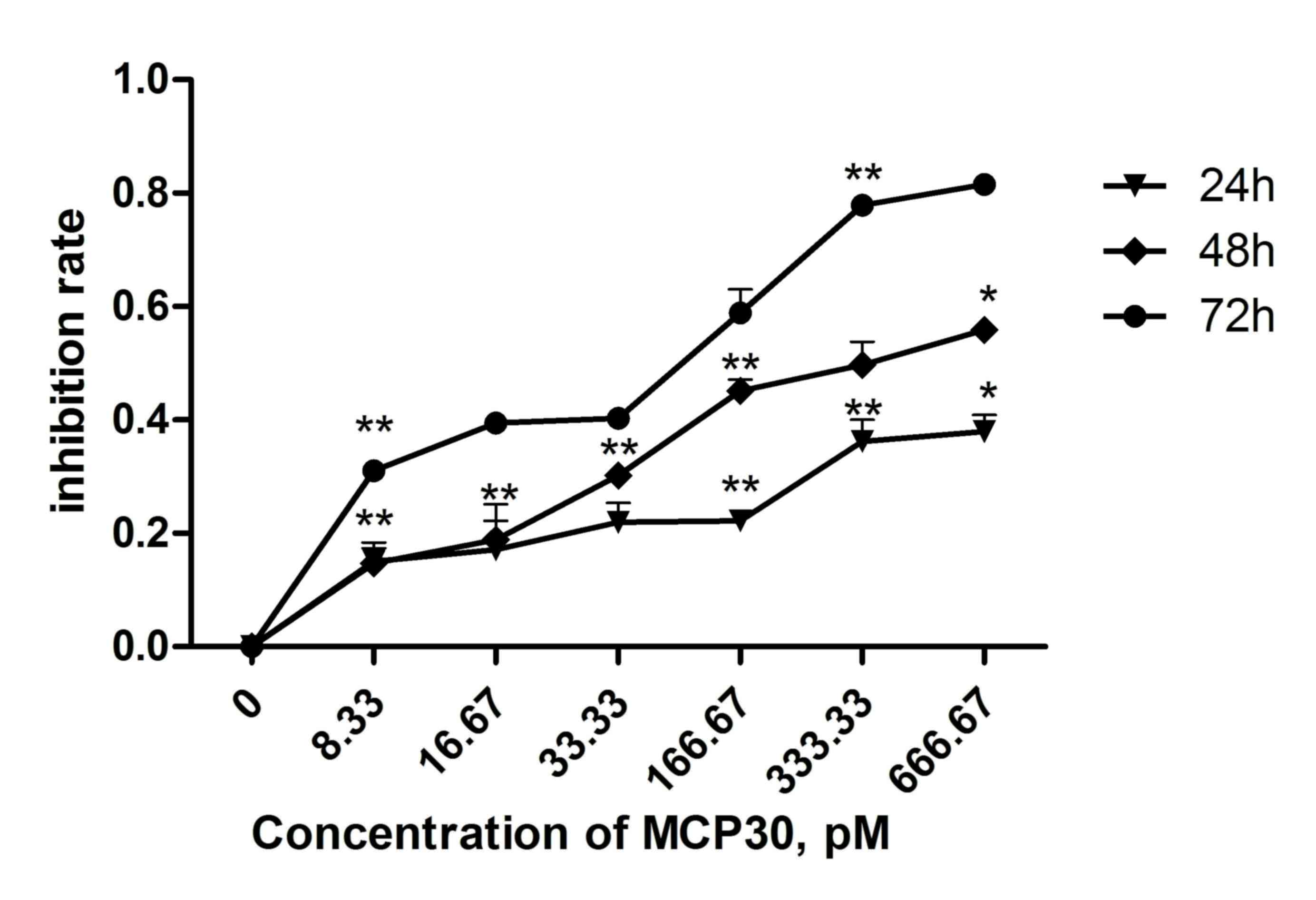
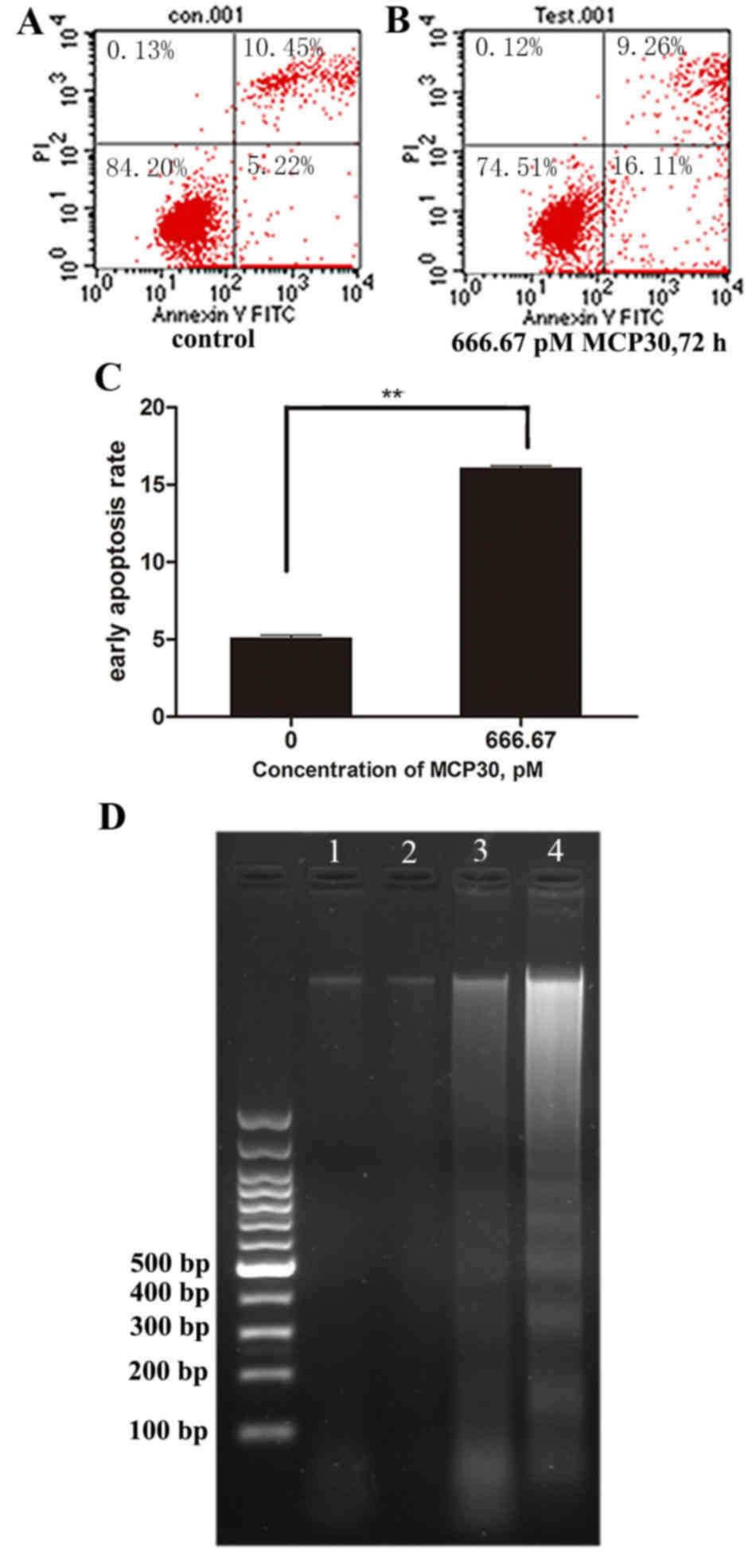
