Introduction
Squamous cell carcinoma of the head and neck (SCCHN)
is a common and highly malignant tumor, typically identified in the
middle or late stages of the disease, with a 5-year survival rate
of 30% (1). SCCHN accounts for
>90% of all head and neck tumors (2). Regional cervical metastasis is one of
the most significant biological behaviors of SCCHN. The extent to
which the lymph nodes of various regions are involved and the sites
of the cervical lymph node metastases are associated with poor
patient prognosis (3,4). Demonstrating the molecular mechanism of
the development of cervical metastasis is necessary in providing
novel strategies of SCCHN therapy.
It is known that chemokines and chemokine receptors
participate in the development of metastasis in various types of
cancer tissue (5,6). Among these chemokine receptors, it has
been observed that chemokine receptor 7 (CCR7) is highly expressed
in metastatic lymph nodes and invasive SCCHN cells, promoting
preferential lymph node metastasis (7). The interactions between CCR7 and its
ligands serve a major role in the malignant metastasis process of
head and neck tumors, and the upregulation of CCR7 significantly
increases the migratory and invasive ability of SCCHN cells
(8). Therefore, characterizing the
specific role of the CCR7 signaling pathway in the malignant
metastasis of SCCHN may be helpful to evaluate whether CCR7 acts as
a novel target in SCCHN therapeutic strategies.
As a non-receptor protein tyrosine kinase, Janus
kinase (Jak) has been demonstrated to serve an important role in
regulating various cell signaling pathways. It was reported that
the tyrosine phosphorylation of the members of the Janus family of
kinases, including Jak1, Jak2, Jak3 and tyrosine kinase 2 (Tyk2),
may be involved in the occurrence and development of prostate and
breast cancer (9). Specifically, it
has been demonstrated that Jak3 is involved in dendritic cell
maturation and migration via the CCR7-mediated signaling pathway
(10,11). However, whether Jak3 was able to be
tyrosine phosphorylated upon stimulation with chemokine ligand 19
(CCL19) in SCCHN cells, and the specific role of Jak3 activation in
the migration and invasion of SCCHN cells, remains unknown.
In the present study, the role of Jak3 in SCCHN
migration and invasion was evaluated. The appropriate dose and
duration of CCL19 (a CCR7 ligand) treatment to activate Jak3
phosphorylation through the CCR7 signaling pathway was
investigated. Furthermore, diverse methodologies were applied to
examine the role of Jak3 activation on the biological behavior of
SCCHN cells, including invasion and migration. In addition, the
association between the expression of phospho-Jak3, lymphatic
metastasis and clinical stage was investigated. The present study
observed that the activation of Jak3, through the interaction of
chemokine receptor CCR7 and its ligand, is significantly involved
in the invasion and migration of metastatic SCCHN. These results
demonstrate the role of CCR7 signaling in metastatic SCCHN and
provide new therapeutic targets for SCCHN.
Materials and methods
Human tumor samples and cell
lines
In total, 70 SCCHN specimens with the adjacent
metastatic (or normal) lymph nodes and 10 normal human oral mucosal
tissue were obtained from the Head and Neck Tumor Center, School of
Stomatology, China Medical University (Shenyang, China). SCCHN
classification, including primary tumors (T), regional lymph nodes
(N), distant metastasis (M) and clinical stage, was determined
according to the rules of the International Union Against Cancer
for Head and Neck Cancer (Tumor node metastasis, TNM
classification, 1997) (12). All
procedures were performed in accordance with the provisions of the
Declaration of Helsinki and approved by the Ethics Committee of the
China Medical University. All the specimens were obtained with the
consent of the patients prior to surgery and in accordance with
Health Insurance Portability. Written informed consent was received
from all individuals.
PCI-37B, a metastatic SCCHN cell line expressing
CCR7, was donated by the University of Pittsburgh Cancer Institute
(Pittsburgh, PA, USA). The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) containing 10% fetal bovine serum,
penicillin, and streptomycin in an atmosphere of 5% CO2
and 95% air at 37°C. The ZM39923 inhibitor treatment at the dose
used did not affect the viability as determined using the Cell
Counting Kit-8 (CCK8; cat. no. C0038; Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer's
protocol.
Reagents and antibodies
CCL19 and monoclonal anti-human CCR7 antibody (10
µg/ml; cat. no. MAB197) were purchased from R&D Systems
(Minneapolis, MN, USA). The Jak3 inhibitor (ZM39923) and
tetramethylrhodamine-labeled phalloidin were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-Jak3 (cat. no.
bs-2808R) and anti-phospho-Jak3 (Tyr785; cat. no. bs-3207R) were
purchased from Beijing Biosynthesis Biotechnology Co., Ltd
(Beijing, China).
Immunohistochemical analysis
All the tumoral and normal specimens were obtained
for histology and immunohistochemistry assay according to the
common protocol (13). Sections of 5
µm thickness were deparaffinized in xylene for 10 min, and
subsequently rehydrated through a graded series of ethanol (100, 95
and 70%) at room temperature. The sections were immersed in 100%
methanol containing 0.3% H2O2 to inhibit
endogenous peroxidase activity for 30 min at room temperature. For
antigen retrieval, sections were put in a jar filled with 10 mM
sodium citrate buffer and heated for 10 min at 95°C using a
microwave oven, and subsequently cooled to room temperature.
