Introduction
Endometrial carcinoma (EC) is one of the most common
female pelvic malignancies; it develops in ~142,000 women worldwide
and is responsible for ~42,000 mortalities each year (1). The 5-year survival rate is 95, 67 or
16%, if the cancer is diagnosed at a local, regional or distant
stage, respectively (2). In China,
the number of women with newly diagnosed endometrial cancer has
also significantly increased annually (3).
Current treatments for EC comprise surgery, hormonal
therapy, radiotherapy and chemotherapy. Young patients (age, ≤40
years) who suffer from endometrial atypical hyperplasia or
well-differentiated EC classified as Federation of Gynecology and
Obstetrics stage IA (intramucous) may choose hormonal treatment if
they decide to preserve fertility (4). However, the risk of non-response, tumor
progression and recurrence remain (5). The cornerstone of treatment for EC is
surgery (6); early-stage patients can
achieve a satisfying outcome, but the outcomes of high-risk
patients are not positive, and the patients also require adjuvant
therapy, such as radiotherapy and/or chemotherapy. Radiotherapy,
including vaginal brachytherapy and pelvic external beam
radiotherapy, is the main method of postoperative adjuvant
treatment, and can decrease the local recurrence rate (7), but no overall survival rate improvement
can be found in the high-risk group (8). The cytotoxic therapies available for the
treatment of advanced-stage, progressive and recurrent disease have
shown limited success (9–11). Therefore, exploration of new
therapeutic strategies continues to be urgently required.
Phosphate and tensin homolog (PTEN) is a tumor
suppressor gene, and loss of function mutations are common and
appear to be important in the pathogenesis of EC (12). Silencing of PTEN is frequently
associated with advanced EC and is likely to play a critical role
in promoting AKT activation (13).
Epidemiological evidence strongly suggests that
diets rich in fruit and vegetables are associated with reduced
risks of cancers (14). Momordica
charantia (MC), often termed bitter melon, grows in tropical
Asia. The fruit has been widely used as food and herbal medicine in
China for centuries. However, little is known about the mechanism
of the effect of MC, which limits the use of MC worldwide.
Recently, scientists have elucidates that MC is capable of
controlling plasma glucose, and has anti-viral, anti-fertility,
immunomodulatory and antitumor effects (15–21). Our
previous study successfully extracted a new protein with a
molecular weight of 30 kDa from MC seeds and termed it MC protein
(MCP30) (22). MCP30 is a ribosome
inactivating protein (RIP), which is a type of protein that can
inhibit protein synthesis in cell system or cell-free system
(23,24).
In the present study, the effects of MCP30 on
proliferation, cell cycle arrest, apoptosis and the AKT signal
pathway in the human endometrial carcinoma Ishikawa H cell line
were investigated in vitro.
Materials and methods
Reagents
RPMI-1640 medium, penicillin and streptomycin were
obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Fat-free milk (5%) was obtained from Bright Dairy (Shanghai,
China). Fetal bovine serum (10%; FBS) was purchased from ZhengJiang
High Technology (Tianjin, China). Tween-20, rabbit primary
anti-p-AKT (dilution, 1:1,000; SAB4301414), anti-AKT (dilution,
1:1,000; SAB4500797) and anti-PTEN (dilution, 1:1,000; SAB4300337)
polyclonal antibodies, horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG secondary antibody (dilution, 1:10,000; A0545) and
GAPDH antibody (dilution, 1:1,000; G9545) were purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). The
chemiluminescent substrate for HRP was obtained from Pierce (Thermo
Fisher Scientific, Inc.). MCP30 was extracted from bitter melon
seeds, prepared by Xiong et al, as previously described
(22).
Cell culture
The Ishikawa H cell line was kindly provided by the
Women's Hospital, School of Medicine, Zhejiang University
(Hangzhou, China), and was cultured in RPMI-1640 medium, which was
supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml
streptomycin, at 37°C in a fully humidified incubator containing 5%
CO2.
Cell viability assay
Cell viability in various concentrations of MCP30
(0, 8.33, 16.67, 33.33, 166.67, 333.33 and 666.67 pM; these
concentrations were selected due to preliminary experiments) for
24, 48 and 72 h was assessed using the Cell Counting Kit-8 (CCK-8
kit; Dojindo Laboratories, Kumamoto, Japan), according to the
manufacturer's protocol. In brief, 10 µl of CCK-8 solution and 100
µl of cell culture supernatants (5×103 cells, log phase)
were added to each well of the 96-well plate (Corning, Inc.,
Corning, NY, USA). The reaction system was incubated at 37°C for 1
h. The absorbance was detected at a 450-nm wavelength using a
microplate reader. Cell growth inhibition was measured using the
following formula: Cell growth inhibition rate (%)=[1-(value of
experimental group-value of blank group)/(value of control
group-value of blank group)]x100.
DNA fragmentation assay
The DNA of cells treated with a series of
concentrations of MCP30 for 72 h was extracted using the selected
DNA Ladder Extraction kit from Aidlab Biotechnologies (Beijing,
China), according to the manufacturer's protocol. The DNA
fragmentation was assayed by electrophoresis on a 1.5% agarose gel
and its pattern was examined on the images obtained under
ultraviolet illumination. Images were captured by Image Lab
Software (Bio-Rad Laboratories, Hercules, CA, USA).