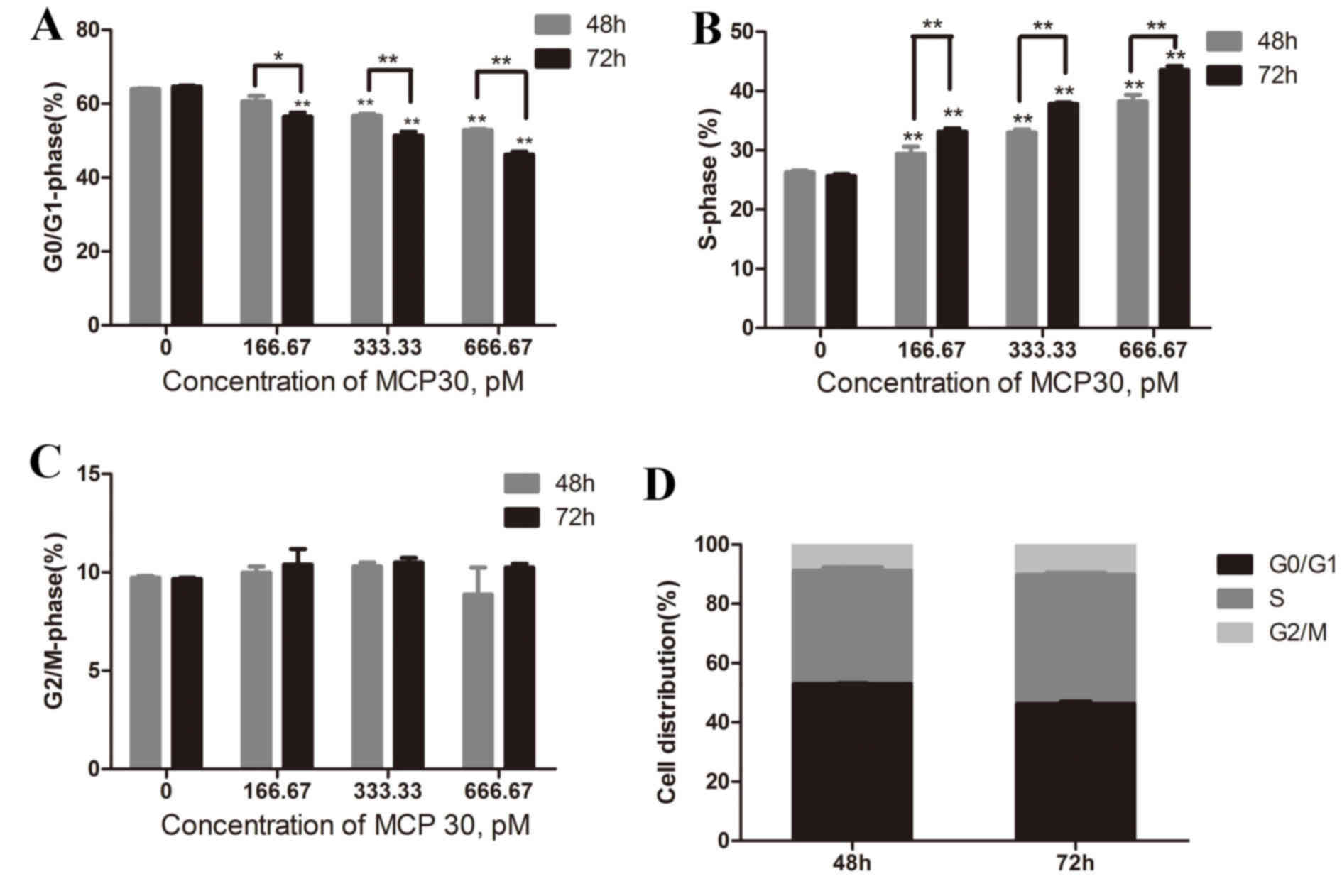
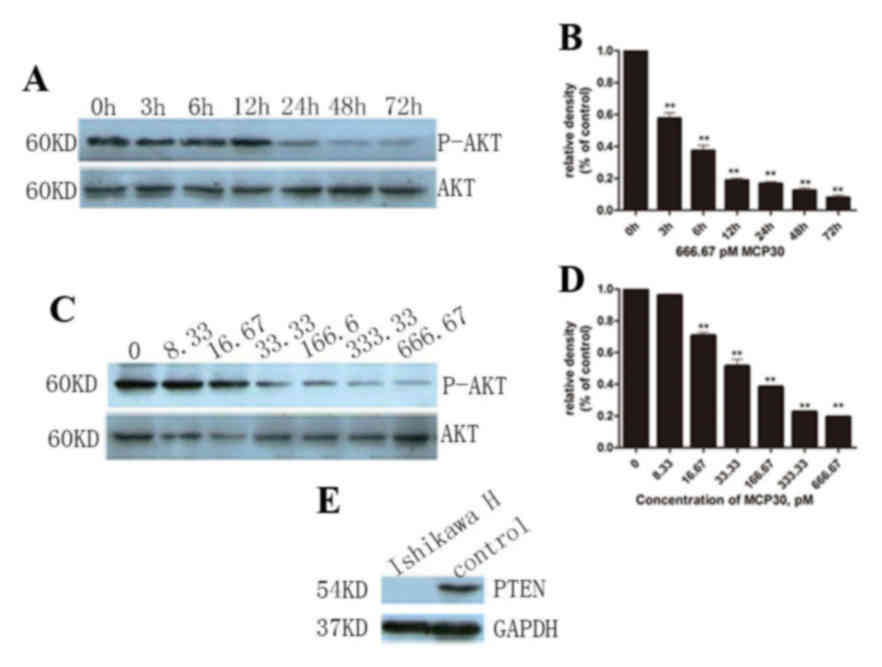
|
1
|
Amant F, Moerman P, Neven P, Timmerman D,
Van Limbergen E and Vergote I: Endometrial cancer. Lancet.
