µg/ml PPAs was associated with the most prominent increase in the
activation of caspase-3/7, −8 and −9. The results indicated that
PCBLs, OPAs and PPAs exerted a strong and concentration-dependent
effect on the induction of active caspase-3/7, −8 and −9
levels.
The majority of caspase-dependent apoptosis is
associated with the mitochondrial pathway (9). The present study demonstrated that
PCBLs, OPAs and PPAs increased the protein expression of the
proapoptotic Bcl-2 family proteins PUMA, Bax and BAD, and decreased
the protein expression of the anti-apoptotic Bcl-2 family protein
Bcl-xL (Fig. 4). However, due to the
low expression level of Bcl-2, an inhibitory effect on this protein
was not observed. PCBLs, OPAs and PPAs were also observed to affect
the extrinsic pathway, primarily via increases in DR5 and Fas
expression levels.
Grape seed extract (GSE) is one of the most well
known nutrition supplements containing proanthocyanidins. A number
of studies have demonstrated that grape seed procyanidins induce
apoptosis in cancer cells through the intrinsic and extrinsic
pathways by downregulating antiapoptotic proteins and upregulating
several proapoptotic factors. This eventually leads to the
activation of caspases-9 and −3 (19–21), which
is in accordance with the results of the present study. The present
study also showed that p53 is associated with OPA- and PPA-induced
apoptosis (Fig. 6). However, several
studies have found that the cytotoxic effect exhibited by overall
GSE is independent of the p53 status of the cancer cell lines
(22,23).
In the present study OPAs and PPAs exhibited strong
effects on OVCAR-3 cell proliferation and apoptosis. Several
studies have reported evidence that monomeric catechins and
proanthocyanidin monomers, dimers and trimers can be absorbed
through human intestinal Caco-2 epithelial cells (24,25).
However, the results of the present study revealed that PPAs
(prodelphinidin tetramers and higher polymerizations) exerted
greater cytotoxic and apoptotic activities compared with OPAs
(dimers and trimers) in general. Similar results were observed by
Miura et al (8), who separated
eight procyanidin fractions according to the degree of
polymerization using normal-phase chromatography, and detected
strong antiproliferative activity of the procyanidin pentamers and
higher degree fractions in B16 and BALB-MC.E12 cells.
Although the precise effect of the degree of
polymerization remains unclear, diversity in prodelphinin
stereochemistry, structure, molecular size, polarity and solubility
may affect the bioavailability of prodelphinidins. Additional
investigation into bioavailability, including analysis of
metabolites and distribution, are required to clarify the
anticancer activity of prodelphinidins, particularly polymers
higher than tetramers, following oral administration in
vivo.
Acknowledgements
The authors would like to thank Dr Kathy Brundage
from the Flow Cytometry Core at West Virginia University for
providing technical help regarding cell apoptosis, Dr Haizhi Huang
for expert technical assistance, and Mrs. Amy Mason-Hopkins for
assistance with writing the study. The present study was supported
by grants from the West Virginia Experimental Program to Stimulate
Competitive Research (grant no. EPS-1003907) and the National
Institutes of Health (grant nos. P20RR016477 and P20GM103434)
awarded to the West Virginia IDeA Network of Biomedical Research
Excellence. The present study was also supported by the Zhejiang
Province Sci-tech Special Commissioner Earmarks (grant no.
2012T2T123).
References
|
1
|
Nandakumar V, Singh T and Katiyar SK:
Multi-targeted prevention and therapy of cancer by
proanthocyanidins. Cancer Lett. 269:378–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prior RL and Gu L: Occurrence and
biological significance of proanthocyanidins in the American diet.
Phytochemistry. 66:2264–2280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de la Iglesia R, Milagro FI, Campión J,
Boqué N and Martínez JA: Healthy properties of proanthocyanidins.
Biofactors. 36:159–168. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen K, Xu C, Zhang B and Ferguson IB: Red
bayberry: Botany and horticulture. Horticultural Rev. 30:83–114.
2004.
|
|
5
|
Zhang SM, Gao ZS, Xu CJ and Chen KS:
Genetic diversity of Chinese bayberry (Myrica rubra Sieb. et
Zucc.) accessions revealed by amplified fragment length
polymorphism. Hort Sci. 44:487–491. 2009.
|
|
6
|
Fu Y, Qiao L, Cao Y, Zhou X, Liu Y and Ye
X: Structural elucidation and antioxidant activities of
proanthocyanidins from Chinese bayberry (Myrica rubra Sieb.