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double staining assay
The cell apoptosis assay was performed using flow
cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA)
and was detected with the Annexin V-FITC/PI Apoptosis Detection kit
(BD Biosciences). Subsequent to culture of cells in 666.67 pM MCP30
for 72 h at 37°C, apoptotic cells were treated with the agents of
the Annexin V-FITC/PI Apoptosis Detection kit (composed of Annexin
V binding buffer, Annexin V-FITC and PI staining solution),
according to the manufacturer's protocol. In brief, cells were
resuspended in 200 µl Annexin V binding buffer and subsequently
incubated with 5 µl Annexin V-FITC and 10 µl PI for 15 min in room
temperature. Subsequently, 200 µl Annexin V binding buffer was
added. After 1 h, the FITC/PI double staining reaction system was
detected using flow cytometry (excitation wavelength, 488 nm;
emission wavelength, 530 nm).
Cell cycle analysis
Cells were seeded in 12-well plates at a density of
4×104 cells per well in 2 ml of complete culture medium.
Subsequent to culturing with MCP30 (166.67, 333.33 or 666.67 pM)
for 48 or 72 h, cells were analyzed using Flow Cytometry Analysis
of Cell Cycle kit (GenMed; Seisa, Plymouth, MN, USA) with a
FACSCalibur Flow Cytometer (BD Biosciences) and distribution of the
cell-cycle phases was determined using CellQuest Software (BD
Biosciences).
Western blot analysis
Total proteins from the cells were prepared by cell
lysis buffer (Applygen Technologies Inc., Beijing, China) and
phenylmethylsulfonyl fluoride (Sigma-Aldrich; Merck Millipore)
inactivated protease. The protein concentration was determined
using the bicinchoninic acid method. Protein extracts were
fractionated on 12% polyacrylamide SDS gel and then transferred to
a polyvinylidene fluoride membrane. The membrane was blocked with
5% fat-free milk in Tris-buffered saline with Tween-20 (0.1%),
followed by incubation with rabbit anti-rat primary anti-p-AKT
(dilution, 1:1,000), anti-AKT (dilution, 1:1,000) and anti-PTEN
(dilution, 1:1,000) polyclonal antibodies at 4°C for 20 h.
Subsequent to washing the membrane with TBST, the membrane was
treated with HRP-conjugated goat anti-rat secondary antibody
IgG-HRP (dilution, 1:10,000) for 1 h at room temperature via
agitation. The enhanced HRP-DAB substrate solution was added to the
membrane and incubated for 5 min. Bands were visualized by
chemiluminescence and exposed to X-ray. The GAPDH antibody
(dilution, 1:1,000) was used as an internal control. The relative
optical density (ratio to GAPDH) of each blot band was quantified
by Quantity One 1-D analysis software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
Each experiment was repeated in triplicate. All data
were analyzed using SPSS Statistics 19.0 (IBM Co., Armonk, NY,
USA), and data were expressed as the mean ± standard deviation. For
comparisons among groups, independent-samples t-test and two-way
analysis of variance were performed, as appropriate. If the test of
homogeneity of variances was satisfied, then Tukey pairwise
comparison was used for post hoc analysis. If not, Dunnet's T3 test
was selected. P<0.05 was considered to indicate a statistically
significant difference.
Results
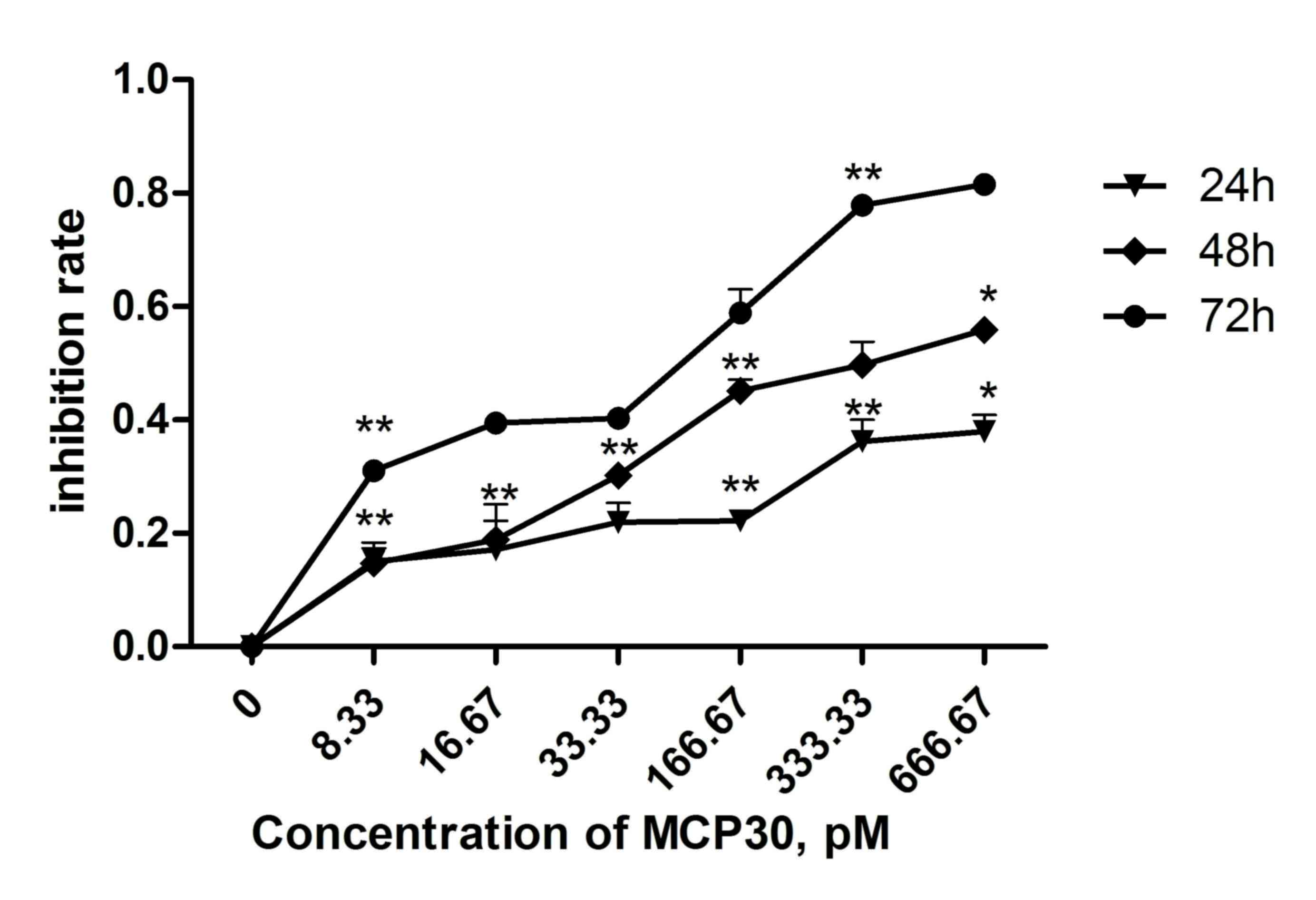
MCP30 decreased the viability of
Ishikawa H cells in a dose- and time-dependent manner
The effect of different concentrations of MCP30 on
cell viability was shown in Fig. 1.