366:491–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society. Cancer Facts
& Figures 2013. American Cancer Society Inc.; Atlanta, GA:
2013
|
|
3
|
Li X, Zheng S, Chen S, Qin F, Lau S and
Chen Q: Trends in gynaecological cancers in the largest obstetrics
and gynaecology hospital in China from 2003 to 2013. Tumour Biol.
36:4961–4966. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laurelli G, Di Vagno G, Scaffa C, Losito
S, Del Giudice M and Greggi S: Conservative treatment of early
endometrial cancer: Preliminary results of a pilot study. Gynecol
Oncol. 120:43–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang CJ, Chao A, Yang LY, Hsueh S, Huang
YT, Chou HH, Chang TC and Lai CH: Fertility-preserving treatment in
young women with endometrial adenocarcinoma: A long-term cohort
study. Int J Gynecol Cancer. 24:718–728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amant F, Moerman P, Neven P, Timmerman D,
Van Limbergen E and Vergote I: Endometrial cancer. Lancet.
366:491–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nout RA, Smit VT, Putter H,
Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik
EM, Mens JW, Slot A, Kroese MC, et al: Vaginal brachytherapy versus
pelvic external beam radiotherapy for patients with endometrial
cancer of high-intermediate risk (PORTEC-2): An open-label,
non-inferiority, randomised trial. Lancet. 375:816–823. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
ASTEC/EN.5 Study Group. Blake P, Swart AM,
Orton J, Kitchener H, Whelan T, Lukka H, Eisenhauer E, Bacon M, Tu
D, et al: Adjuvant external beam radiotherapy in the treatment of
endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials):
Pooled trial results, systematic review, and meta-analysis. Lancet.
373:137–146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Humber CE, Tierney JF, Symonds RP,
Collingwood M, Kirwan J, Williams C and Green JA: Chemotherapy for
advanced, recurrent or metastatic endometrial cancer: A systematic
review of Cochrane collaboration. Ann Oncol. 18:409–420. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amadio G, Masciullo V, Stefano L and
Scambia G: An update on the pharmacotherapy for endometrial cancer.
Expert Opin Pharmacother. 14:2501–2509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Homesley HD, Filiaci V, Gibbons SK, Long
HJ, Cella D, Spirtos NM, Morris RT, DeGeest K, Lee R and Montag A:
A randomized phase III trial in advanced endometrial carcinoma of
surgery and volume directed radiation followed by cisplatin and
doxorubicin with or without paclitaxel: A gynecologic oncology
group study. Gynecol Oncol. 112:543–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mutter GL, Lin MC, Fitzgerald JT, Kum JB,
Baak JP, Lees JA, Weng LP and Eng C: Altered PTEN expression as a
diagnostic marker for the earliest endometrial precancers. J Natl
Cancer Inst. 92:924–930. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Terakawa N, Kanamori Y and Yoshida S: Loss
of PTEN expression followed by Akt phosphorylation is a poor
prognostic factor for patients with endometrial cancer. Endocr
Relat Cancer. 10:203–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai Y, Luo Q, Sun M and Corke H:
Antioxidant activity and phenolic compounds of 112 traditional
Chinese medicinal plants associated with anticancer. Life Sci.
74:2157–2184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grover JK and Yadav SP: Pharmacological
actions and potential uses of Momordica charantia: A review. J
Ethnopharmacol. 93:123–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan MJ, Ye JM, Turner N, Hohnen-Behrens C,
Ke CQ, Tang CP, Chen T, Weiss HC, Gesing ER, Rowland A, et al:
Antidiabetic activities of triterpenoids isolated from bitter melon
associated with activation of the AMPK pathway. Chem Biol.