et Zucc.) leaves. PLoS One. 9:e961622014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang H, Ye X, Liu D, Chen J, Zhang J, Shen
Y and Yu D: Characterization of unusual proanthocyanidins in leaves
of bayberry (Myrica rubra Sieb. et Zucc.). J Agric Food
Chem. 59:1622–1629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miura T, Chiba M, Kasai K, Nozaka H,
Nakamura T, Shoji T, Kanda T, Ohtake Y and Sato T: Apple
procyanidins induce tumor cell apoptosis through mitochondrial
pathway activation of caspase-3. Carcinogenesis. 29:585–593. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han MH, Lee WS, Jung JH, Jeong JH, Park C,
Kim HJ, Kim G, Jung JM, Kwon TK, Kim GY, et al: Polyphenols
isolated from Allium cepa L. induces apoptosis by suppressing IAP-1
through inhibiting PI3K/Akt signaling pathways in human leukemic
cells. Food Chem Toxicol. 62:382–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai GH, Meng GM, Tong YL, Chen X, Ren ZM,
Wang K and Yang F: Growth-inhibiting and apoptosis-inducing
activities of Myricanol from the bark of Myrica rubra in
human lung adenocarcinoma A549 cells. Phytomedicine. 21:1490–1496.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaur M, Mandair R, Agarwal R and Agarwal
C: Grape seed extract induces cell cycle arrest and apoptosis in
human colon carcinoma cells. Nutr Cancer. 60:(Suppl 1). S2–S11.
2008. View Article : Google Scholar
|
|
12
|
Kroemer G and Martin SJ:
Caspase-independent cell death. Nat Med. 11:725–730. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang C, Ma WY, Goranson A and Dong Z:
Resveratrol suppresses cell transformation and induces apoptosis
through a p53-dependent pathway. Carcinogenesis. 20:237–242. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guan YQ, Li Z, Yang A, Huang Z, Zheng Z,
Zhang L, Li L and Liu JM: Cell cycle arrest and apoptosis of
OVCAR-3 and MCF-7 cells induced by co-immobilized TNF-α plus IFN-γ
on polystyrene and the role of p53 activation. Biomaterials.
33:6162–6171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin HY, Delmas D, Vang O, Hsieh TC, Lin S,
Cheng GY, Chiang HL, Chen CE, Tang HY, Crawford DR, et al:
Mechanisms of ceramide-induced COX-2-dependent apoptosis in human
ovarian cancer OVCAR-3 cells partially overlapped with resveratrol.
J Cell Biochem. 114:1940–1954. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li B, Gao Y, Rankin GO, Rojanasakul Y,
Cutler SJ, Tu Y and Chen YC: Chaetoglobosin K induces apoptosis and
G2 cell cycle arrest through p53-dependent pathway in
cisplatin-resistant ovarian cancer cells. Cancer Lett. 356:418–433.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang X, Fraser M, Moll UM, Basak A and
Tsang BK: Akt-mediated cisplatin resistance in ovarian cancer:
Modulation of p53 action on caspase-dependent mitochondrial death
pathway. Cancer Res. 66:3126–3136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia M, Knezevic D and Vassilev LT: p21
does not protect cancer cells from apoptosis induced by
nongenotoxic p53 activation. Oncogene. 30:346–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsu CP, Lin YH, Chou CC, Zhou SP, Hsu YC,
Liu CL, Ku FM and Chung YC: Mechanisms of grape seed
procyanidin-induced apoptosis in colorectal carcinoma cells.
Anticancer Res. 29:283–289. 2009.PubMed/NCBI
|
|
20
|
Singh T, Sharma SD and Katiyar SK: Grape
proanthocyanidins induce apoptosis by loss of mitochondrial
membrane potential of human non-small cell lung cancer cells in
vitro and in vivo. PLoS One. 6:e274442011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dinicola S, Cucina A, Antonacci D and
Bizzarri M: Anticancer effects of grape seed extract on human
cancers: A review. J Carcinogenesis Mutagenesis. 8:60–70. 2014.
|
|
22
|
Dinicola S, Cucina A, Pasqualato A,
D'Anselmi F, Proietti S, Lisi E, Pasqua G, Antonacci D and Bizzarri
M: Antiproliferative and apoptotic effects triggered by Grape Seed
Extract (GSE) versus epigallocatechin and procyanidins on colon
cancer cell lines. Int J Mol Sci. 13:651–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prasad R and Katiyar SK: Bioactive
phytochemical proanthocyanidins inhibit growth of head and neck
squamous cell carcinoma cells by targeting multiple signaling
molecules. PLoS One. 7:e464042012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deprez S, Mila I, Huneau JF, Tome D and
Scalbert A: Transport of proanthocyanidin dimer, trimer, and
polymer across monolayers of human intestinal epithelial Caco-2
cells. Antioxid Redox Signal. 3:957–967. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Faria A, Mateus N, de Freitas V and Calhau
C: Modulation of MPP+ uptake by procyanidins in Caco-2 cells:
Involvement of oxidation/reduction reactions. FEBS Lett.
580:155–160. 2006. View Article : Google Scholar : PubMed/NCBI
|