Subsequently, sections were blocked via incubation with normal goat
serum (cat. no. KIT-9706, Fuzhou Maixin Biotech Co., Ltd., Fuzhou,
China) for 10 min at room temperature and incubated with rabbit
polyclonal anti-phospho-Jak3 antibody (dilution, 1:100) overnight
at 4°C. The sections were washed and then incubated with 50 µl
undiluted biotinylated labeled secondary antibody (cat. no.
KIT-9706, Fuzhou Maixin Biotech Co., Ltd.) for 1 h at room
temperature, subsequent to the incubation of primary antibody.
Then, following washing three times with PBS, sections were further
incubated with a complex of avidin/streptavidin-peroxidase for 10
min at room temperature. Following diaminobenzidine development,
the sections were then counterstained with hematoxylin for
histology. Negative controls were conducted by exchange of primary
antibody for PBS. Images were captured of the stained slides and
they were analyzed by microscopy (Nikon Eclipse 80i; Nikon
Corporation, Tokyo, Japan) at a magnification of ×100. Tumors were
classified according to the percentage of positive cells: Negative
(−), ≤10% or no staining; weakly positive (+), 11–50%; positive
(++), 51–75%; or strongly positive (+++), >75%. For each
experimental condition, ≥5 randomly selected fields were
analyzed.
Western blot assay
To explore whether Jak3 is phosphorylated by CCL19,
the protein expression of phospho-Jak3 and Jak3 was determined
using western blot analysis. PCI-37B cells were exposed to CCL19 at
a concentration of 200 ng/ml for 0, 0.25, 1, 5, 10, 15 and 30 min.
The cells were lysed with ice-cold RIPA lysis buffer (Beijing
Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) and
centrifuged at 13,400 × g at 4°C, for 30 min. Using a BCA Protein
assay kit, the protein concentration of the supernatants was
determined. The supernatant in the aliquots, which contained equal
amounts of total protein (20 µg) were denatured and electrophoresed
by 10% SDS-PAGE. Separated proteins were transferred to
nitrocellulose filters. The membrane was blocked for 1 h at room
temperature with 5% non-fat dried milk and subsequently incubated
with rabbit polyclonal anti-Jak3 and anti-phospho-Jak3 antibody
(both dilutions, 1:500) at 4°C overnight. The primary antibodies
were labeled for 1 h with a horseradish peroxidase-conjugated
secondary antibody (Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd., Beijing, China). β-actin (dilution, 1:1,000; cat. no.
bs-0061R; Beijing Biosynthesis Biotechnology Co., Ltd.) served as
an internal control. Bands were visualized using enhanced
chemiluminescence with the BeyoECL Plus kit (P0018, Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol. Subsequent to the determination of the time point of Jak3
activation, the cells were treated with Jak3 inhibitor (ZM39923, 10
µM) for 24 h or monoclonal anti-human CCR7 antibody (10 µg/ml) was
used as an effective CCR7 inhibitor for 4 h at 37°C. Subsequent to
the cells being administrated with CCL19 for 5 min, the cells were
harvested and subjected to western blot analysis, as described
above, to determine Jak3 and Phospho-Jak3 protein expression.
Wound healing assay
Wound-healing assays were performed to investigate
the cellular migration ability by measuring cell movement into a
scraped cell-free area. The study groups consisted of control
cells, cells treated with CCL19 alone and cells pretreated with
ZM39923 for 24 h followed by CCL19. Cells were grown in 24-well
plates and a cell-free area was created using a pipette tip.
Wounded monolayers were washed with serum-free DMEM (Hyclone; GE
Healthcare Life Sciences; Logan, UT, USA). Wound closure was
observed after 24 h and images were captured using a microscope
(Nikon TE2000-S Eclipse; Nikon Corporation) at magnification, ×100.
The result was calculated as the percentage of the remaining
cell-free space in comparison with the initial wound space at 0 h.
The experiments were performed in triplicate and between 4 and 5
scratches/well were analyzed.
Transwell and Matrigel assay
Transwell filter insert chambers (24 chambers with 8
µm pore size) were used to evaluate the biological behavior of
PCI-37B cellular migration and invasion. The cell suspensions
(2×105 cell/200 µl) were added to the upper chamber.
Aliquots of the corresponding reagents (anti-CCR7 antibody or
ZM39923) were added to the wells. Subsequently, CCL19 (aliquots of
500 ng/ml) was added to the lower chamber. The cells were subjected
to 24 h of incubation at 37°C, and then cells in the lower well
were fixed with ice-cold methanol for 30 min at room temperature,
then stained with 0.1% crystal violet. For the cellular invasion
assay, the procedure was performed similarly as aforementioned, but
the upper chamber was precoated with 500 ng/µl Matrigel solution
(BD Biosciences, Franklin Lakes, NJ, USA). For each experimental
condition, ≥5 randomly selected fields were analyzed by microscopy
(Nikon TE2000-S Eclipse; Nikon Corporation, Tokyo, Japan) at a
magnification of ×200.