MCP30 significantly decreased the cell viability in a dose- and
time-dependent manner (two-way analysis of variance; time,
F=89.529, P<0.001; concentration, F=56.119, P<0.001).
Following a 72 h incubation with 33.33 pM MCP30, the viability of
the cells was reduced by 40.26% (33.33 pM group vs. control group).
Additionally, 166.67 pM MCP30 reduced cell viability by 58.84%
(166.67 pM group vs. control group). The half-maximal inhibitory
concentration of MCP30 for 72 h was ~62.00 pM (data not shown).
These results indicated that treatment with MCP30 decreased the
viability of Ishikawa H cells.
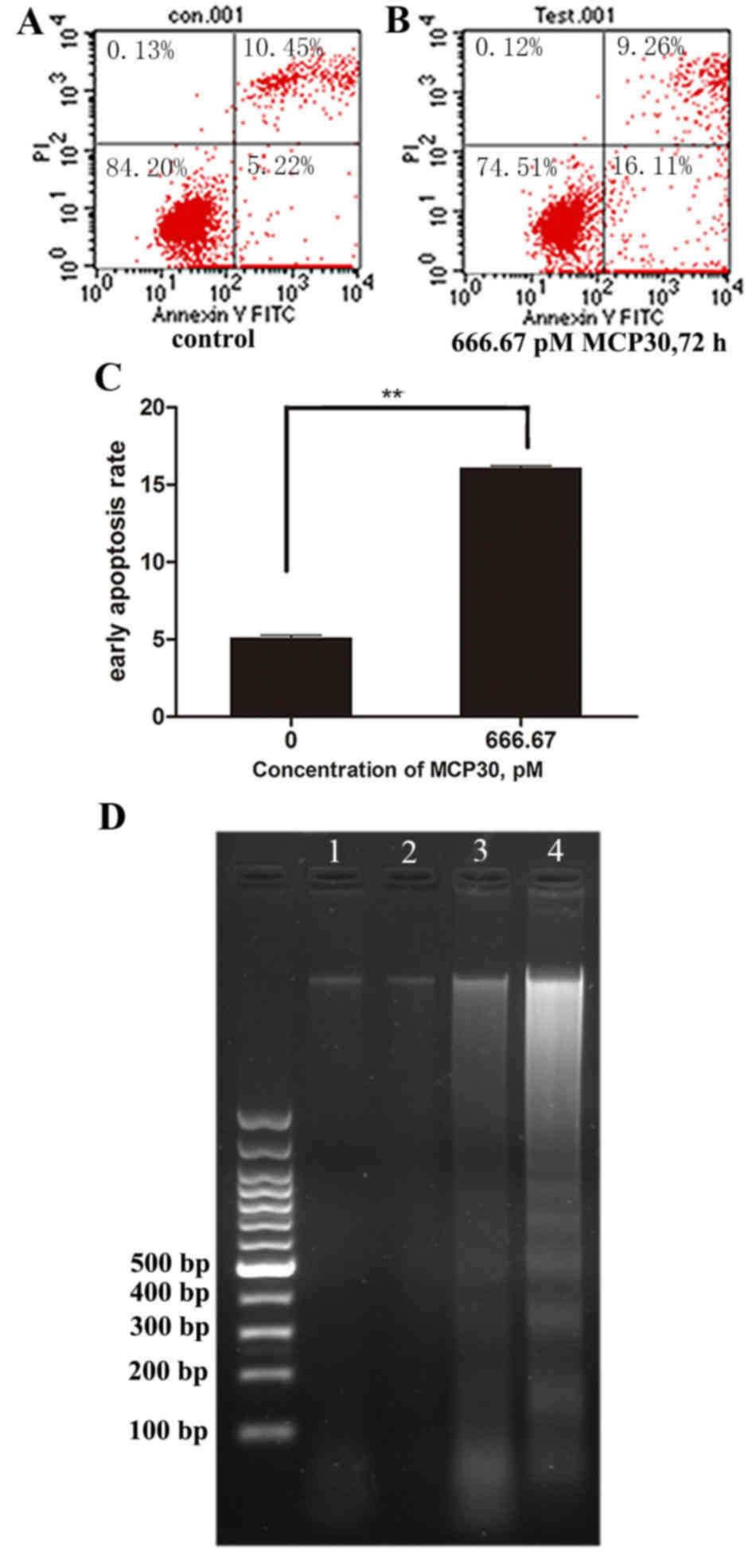
MCP30 induced early apoptosis in
Ishikawa H cells
The results of Annexin FITC/PI staining revealed
that cell viability decrease was associated with early apoptosis.