15:263–273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee-Huang S and Huang PL, Nara PL, Chen
HC, Kung HF, Huang P, Huang HI and Huang PL: MAP 30: A new
inhibitor of HIV-1 infection and replication. FEBS Lett. 272:12–18.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adewale OO, Oduyemi OI and Ayokunle O:
Oral administration of leaf extracts of Momordica charantia affect
reproductive hormones of adult female Wistar rats. Asian Pac J Trop
Biomed. 4:(Suppl 1). S521–S524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng YY, Yi Y, Zhang LF, Zhang RF, Zhang
Y, Wei ZC, Tang XJ and Zhang MW: Immunomodulatory activity and
partial characterisation of polysaccharides from Momordica
charantia. Molecules. 19:13432–13447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan JM, Luo J, Xu J, Zhu S, Zhang Q, Gao
DF, Xu YB and Zhang GP: Effects of recombinant MAP30 on cell
proliferation and apoptosis of human colorectal carcinoma LoVo
cells. Mol Biotechnol. 39:79–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang CZ, Fang EF, Zhang HT, Liu LL and
Yun JP: Momordica Charantia lectin exhibits antitumor activity
towards hepatocellular carcinoma. Invest New Drugs. 33:1–11. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiong SD, Yu K, Liu XH, Yin LH,
Kirschenbaum A, Yao S, Narla G, DiFeo A, Wu JB, Yuan Y, et al:
Ribosome-inactivating proteins isolated from dietary bitter melon
induce apoptosis and inhibit histone deacetylase-1 selectively in
premalignant and malignant prostate cancer cells. Int J Cancer.
125:774–782. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Endo Y, Mitsui K, Motizuki M and Tsurugi
K: The mechanism of action of ricin and related toxic lectins on
eukaryotic ribosomes. The site and the characteristics of the
modification in 28 S ribosomal RNA caused by the toxins. J Biol
Chem. 262:5908–5912. 1987.PubMed/NCBI
|
|
24
|
Olsnes S and Pihl A: Treatment of abrin
and ricin with -mercaptoethanol opposite effects on their toxicity
in mice and their ability to inhibit protein synthesis in a
cell-free system. FEBS Lett. 28:48–50. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitsuhashi A, Sato Y, Kiyokawa T,
Koshizaka M, Hanaoka H and Shozu M: Phase II study of
medroxyprogesterone acetate plus metformin as a fertility-sparing
treatment for atypical endometrial hyperplasia and endometrial
cancer. Ann Oncol. 27:262–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fong P and Meng LR: Effect of mTOR
inhibitors in nude mice with endometrial carcinoma and variable
PTEN expression status. Med Sci Monit Basic Res. 20:146–152. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng W, Yang CX, Zhang L, Fang Y and Yan
M: Curcumin promotes the apoptosis of human endometrial carcinoma
cells by downregulating the expression of androgen receptor through
Wnt signal pathway. Eur J Gynaecol Oncol. 35:718–723.
2014.PubMed/NCBI
|
|
28
|
Ray RB, Raychoudhuri A, Steele R and
Nerurkar P: Bitter melon (Momordica charantia) extract inhibits
breast cancer cell proliferation by modulating cell cycle
regulatory genes and promotes apoptosis. Cancer Res. 70:1925–1931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pitchakarn P, Ogawa K, Suzuki S, Takahashi
S, Asamoto M, Chewonarin T, Limtrakul P and Shirai T: Momordica
charantia leaf extract suppresses rat prostate cancer progression
in vitro and in vivo. Cancer Sci. 101:2234–2240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chipps ES, Jayini R, Ando S, Protzman AD,
Muhi MZ, Mottaleb MA, Malkawi A and Islam MR: Cytotoxicity analysis
of active components in bitter melon (Momordica charantia) seed
extracts using human embryonic kidney and colon tumor cells. Nat
Prod Commun. 7:1203–1208. 2012.PubMed/NCBI
|
|
31
|
Fang EF, Zhang CZ, Wong JH, Shen JY, Li CH
and Ng TB: The MAP30 protein from bitter gourd (Momordica
charantia) seeds promotes apoptosis in liver cancer cells in vitro
and in vivo. Cancer Lett. 324:66–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Albitar L, Pickett G, Morgan M, Davies S
and Leslie KK: Models representing type I and type II human
endometrial cancers: Ishikawa H and Hec50co cells. Gynecol Oncol.
106:52–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yeung HW, Li WW, Feng Z, Barbieri L and
Stirpe F: Trichosanthin, alpha-momorcharin and beta-momorcharin:
Identity of abortifacient and ribosome-inactivating proteins. Int J
Pept Protein Res. 31:265–268. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Walsh MJ, Dodd JE and Hautbergue GM:
Ribosome-inactivating proteins: Potent poisons and molecular tools.
Virulence. 4:774–784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Olsnes S and Pihl A: Different biological
properties of the two constituent peptide chains of ricin, a toxic
protein inhibiting protein synthesis. Biochemistry. 12:3121–3126.