Immunofluorescence staining
The present study examined morphological changes in
the actin cytoskeleton of SCCHN cells, which is required for tumor
cell metastasis. PCI-37B cells were cultured in 24-well plates
treated with 10 µg/ml CCR7 antibody for 4 h or 10 µM ZM39923 for 24
h at 37°C, followed by treatment with CCL19 (final concentration of
500 ng/ml) for 30 min at 37°C. Cells were fixed in 4%
paraformaldehyde for 10 min, permeabilized using 0.1% Triton X-100
for 5 min at 37°C, stained with rhodamine-labeled phalloidin and
diluted to a final concentration of 10 µg/ml in PBS (containing 1%
bovine serum albumin; R&D Systems, Inc., Minneapolis, MN, USA)
for 1 h at 37°C. Subsequently, the samples were washed 3 times for
10 min. F-actin distribution was evaluated by fluorescence
microscopy at 495 nm.
Statistical analysis
All experiments were replicated ≥3 times. Data are
expressed as the mean ± standard deviation. Differences were
evaluated using the Student t-test or χ2 test. P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed with SPSS version 13.0
(SPSS, Inc., Chicago, IL, USA).
Results
Phospho-Jak3 is significantly
expressed in tumor tissues and metastatic lymph nodes
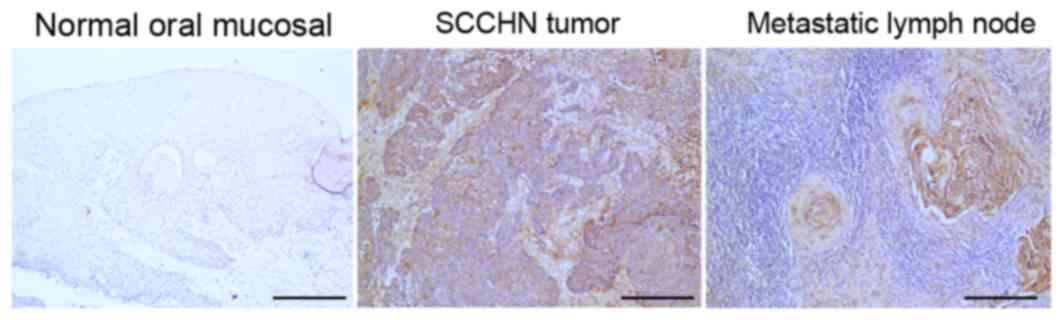
In SCCHN tumor tissues, metastatic lymph nodes,
normal oral mucosal tissues, the expression of phospho-Jak3 was
investigated by immunohistochemical staining. The
immunohistochemistry results revealed that the number of stained
cells, expressing phospho-Jak3, was low or absent in normal oral
mucosal tissues. By contrast, the staining of phospho-Jak3
presented in the cell cytoplasm in tumor cells and metastatic lymph
node cells (Fig. 1). Additionally,
the present study demonstrated that phospho-Jak3 expression was
significantly associated with cervical lymph node metastasis and
SCCHN clinical stage (P<0.01; Table
I).
 | Table I.Association between phospho-Jak3
expression and clinicopathological factors of SCCHN. |
Table I.
Association between phospho-Jak3
expression and clinicopathological factors of SCCHN.
|
|
| Phospho-Jak3
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | No. of patients | Positive | Negative | χ2
test | P-value |
|---|
| Age, years |
|
|
|
| >0.05 |
|
≥60 | 39 | 21 | 18 | 0.125 |
|
|
<60 | 31 | 18 | 13 |
|
|
| Tumor size |
|
|
|
| >0.05 |
|
T1/T2 | 55 | 30 | 25 | 0.142 |
|
|
T3/T4 | 15 | 9 | 6 |
|
|
| Clinical stage |
|
|
|
| <0.01 |
|
I/II | 33 | 10 | 23 | 16.339a |
|
|
III/IV | 37 | 29 | 8 |
|
|
| Nodal
metastasis |
|
|
|
| <0.01 |
| No | 35 | 12 | 23 | 13.027a |
|
|
Yes | 35 | 27 | 8 |
|
|
Jak3 phosphorylation induced by CCL19
in CCR7-expressing SCCHN cells
To investigate whether Jak3 is involved in SCCHN
metastasis mediated by the interaction between CCR7 and its
ligands, the expression of phosphorylated Jak3 protein was examined
in the metastatic SCCHN cell line as determined by western blot
assay. Firstly, the PCI-37B cells were pretreated with CCL19 for 0,
0.25, 1, 5, 10, 15 and 30 min, respectively. The results
demonstrated that the expression of phospho-Jak3 protein was
significantly modulated by CCL19 administration in a time-dependent
manner. As presented in Fig. 2A, the
increased expression of phosphorylated Jak3 protein appeared at 1
min, and the maximal expression was identified at 5 min subsequent
to treating by CCL19, which indicated that Jak3 activation by CCL19
at an appropriate dose was transient and reversible in the
metastatic SCCHN cell line. Additionally, the effect of CCR7 in
regulating Jak3 activation treated by CCL19 in the PCI-37B cells
was investigated. CCL19 induced a marked increased expression of
phosphorylated Jak3 protein after 5 min administration (Fig. 2A). By contrast, the phosphorylation of
the molecule was blocked by CCR7 antibody or ZM39923 compared with
CCL19 alone (Fig. 2B). The action of
CCL19 was counteracted when the CCR7 blocker was used, which
suggested CCL19 application was associated with CCR7 activation.