The early apoptotic rates of Ishikawa H cells treated with MCP30 at
666.67 pM reached to 16.07±0.15% following 72 h of treatment. By
contrast, the control cells showed early apoptosis rates of only
5.08±0.19% (t=76.589; P<0.001) (Fig.
2). Furthermore, DNA ladder was observed in cells treated with
333.33 pM and 666.67 pM MCP30 following 72 h of treatment (Fig. 2D; lanes 3 and 4), while no DNA ladder
was found in the blank control and 8.33 pM groups (lanes 1 and
2).
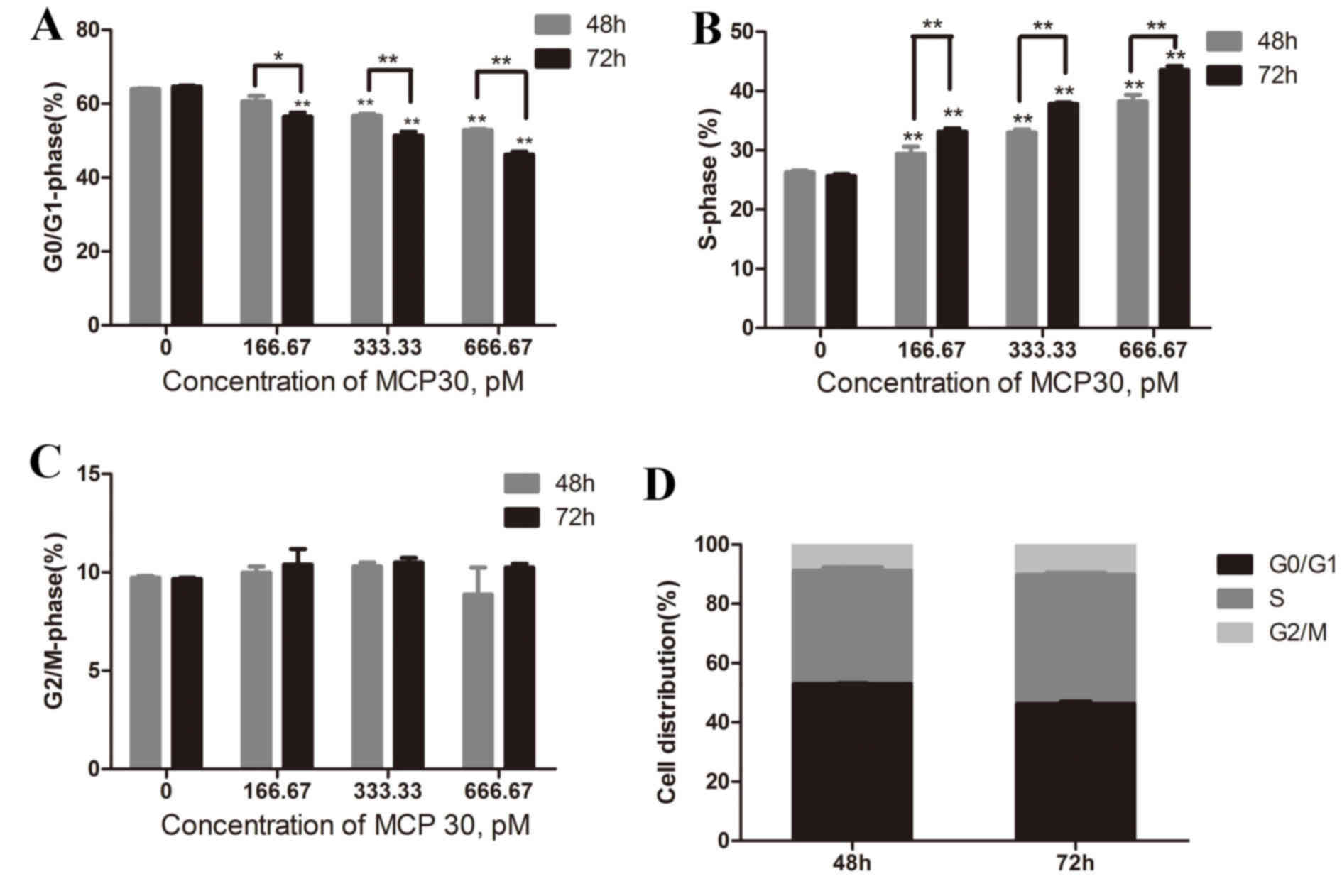
MCP30 affected the cell cycle
distribution of Ishikawa H cells in a time- and
concentration-dependent manner
Cell cycle analysis was performed by flow cytometry
(Fig. 3). Treatment with different
concentrations (166.67, 333.33 and 666.67 pM) of MCP30 for 48 h
resulted in the distribution of the cell phase changing so that the
higher the concentration added, the lower the G0/G1-phase rate and
the higher the S-phase rate (G0/G1-phase rate, F=106.866,
P<0.001; S-phase rate, F=99.686, P<0.001). The G2/M-phase
rate remained consistent. A similar result was obtained when the
time of treatment was prolonged to 72 h (G0/G1-phase rate,
F=169.836, P<0.001; S-phase rate, F=742.190, P<0.001). This
indicated that MCP30 blocks Ishikawa H cells from progressing
between the S-phase and the G2/M-phase in a time- and
concentration-dependent manner.