1973. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stirpe F, Barbieri L, Battelli MG, Soria M
and Lappi DA: Ribosome-inactivating proteins from plants: Present
status and future prospects. Biotechnology (NY). 10:405–412. 1992.
View Article : Google Scholar
|
|
37
|
de Virgilio M, Lombardi A, Caliandro R and
Fabbrini MS: Ribosome-inactivating proteins: From plant defense to
tumor attack. Toxins (Basel). 2:2699–2737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hartwell LH and Weinert TA: Checkpoints:
Controls that ensure the order of cell cycle events. Science.
246:629–634. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang S, Zheng Y, Yan J, Zhu Z, Wu Z and
Ding Y: Alpha-momorcharin: A ribosome-inactivating protein from
Momordica charantia, possessing DNA cleavage properties. Protein
Pept Lett. 20:1257–1263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang D, Chen B, Zhou J, Zhou L, Li Q, Liu
F, Chou KY, Tao L and Lu LM: Low concentrations of trichosanthin
induce apoptosis and cell cycle arrest via c-Jun N-terminal protein
kinase/mitogen-activated protein kinase activation. Mol Med Rep.
11:349–356. 2015.PubMed/NCBI
|
|
41
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Banerji S, Cibulskis K, Rangel-Escareno C,
Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY,
Sougnez C, Zou L, et al: Sequence analysis of mutations and
translocations across breast cancer subtypes. Nature. 486:405–409.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oda K, Stokoe D, Taketani Y and McCormick
F: High frequency of coexistent mutations of PIK3CA and PTEN genes
in endometrial carcinoma. Cancer Res. 65:10669–10673. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stambolic V, Suzuki A, de la Pompa JL,
Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM,
Siderovski DP and Mak TW: Negative regulation of PKB/Akt-dependent
cell survival by the tumor suppressor PTEN. Cell. 95:29–39. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Somasagara RR, Deep G, Shrotriya S, Patel
M, Agarwal C and Agarwal R: Bitter melon juice targets molecular
mechanisms underlying gemcitabine resistance in pancreatic cancer
cells. Int J Oncol. 46:1849–1857. 2015.PubMed/NCBI
|
|
47
|
Matulonis U, Vergote I, Backes F, Martin
LP, McMeekin S, Birrer M, Campana F, Xu Y, Egile C and Ghamande S:
Phase II study of the PI3K inhibitor pilaralisib (SAR245408; XL147)
in patients with advanced or recurrent endometrial carcinoma.
Gynecol Oncol. 136:246–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jimeno A, Bauman JE, Weissman C, Adkins D,
Schnadig I, Beauregard P, Bowles DW, Spira A, Levy B, Seetharamu N,
et al: A randomized, phase 2 trial of docetaxel with or without
PX-866, an irreversible oral phosphatidylinositol 3-kinase
inhibitor, in patients with relapsed or metastatic head and neck
squamous cell cancer. Oral Oncol. 51:383–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Konopleva MY, Walter RB, Faderl SH,
Jabbour EJ, Zeng Z, Borthakur G, Huang X, Kadia TM, Ruvolo PP,
Feliu JB, et al: Preclinical and early clinical evaluation of the
oral AKT inhibitor, MK-2206, for the treatment of acute myelogenous
leukemia. Clin Cancer Res. 20:2226–2235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Massarelli E, Lin H, Ginsberg LE, Tran HT,
Lee JJ, Canales JR, Williams MD, Blumenschein GR Jr, Lu C, Heymach
JV, et al: Phase II trial of everolimus and erlotinib in patients
with platinum-resistant recurrent and/or metastatic head and neck
squamous cell carcinoma. Ann Oncol. 26:1476–1480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Oza AM, Pignata S, Poveda A, McCormack M,
Clamp A, Schwartz B, Cheng J, Li X, Campbell K, Dodion P and
Haluska FG: Randomized phase II trial of ridaforolimus in advanced
endometrial carcinoma. J Clin Oncol. 33:3576–3582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bauman JE, Arias-Pulido H, Lee SJ,
Fekrazad MH, Ozawa H, Fertig E, Howard J, Bishop J, Wang H, Olson
GT, et al: A phase II study of temsirolimus and erlotinib in
patients with recurrent and/or metastatic, platinum-refractory head
and neck squamous cell carcinoma. Oral Oncol. 49:461–467. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Stirpe F: Ribosome-inactivating proteins:
From toxins to useful proteins. Toxicon. 67:12–16. 2013. View Article : Google Scholar : PubMed/NCBI
|