These findings strongly suggested that the CCL19/CCR7 mediated
signaling induced phosphorylated activation of Jak3 in PCI-37B
cells.
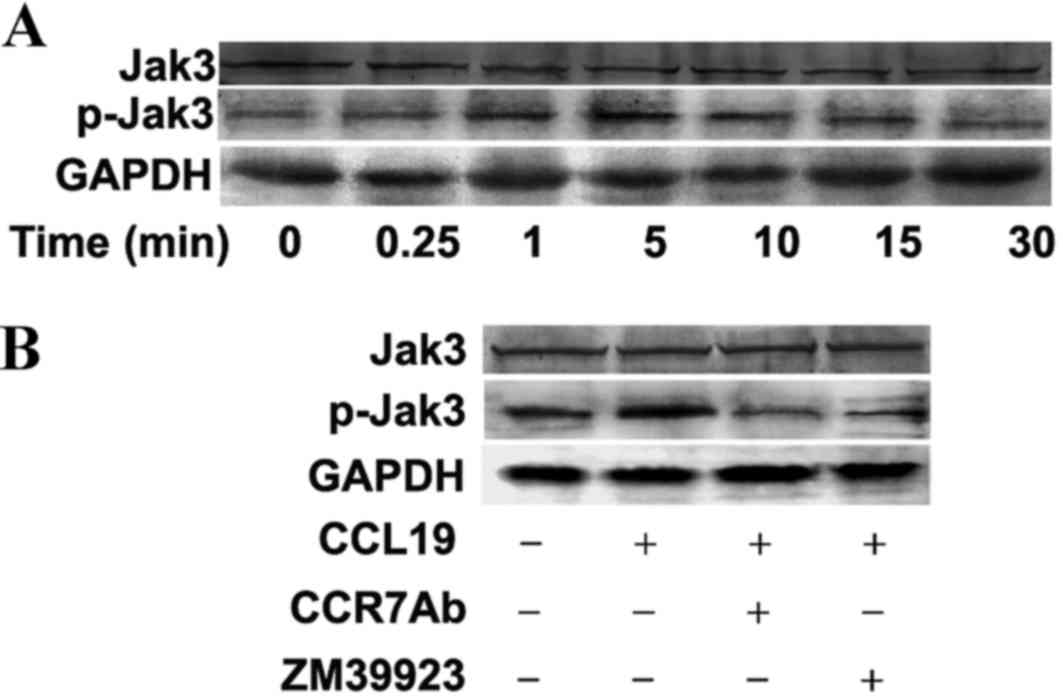 | Figure 2.Jak3 phosphorylation induced by CCL19
in CCR7-expressing SCCHN cells was examined by western blot assay.
(A) Time-course effect of CCL19 on Jak3 phosphorylation examined in
PCI-37B cells. PCI-37B cells of control and CCL19 groups were
treated with vehicle or 200 ng/ml CCL19 for 0, 0.25, 1 5, 10, 15
and 30 min, respectively. (B) Effect of CCR7 Ab and ZM39923 on
CCL19-induced Jak3 activation in PCI-37B cells. PCI-37B cells
pretreated with CCR7 Ab or ZM39923 followed by CCL19 were
stimulated with 200 ng/ml CCL19 for 5 min. Jak3, Janus activated
kinase-3; CCL19, chemokine ligand 19; CCR7, chemokine receptor 7;
SCCHN, squamous cell carcinoma of the head and neck; Ab,
antibody. |
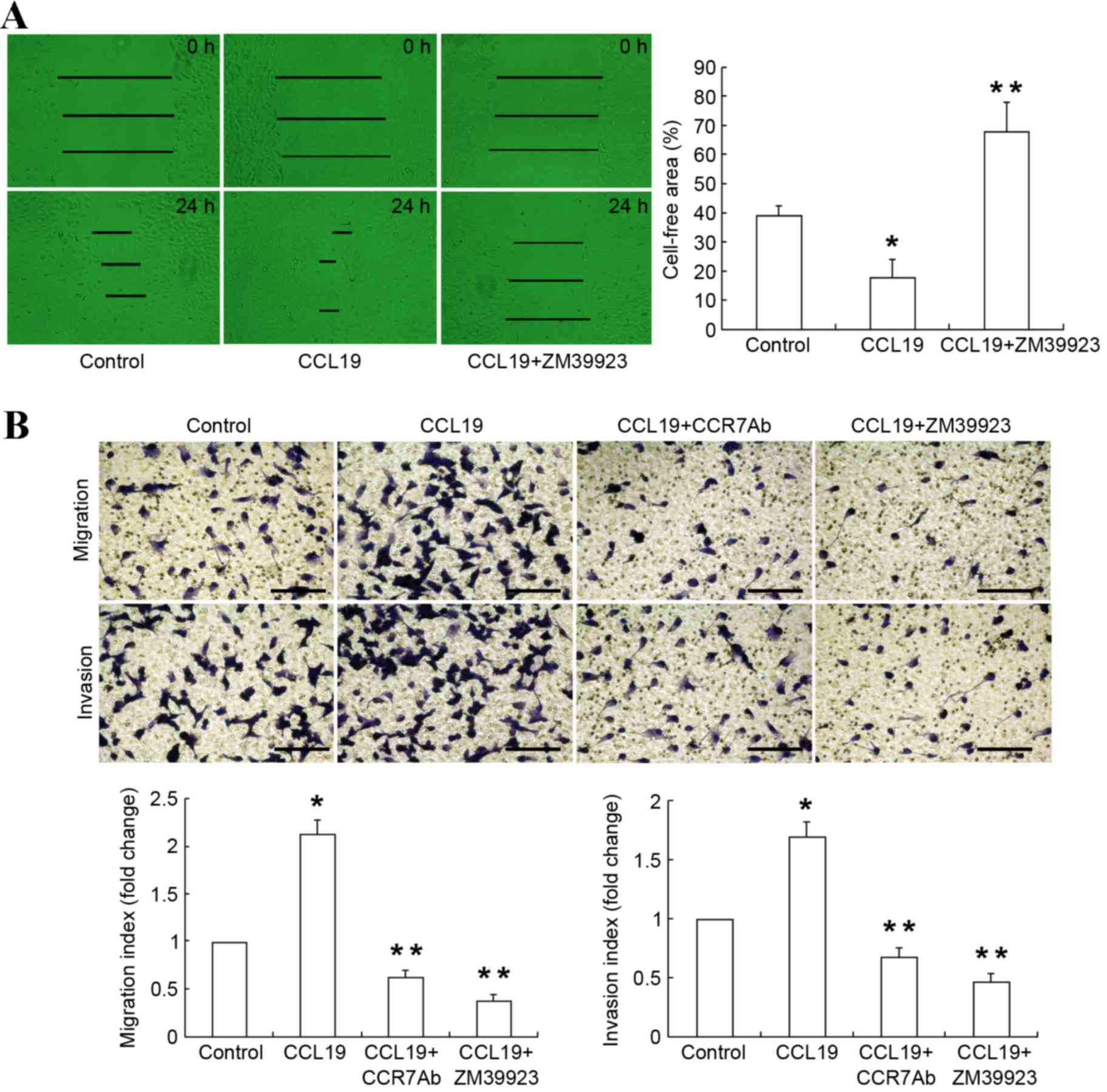
Jak3 activation promotes the migration
and invasion of PCI-37B cells
To evaluate whether Jak3 phosphorylation has an
effect on the biological behavior of SCCHN cells, the present study
analyzed the migration and invasion capability of SCCHN cells by
wound healing assay and the Transwell assay. To determine PCI-37B
cell migration rate, the scratch-wound assay was performed. Cells
were grown in 24-well plates in DMEM to confluence for 24 h,
following the introduction of a wound by scratching. The present
study demonstrated that subsequent to 24 h cells gradually migrated
into the wound space along the wound edge. Compared with the
control group, wound closure was significantly promoted in the
PCI-37B cells upon addition of CCL19 at the 24 h (P<0.05;
Fig. 3A), which exhibits that CCL19