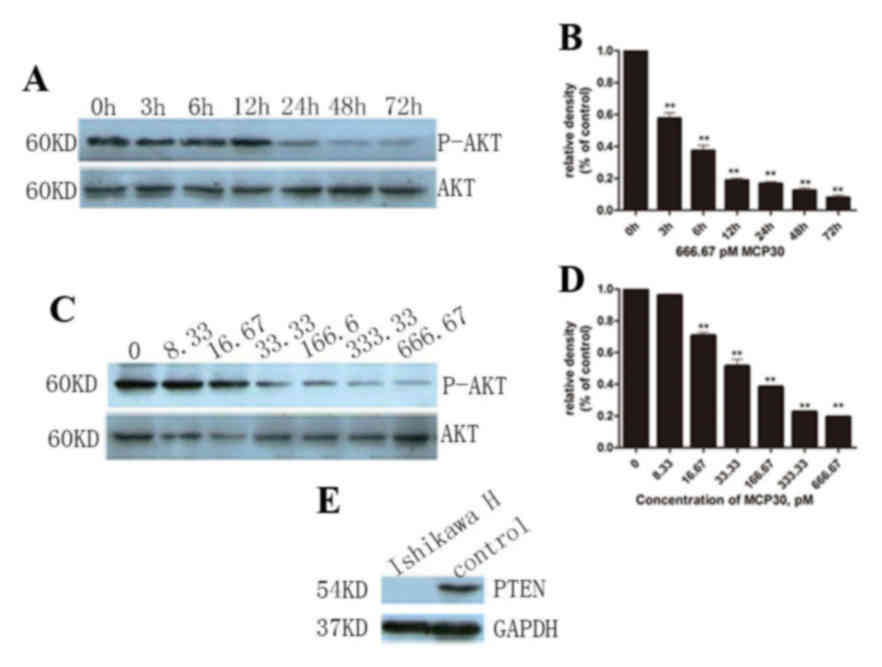
MCP30 induced Ishikawa H cell
apoptosis and S-phase arrest through the AKT signaling pathway
Finally, in order to evaluate the effect of culture
time on the P-AKT expression level, 666.67 pM MCP30 was added to
the cell culture system for various time points (0, 3, 6, 12, 24,
48 and 72 h). With increased culture time, the P-AKT level
decreased (F=286.582, P<0.001), but this change stopped when the
time reached 12 h, and no subsequent decrease was observed
(Fig. 4A and B). Thus, it was
hypothesized that 12 h is the best culture time for cells with
MCP30. The levels of P-AKT were detected by western blot analysis
following incubation with MCP30 for 72 h (Fig. 4C and D). MCP30 treatment decreased the
levels of P-AKT in a dose-dependent manner (F=975.799; P<0.001).
Furthermore, it was verified that Ishikawa H Cells lost PTEN
expression (Fig. 4E).
Discussion
Endometrial carcinoma (EC) is a leading female
pelvic malignancy, and the incidence rates of endometrial cancer
are increasing in Chinese women (3).
Although the mortality rate of EC has been significantly decreased
due to adjuvant therapies, the increased incidence, high relapse
rate and metastasis rate following treatment result in EC remaining
a major clinical hurdle (25).
Current treatments for EC comprise surgical resection, radiotherapy
hormonal therapy and chemotherapy; for the latter, there are
numerous studies investigating synthesized and natural medicinal
components (26,27). However, currently available and newly
found drugs for the treatment of patients with advanced-stage,
progressive or recurrent disease have shown limited success
(9–11). Therefore, exploration of new effective
drugs continues to be urgently required.
A large variety of natural compounds exhibit
antitumor effects, a number of these compounds have been used as
traditional herbs and are present in our daily diet (14). The plant MC, also termed bitter melon,
grows in tropical Asia, where it is utilized as medicinal herb and
food for centuries. Previously, studies have found that the protein
extracted from MC seeds have numerous pharmacological properties,
such as plasma glucose control (16),
and antiviral (17), anti-fertility
(18), immunomodulatory (19) and antitumor activities (28–31).
However, to the best of our knowledge, studies investigating the
effect of MCP30 on endometrial cancer have not yet been published.
In the present study, it was found that MCP30 exhibited potent
cytotoxic activity in EC cells.
EC is classified into two types (types 1 and 2),
with the most common lesions (type 1) typically being
hormone-sensitive (6). Therefore, the
Ishikawa H cell line, a type of estrogen-dependent endometrial
cancer cell line (32), was chosen.
The CCK-8 results showed that MCP30 inhibited cell viability in a
dose- and time-dependent manner. In the cell cycle experiment, it
appeared that MCP30 may induce S-phase arrest in EC cells. In
addition, previous studies have suggested that MCP30 is a type I
RIP (33). At present, it is
acknowledged that RIPs are classified into two major types
(34). Type I RIPs consist of only a
single rRNA-cleaving domain and have a molecular weight ~30 kDa,
while type II RIPs have another B chain, which make them manifest
marked cytotoxicity, such as ricin (35). Proteins that are classed as RIPs are
mainly present in plants (36), and
have the ability to inhibit protein synthesis in a cell system or
cell-free system (24,37). RIPs have been shown to exhibit RNA
N-glycosidase activity and to modify two nucleoside residues, G4323
and A4324, in 28 S rRNA of the eukaryotic 60 S ribosomal subunit,
resulting in the failure of combination with elongation factor and
making RIPs protein synthesis inhibitors (23). It is well known that S-phase is a
period for DNA duplication and the synthesis of histones and other
necessary proteins (38). If either
of the synthesis processes is interrupted, cells arrest in S-phase.