enhanced the migration of PCI-37B cells. A special inhibitor of
Jak3, ZM39923 significantly blocked the CCL19 induced wound closure
rate compared with CCL19 alone (P<0.05; Fig. 3A). The wound-healing assay proved that
Jak3 activates the ability of migration of SCCHN cells.
Furthermore, the migration and invasion assays
validated that CCL19 significantly accelerated tumor progression by
increasing the migration and invasion ability of the PCI-37B cells.
Compared to CCL19 alone, PCI-37B cells pretreated with CCR7
antibody followed by CCL19 resulted a significantly decrease in
migration and invasion ability (P<0.05; Fig. 3B). Similarly, ZM39923 followed by
CCL19 also significantly decreased the migration and invasion of
PCI-37B cells compared with CCL19 alone (P<0.05; Fig. 3B). These results indicated that Jak3
performs an important role in the metastatic activity mediated
through CCR7 in the metastatic SCCHN cell line.
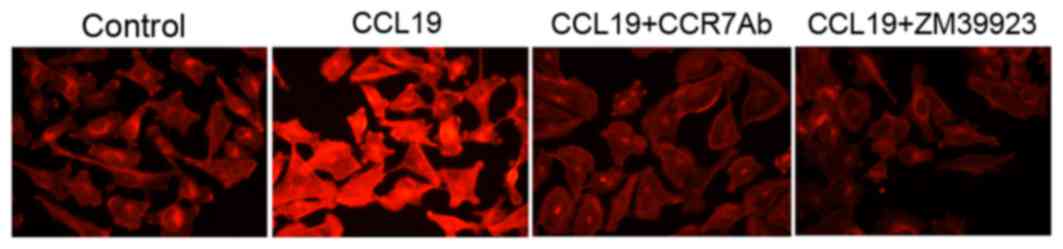
Jak3 activation participates in
F-actin rearrangement induced by CCL19 in CCR7-expressing SCCHN
cells
F-actin, as the major component of cytoskeleton,
performs a crucial role in tumor cell migration and motility. The
rhodamine-labeled phalloidin staining demonstrated that there was a
redistribution and intense impression of F-actin within the cells
stimulated by CCL19. Evident pseudopodia formation was observed,
which contributes to enhancing the migration and motility of
PCI-37B cells. The reorganization of the F-actin was inhibited in
the cells pretreated with CCR7 antibody or ZM39923, which indicated
that Jak3 activation may be one of the mechanisms of F-actin
polymerization induced by CCR7 and its ligands interaction
(Fig. 4).