Wang et al reported that MCP30 has DNase-like enzymatic
activity and can nick closed circular Pet-32a(+) plasmid DNA to
open circular conformation, making plasmid DNA exhibit a linear
formation (39). Our previous study
has also revealed that MCP30 has potential histone deacetylase
inhibitor function that selectively increases histone acetylation
in neoplastic prostate cell lines (22). Zhang et al found that low
concentrations of trichosanthin, another type 1 RIP that shares 59%
sequence similarity with MCP30, induces apoptosis and S-phase cell
cycle arrest in two laryngeal cancer cell lines (40). Additional studies investigating the
effect of MCP30 on certain S cell cycle regulating proteins, such
as cyclin A, checkpoint kinase (Chk) 1, Chk2 and p53, are
required.
It was revealed in the present study that MCP30
exhibited cell cycle arrest and apoptosis-inducing activities. Flow
cytometry analysis using Annexin V/PI showed that MCP30
dose-dependently induces early apoptosis in the Ishikawa H cell
line. Subsequently, typical DNA fragmentation ladders were found
subsequent to treatment. The AKT pathway has been widely studied
and plays an important role in cellular growth and survival. This
pathway is commonly considered to be an important target for cancer
chemotherapy (41). AKT has been
reported as overexpressed in numerous malignancies (42,43),
including EC (44). PTEN, a tumor
suppressor gene, is the major negative regulator of the AKT pathway
(45). Loss of function mutations of
PTEN are common and appear to be important in the pathogenesis of
type I EC (12). In the present
study, PTEN loss was also verified in the Ishikawa H cell line,
which is consistent with previous findings (32). There was an apparent negative
associated between MCP30 concentrations and the P-AKT level
(Fig. 4B). Previously, Somasagara
et al found MCP30 effectively decreased AKT phosphorylation
and viability of gemcitabine-resistant pancreatic cancer cells
(46). Overall, in the present study
MCP30 showed cytotoxicity to EC cells, partially through decreasing
activation of the AKT pathway.
Previously, extensive efforts in developing
inhibitors of the AKT pathway as therapeutic agents to treat
cancers in which the AKT pathway is hyperactivated have been
thwarted by unacceptable toxicity or poor pharmacokinetics
(47–52). MCP30 as a type I RIP, devoid of a
cell-binding B chain, have less cytotoxic effects than the majority
of type II RIPs (53). These
observations suggested that MCP30 has good potential as a cytotoxic
agent against EC cells and warrants additional investigation.
Glossary
Abbreviations
Abbreviations:
|
MCP30
|
Momordica charantia protein
|
|
PTEN
|
phosphatase and tensin homolog
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
propidium iodide
|
|
EC
|
endometrial carcinoma
|
|
MC
|
Momordica charantia
|
|
RIPs
|
ribosome inactivating proteins
|
References
|
1
|
Amant F, Moerman P, Neven P, Timmerman D,
Van Limbergen E and Vergote I: Endometrial cancer. Lancet.
366:491–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society. Cancer Facts
& Figures 2013. American Cancer Society Inc.; Atlanta, GA:
2013
|
|
3
|
Li X, Zheng S, Chen S, Qin F, Lau S and
Chen Q: Trends in gynaecological cancers in the largest obstetrics
and gynaecology hospital in China from 2003 to 2013. Tumour Biol.
36:4961–4966. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laurelli G, Di Vagno G, Scaffa C, Losito
S, Del Giudice M and Greggi S: Conservative treatment of early
endometrial cancer: Preliminary results of a pilot study. Gynecol
Oncol. 120:43–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang CJ, Chao A, Yang LY, Hsueh S, Huang
YT, Chou HH, Chang TC and Lai CH: Fertility-preserving treatment in
young women with endometrial adenocarcinoma: A long-term cohort
study. Int J Gynecol Cancer. 24:718–728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amant F, Moerman P, Neven P, Timmerman D,
Van Limbergen E and Vergote I: Endometrial cancer. Lancet.
366:491–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nout RA, Smit VT, Putter H,
Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik
EM, Mens JW, Slot A, Kroese MC, et al: Vaginal brachytherapy versus
pelvic external beam radiotherapy for patients with endometrial
cancer of high-intermediate risk (PORTEC-2): An open-label,
non-inferiority, randomised trial. Lancet. 375:816–823. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
ASTEC/EN.5 Study Group. Blake P, Swart AM,
Orton J, Kitchener H, Whelan T, Lukka H, Eisenhauer E, Bacon M, Tu
D, et al: Adjuvant external beam radiotherapy in the treatment of
endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials):
Pooled trial results, systematic review, and meta-analysis. Lancet.
373:137–146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Humber CE, Tierney JF, Symonds RP,
Collingwood M, Kirwan J, Williams C and Green JA: Chemotherapy for
advanced, recurrent or metastatic endometrial cancer: A systematic
review of Cochrane collaboration. Ann Oncol. 18:409–420. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amadio G, Masciullo V, Stefano L and
Scambia G: An update on the pharmacotherapy for endometrial cancer.