Discussion
The present experiments demonstrated that Jak3 is
involved in modulating the migration and invasion induced by
chemokine receptor CCR7 in metastatic SCCHN. The results of the
present study may provide valuable insight into illustrating the
complicated mechanisms of CCR7 signaling, through which tumor
metastasis is promoted. The present study identified high
expression of phospho-Jak3 in tumor tissues and metastatic lymph
nodes. Phospho-Jak3 expression was associated with cervical lymph
node metastasis and clinical stage of SCCHN. Additionally, the
western blot analysis results demonstrated that Jak3 may be
activated by CCR7 in PCI-37B cells. Furthermore, the wound healing
and Transwell assays validated the hypothesis that Jak3 serves an
important role in CCR7-induced malignant biological behavior of
SCCHN cells. Additionally, Jak3 inhibitor blocked F-actin
rearrangement stimulated by CCL19 in CCR7-expressing SCCHN cells.
Based on these results, the role of Jak3 was characterized in
regulating the migration and invasion of SCCHN.
SCCHN, as a common malignant tumor of the head and
neck, is characterized by a high degree of malignancy, high
incidence of recurrence and metastasis, and high mortality. The
combined treatment consisting of surgery or radiotherapy with
postoperative adjuvant chemotherapy exhibits an improved outcome to
prolong the overall survival in the treatment of SCCHN, but the
prognosis remains far from optimistic due to the complexity and
inscrutability of SCCHN (14,15). Development of novel effective
therapeutic strategies is urgent to improve life quality and reduce
quantity of patients with recurrent or metastatic SCCHN. The low
5-year survival of SCCHN particularly depends on the regional nodal
metastasis in the neck, and thus it is crucial to explore the
associated mechanisms of cervical metastasis process of SCCHN to
improve outcomes for these patients (16). Numerous clinical and experimental
studies identified that chemokines and chemokine receptors have
significantly affected the development, invasion and metastasis of
a variety of tumors, apart from performing a role in the immune
system. Presently, targeted-drug therapy is a promising adjuvant
treatment approach in order to produce anti-tumor effects and
result in an improved prognosis for the patient (17). Studies have suggested that chemokines
and chemokine receptors act as potential therapeutic targets to
inhibit tumor growth in malignant tumors (18). In particular, the CCL19/21-CCR7
signaling pathway has been associated with regional lymphatic nodal
metastasis in a variety of tumors, including breast, gastric, and
non-small cell lung cancer and melanoma (19,20). CCR7,
as a seven-transmembrane domain G-protein-coupled receptor,
presented a high expression in metastatic SCCHN and contributed to
tumor progression and poor prognosis (21). Targeting of CCR7 and associated
downstream molecules may control the migration and metastasis of
SCCHN tumors (22). However,
corresponding activation cascades of CCR7 pathway have not been
accurately identified in detail in SCCHN.
The Jak family consists of 4 isoforms (Jak1, Jak2,
Jak3 and Tyk2) and performs a pivotal role in the cytokine
signaling pathway, modulating diverse cellular development,
proliferation and differentiation (23). Among the Jaks, Jak3 that mainly exists
in the lymphatic system and hematopoetic tissues, is a crucial
tyrosine kinase in cell signal transduction for lymphocyte
development and proliferation in the immune response (24). Jak3 is rapidly activated in
phosphorylated form via cytokines by binding to their cell-surface
receptors (25). Targeting
Jak3-linked signal transduction pathways has shown effective immune
suppression and anti-cancer effects (26,27). The
activation of the Jak3 pathway participates in the proliferation of
certain types of cancer including cervical cancer (28). The selective Jak3 inhibitors have been
proposed as potential therapeutic reagents targeting certain
cytokine-associated diseases, unlike other Jak inhibitors
possessing certain unintended side effects (29,30).
However, little is known regarding the role of the Jak3 signaling
in SCCHN. To explore this problem, in the present study, the
expression levels of Jak3 phosphorylation were detected at the
indicated time points subsequent to the pretreatment of CCL19 at a
dose of 200 ng/ml in a human SCCHN cell line, PCI-37B cultured in
serum-free medium. The results of the western blot analysis
revealed that CCL19 resulted in an increased phosphorylation of
Jak3 in a time-dependent manner in PCI-37B cells. Additionally,
immunohistochemical analysis demonstrated that there is an
increased expression of phospho-Jak3 in tumor tissues and
metastatic lymph nodes compared with the normal tissues.
Additionally, the increased phospho-Jak3 expression was associated
with condition of nodal metastasis and advanced stage, but not with
age and tumor size (P>0.05). CCR7 was an independent risk factor
for a higher nodal stage, recurrence and poor prognosis for SCCHN
patients. Rivas-Caicedo et al (10) reported that Jak3 serves an important
role in the cellular migration and function via the CCR7 pathway in
the dendritic cell. The present study hypothesized that CCL19, by
interacting with its receptor, CCR7, stimulated the activation of
Jak3 in SCCHN. In the present study, PCI-37B cells were treated
with or without Jak3 inhibitor ZM39923 or the CCR7 antibody
followed by CCL19. The activation of Jak3 was inhibited by ZM39923
or the CCR7 antibody. As a result, the present study preliminarily
proved that the interaction between CCR7 and its ligand, CCL19,
enhanced Jak3 phosphorylation in the SCCHN cell line.
Previous studies have demonstrated that CCR7
expression is recognized as a key marker for predicting lymph nodal
metastasis and tumor progression in tumor cells of breast and
gastric cancer, as well as malignant melanoma (31–33). The
authors previously investigated the role of CCR7 in tumor growth
and metastasis in SCCHN (34,35). Although it was shown that Jak3 is
phosphorylated by the CCL19/CCR7 pathway in SCCHN tumor, additional
descriptions of the role of Jak3 in SCCHN are required. To test
whether Jak3 activation was involved in CCR7-mediated migration and
invasion of SCCHN cell lines, the biological behavior in SCCHN was
analyzed by inhibiting Jak3 signaling using specific inhibitor
ZM39923. Cell mobility and migration was detected using a wound
healing assay. The cellular migration rate was increased by CCL19
application at 24 h subsequent to scratching SCCHN cell layer,
suggesting that migration may be increased by CCR7 activation
(13,35). These effects were blocked by the
administration of ZM39923. Accordingly, the results of Transwell
migration and invasion assays were consistent with the findings
obtained by the wound healing assay. In addition, migration and
invasion of cancer cells are functionally facilitated by actin
cytoskeleton reorganization, and chemotherapeutic agents targeting
the integrity of actin cytoskeleton were used to attenuate prostate
cancer progression (36). In this
study, an actin polymerization assay verified that ZM39923 also
counteracted the effects of F-actin polymerization and pseudopodia
formation as mediated by CCL19 in CCR7-expressing SCCHN cells. The
alternations of cytoskeleton are the early events of migration, and
are essential for the invasion and metastasis of tumor cells
(37,38). Therefore, all assays emphasized the
importance of Jak3 activation in the migration and invasion of
SCCHN cells mediated by CCR7 signaling, which revealed one of the
mechanisms for the role of CCR7 in metastatic SCCHN. Additional
studies investigating the response of molecules downstream of Jak3
induced by CCR7 in SCCHN tumor cells need to be performed in the
future.
In conclusion, the present study highlights the
growing importance of the Jak3 signaling pathway in the metastasis
of malignant head and neck tumors mediated by the interactions of
chemokine receptor CCR7 and its ligands. The results of the present
study also contribute to the illustration of the complicated
genetic regulation mechanism of CCR7 and provide a novel target for
the treatment of SCCHN.
Acknowledgements
The present study was funded with the following
grants: Science Public Welfare Research Fund Projects of Liaoning
Province (grant nos., 2013001017 and 2011002001); Natural Science
Foundation, China (grant nos., 81201800 and 81372877); and,
Shenyang Science and Technology Plan Projects China (grant no.,
F12-277-1-68).
Glossary
Abbreviations
Abbreviations:
|
SCCHN
|
squamous cell carcinoma of the head
and neck
|
|
Jak3
|
Janus activated kinase-3
|
|
CCR7
|
chemokine receptor 7
|
References
|
1
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer Statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cognetti DM, Weber RS and Lai SY: Head and
neck cancer: An evolving treatment paradigm. Cancer. 113:(7 Suppl).
S1911–S1932. 2008. View Article : Google Scholar
|
|
3
|
Inglehart RC, Scanlon CS and D'Silva NJ:
Reviewing and reconsidering invasion assays in head and neck
cancer. Oral Oncol. 50:1137–1143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kiyota N, Tahara M and Fujii M: Adjuvant
treatment for post-operative head and neck squamous cell carcinoma.
Jpn J Clin Oncol. 45:2–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sarvaiya PJ, Guo D, Ulasov I, Gabikian P
and Lesniak MS: Chemokines in tumor progression and metastasis.
Oncotarget. 4:2171–2185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruffini PA, Morandi P, Cabioglu N,
Altundag K and Cristofanilli M: Manipulating the
chemokine-chemokine receptor network to treat cancer. Cancer.
109:2392–2404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Xi L, Hunt JL, Gooding W,
Whiteside TL, Chen Z, Godfrey TE and Ferris RL: Expression pattern
of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma
of the head and neck identifies a novel metastatic phenotype.