Expert Opin Pharmacother. 14:2501–2509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Homesley HD, Filiaci V, Gibbons SK, Long
HJ, Cella D, Spirtos NM, Morris RT, DeGeest K, Lee R and Montag A:
A randomized phase III trial in advanced endometrial carcinoma of
surgery and volume directed radiation followed by cisplatin and
doxorubicin with or without paclitaxel: A gynecologic oncology
group study. Gynecol Oncol. 112:543–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mutter GL, Lin MC, Fitzgerald JT, Kum JB,
Baak JP, Lees JA, Weng LP and Eng C: Altered PTEN expression as a
diagnostic marker for the earliest endometrial precancers. J Natl
Cancer Inst. 92:924–930. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Terakawa N, Kanamori Y and Yoshida S: Loss
of PTEN expression followed by Akt phosphorylation is a poor
prognostic factor for patients with endometrial cancer. Endocr
Relat Cancer. 10:203–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai Y, Luo Q, Sun M and Corke H:
Antioxidant activity and phenolic compounds of 112 traditional
Chinese medicinal plants associated with anticancer. Life Sci.
74:2157–2184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grover JK and Yadav SP: Pharmacological
actions and potential uses of Momordica charantia: A review. J
Ethnopharmacol. 93:123–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan MJ, Ye JM, Turner N, Hohnen-Behrens C,
Ke CQ, Tang CP, Chen T, Weiss HC, Gesing ER, Rowland A, et al:
Antidiabetic activities of triterpenoids isolated from bitter melon
associated with activation of the AMPK pathway. Chem Biol.
15:263–273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee-Huang S and Huang PL, Nara PL, Chen
HC, Kung HF, Huang P, Huang HI and Huang PL: MAP 30: A new
inhibitor of HIV-1 infection and replication. FEBS Lett. 272:12–18.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adewale OO, Oduyemi OI and Ayokunle O:
Oral administration of leaf extracts of Momordica charantia affect
reproductive hormones of adult female Wistar rats. Asian Pac J Trop
Biomed. 4:(Suppl 1). S521–S524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng YY, Yi Y, Zhang LF, Zhang RF, Zhang
Y, Wei ZC, Tang XJ and Zhang MW: Immunomodulatory activity and
partial characterisation of polysaccharides from Momordica
charantia. Molecules. 19:13432–13447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan JM, Luo J, Xu J, Zhu S, Zhang Q, Gao
DF, Xu YB and Zhang GP: Effects of recombinant MAP30 on cell
proliferation and apoptosis of human colorectal carcinoma LoVo
cells. Mol Biotechnol. 39:79–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang CZ, Fang EF, Zhang HT, Liu LL and
Yun JP: Momordica Charantia lectin exhibits antitumor activity
towards hepatocellular carcinoma. Invest New Drugs. 33:1–11. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiong SD, Yu K, Liu XH, Yin LH,
Kirschenbaum A, Yao S, Narla G, DiFeo A, Wu JB, Yuan Y, et al:
Ribosome-inactivating proteins isolated from dietary bitter melon
induce apoptosis and inhibit histone deacetylase-1 selectively in
premalignant and malignant prostate cancer cells. Int J Cancer.
125:774–782. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Endo Y, Mitsui K, Motizuki M and Tsurugi
K: The mechanism of action of ricin and related toxic lectins on
eukaryotic ribosomes. The site and the characteristics of the
modification in 28 S ribosomal RNA caused by the toxins. J Biol
Chem. 262:5908–5912. 1987.PubMed/NCBI
|
|
24
|
Olsnes S and Pihl A: Treatment of abrin
and ricin with -mercaptoethanol opposite effects on their toxicity
in mice and their ability to inhibit protein synthesis in a
cell-free system. FEBS Lett. 28:48–50. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitsuhashi A, Sato Y, Kiyokawa T,
Koshizaka M, Hanaoka H and Shozu M: Phase II study of
medroxyprogesterone acetate plus metformin as a fertility-sparing
treatment for atypical endometrial hyperplasia and endometrial
cancer. Ann Oncol. 27:262–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fong P and Meng LR: Effect of mTOR
inhibitors in nude mice with endometrial carcinoma and variable
PTEN expression status. Med Sci Monit Basic Res. 20:146–152. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng W, Yang CX, Zhang L, Fang Y and Yan
M: Curcumin promotes the apoptosis of human endometrial carcinoma
cells by downregulating the expression of androgen receptor through
Wnt signal pathway. Eur J Gynaecol Oncol. 35:718–723.
2014.PubMed/NCBI
|
|
28
|
Ray RB, Raychoudhuri A, Steele R and
Nerurkar P: Bitter melon (Momordica charantia) extract inhibits
breast cancer cell proliferation by modulating cell cycle
regulatory genes and promotes apoptosis. Cancer Res. 70:1925–1931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pitchakarn P, Ogawa K, Suzuki S, Takahashi
S, Asamoto M, Chewonarin T, Limtrakul P and Shirai T: Momordica
charantia leaf extract suppresses rat prostate cancer progression
in vitro and in vivo. Cancer Sci. 101:2234–2240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chipps ES, Jayini R, Ando S, Protzman AD,
Muhi MZ, Mottaleb MA, Malkawi A and Islam MR: Cytotoxicity analysis
of active components in bitter melon (Momordica charantia) seed
extracts using human embryonic kidney and colon tumor cells. Nat
Prod Commun. 7:1203–1208. 2012.PubMed/NCBI
|
|
31
|
Fang EF, Zhang CZ, Wong JH, Shen JY, Li CH
and Ng TB: The MAP30 protein from bitter gourd (Momordica
charantia) seeds promotes apoptosis in liver cancer cells in vitro
and in vivo. Cancer Lett. 324:66–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Albitar L, Pickett G, Morgan M, Davies S
and Leslie KK: Models representing type I and type II human
endometrial cancers: Ishikawa H and Hec50co cells. Gynecol Oncol.
106:52–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yeung HW, Li WW, Feng Z, Barbieri L and
Stirpe F: Trichosanthin, alpha-momorcharin and beta-momorcharin:
Identity of abortifacient and ribosome-inactivating proteins. Int J
Pept Protein Res. 31:265–268. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Walsh MJ, Dodd JE and Hautbergue GM:
Ribosome-inactivating proteins: Potent poisons and molecular tools.
Virulence. 4:774–784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Olsnes S and Pihl A: Different biological
properties of the two constituent peptide chains of ricin, a toxic
protein inhibiting protein synthesis. Biochemistry. 12:3121–3126.
1973. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stirpe F, Barbieri L, Battelli MG, Soria M
and Lappi DA: Ribosome-inactivating proteins from plants: Present
status and future prospects. Biotechnology (NY). 10:405–412. 1992.
View Article : Google Scholar
|
|
37
|
de Virgilio M, Lombardi A, Caliandro R and
Fabbrini MS: Ribosome-inactivating proteins: From plant defense to
tumor attack. Toxins (Basel). 2:2699–2737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hartwell LH and Weinert TA: Checkpoints:
Controls that ensure the order of cell cycle events. Science.
246:629–634. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang S, Zheng Y, Yan J, Zhu Z, Wu Z and
Ding Y: Alpha-momorcharin: A ribosome-inactivating protein from
Momordica charantia, possessing DNA cleavage properties. Protein
Pept Lett. 20:1257–1263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang D, Chen B, Zhou J, Zhou L, Li Q, Liu
F, Chou KY, Tao L and Lu LM: Low concentrations of trichosanthin
induce apoptosis and cell cycle arrest via c-Jun N-terminal protein
kinase/mitogen-activated protein kinase activation. Mol Med Rep.
11:349–356. 2015.PubMed/NCBI
|
|
41
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Banerji S, Cibulskis K, Rangel-Escareno C,
Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY,
Sougnez C, Zou L, et al: Sequence analysis of mutations and
translocations across breast cancer subtypes. Nature. 486:405–409.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oda K, Stokoe D, Taketani Y and McCormick
F: High frequency of coexistent mutations of PIK3CA and PTEN genes
in endometrial carcinoma. Cancer Res. 65:10669–10673. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stambolic V, Suzuki A, de la Pompa JL,
Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM,
Siderovski DP and Mak TW: Negative regulation of PKB/Akt-dependent
cell survival by the tumor suppressor PTEN. Cell. 95:29–39. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Somasagara RR, Deep G, Shrotriya S, Patel
M, Agarwal C and Agarwal R: Bitter melon juice targets molecular
mechanisms underlying gemcitabine resistance in pancreatic cancer
cells. Int J Oncol. 46:1849–1857. 2015.PubMed/NCBI
|
|
47
|
Matulonis U, Vergote I, Backes F, Martin
LP, McMeekin S, Birrer M, Campana F, Xu Y, Egile C and Ghamande S:
Phase II study of the PI3K inhibitor pilaralisib (SAR245408; XL147)
in patients with advanced or recurrent endometrial carcinoma.
Gynecol Oncol. 136:246–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jimeno A, Bauman JE, Weissman C, Adkins D,
Schnadig I, Beauregard P, Bowles DW, Spira A, Levy B, Seetharamu N,
et al: A randomized, phase 2 trial of docetaxel with or without
PX-866, an irreversible oral phosphatidylinositol 3-kinase
inhibitor, in patients with relapsed or metastatic head and neck
squamous cell cancer. Oral Oncol. 51:383–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Konopleva MY, Walter RB, Faderl SH,
Jabbour EJ, Zeng Z, Borthakur G, Huang X, Kadia TM, Ruvolo PP,
Feliu JB, et al: Preclinical and early clinical evaluation of the
oral AKT inhibitor, MK-2206, for the treatment of acute myelogenous
leukemia. Clin Cancer Res. 20:2226–2235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Massarelli E, Lin H, Ginsberg LE, Tran HT,
Lee JJ, Canales JR, Williams MD, Blumenschein GR Jr, Lu C, Heymach
JV, et al: Phase II trial of everolimus and erlotinib in patients
with platinum-resistant recurrent and/or metastatic head and neck
squamous cell carcinoma. Ann Oncol. 26:1476–1480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Oza AM, Pignata S, Poveda A, McCormack M,
Clamp A, Schwartz B, Cheng J, Li X, Campbell K, Dodion P and
Haluska FG: Randomized phase II trial of ridaforolimus in advanced
endometrial carcinoma. J Clin Oncol. 33:3576–3582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bauman JE, Arias-Pulido H, Lee SJ,
Fekrazad MH, Ozawa H, Fertig E, Howard J, Bishop J, Wang H, Olson
GT, et al: A phase II study of temsirolimus and erlotinib in
patients with recurrent and/or metastatic, platinum-refractory head
and neck squamous cell carcinoma. Oral Oncol. 49:461–467. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Stirpe F: Ribosome-inactivating proteins:
From toxins to useful proteins. Toxicon. 67:12–16. 2013. View Article : Google Scholar : PubMed/NCBI
|