Cancer Res. 64:1861–1866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Seethala RR, Zhang Q, Gooding W,
van Waes C, Hasegawa H and Ferris RL: Autocrine and paracrine
chemokine receptor 7 activation in head and neck cancer:
Implications for therapy. J Natl Cancer Inst. 100:502–512. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Babon JJ, Lucet IS, Murphy JM, Nicola NA
and Varghese LN: The molecular regulation of Janus kinase (JAK)
activation. Biochem J. 462:1–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rivas-Caicedo A, Soldevila G, Fortoul TI,
Castell-Rodríguez A, Flores-Romo L and García-Zepeda EA: Jak3 is
involved in dendritic cell maturation and CCR7-dependent migration.
PLoS One. 4:e70662009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
García-Zepeda EA, Licona-Limón I,
Jiménez-Sólomon MF and Soldevila G: Janus kinase 3-deficient T
lymphocytes have an intrinsic defect in CCR7-mediated homing to
peripheral lymphoid organs. Immunology. 122:247–260. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loh KC, Greenspan FS, Gee L, Miller TR and
Yeo PP: Pathological tumor-node-metastasis (pTNM) staging for
papillary and follicular thyroid carcinomas: A retrospective
analysis of 700 patients. J Clin Endocrinol Metab. 82:3553–3562.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu FY, Safdar J, Li ZN, Fang QG, Zhang X,
Xu ZF and Sun CF: CCR7 regulates cell migration and invasion
through MAPKs in metastatic squamous cell carcinoma of head and
neck. Int J Oncol. 45:2502–2510. 2014.PubMed/NCBI
|
|
14
|
Winquist E, Al-Rasheedy I, Nichols AC,
Palma DA and Stitt L: Temporal changes in the efficacy of
chemotherapy for recurrent or metastatic squamous cell carcinoma of
the head and neck: A systematic review and meta-analysis. Cancer
Treat Rev. 40:1073–1079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nelke KH, Pawlak W, Gerber H and
Leszczyszyn J: Head and Neck Cancer Patients' Quality of Life. Adv
Clin Exp Med. 23:1019–1027. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noorlag R, van Kempen PM, Stegeman I,
Koole R, van Es RJ and Willems SM: The diagnostic value of 11q13
amplification and protein expression in the detection of nodal
metastasis from oral squamous cell carcinoma: A systematic review
and meta-analysis. Virchows Arch. 466:363–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee HJ, Song IC, Yun HJ, Jo DY and Kim S:
CXC chemokines and chemokine receptors in gastric cancer: From
basic findings towards therapeutic targeting. World J
Gastroenterol. 20:1681–1693. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh JK, Farnie G, Bundred NJ, Simões BM,
Shergill A, Landberg G, Howell SJ and Clarke RB: Targeting CXCR1/2
significantly reduces breast cancer stem cell activity and
increases the efficacy of inhibiting HER2 via HER2-dependent and
-independent mechanisms. Clin Cancer Res. 19:643–656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kakinuma T and Hwang ST: Chemokines,
chemokine receptors, and cancer metastasis. J Leukoc Biol.
79:639–651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Zhang X, Thomas SM, Grandis JR,
Wells A, Chen ZG and Ferris RL: Chemokine receptor 7 activates
phosphoinositide-3 kinase-mediated invasive and prosurvival
pathways in head and neck cancer cells independent of EGFR.
Oncogene. 24:5897–5904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mburu YK, Egloff AM, Walker WH, Wang L,
Seethala RR, van Waes C and Ferris RL: Chemokine Receptor 7 (CCR7)
gene expression is regulated by NF-B and Activator Protein 1 (AP1)
in metastatic squamous cell carcinoma of head and neck (SCCHN). J
Biol Chem. 287:3581–3590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signaling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamaoka K, Maeshima K, Kubo S and Tanaka
Y: Regulation of inflammation through JAK3-Stat6 pathway in
dendritic cells. Nihon Rinsho Meneki Gakkai Kaishi. 35:62–68.
2012.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhavsar SK, Gu S, Bobbala D and Lang F:
Janus kinase 3 is expressed in erythrocytes, phosphorylated upon
energy depletion and involved in the regulation of suicidal
erythrocyte death. Cell Physiol Biochem. 27:547–556. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Säemann MD, Zeyda M, Stulnig TM, Böhmig
GA, Wekerle T, Hörl WH and Zlabinger GJ: Janus kinase-3 (JAK3)
inhibition: A novel immunosuppressive option for allogeneic
transplantation. Transpl Int. 17:481–489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uckun FM, Vassilev A, Dibirdik I and
Tibbles H: Targeting JAK3 tyrosine kinase-linked signal
transduction pathways with rationally-designed inhibitors.
Anticancer Agents Med Chem. 7:612–623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Valle-Mendiola A, Weiss-Steider B,
Rocha-Zavaleta L and Soto-Cruz I: IL-2 enhances cervical cancer
cells proliferation and JAK3/STAT5 phosphorylation at low doses,
while at high doses IL-2 has opposite effects. Cancer Invest.
32:115–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Treliński J and Robak T: JAK inhibitors:
Pharmacology and clinical activity in chronic myeloprolipherative
neoplasms. Curr Med Chem. 20:1147–1161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghoreschi K, Laurence A and O'Shea JJ:
Janus kinases in immune cell signaling. Immunol Rev. 228:273–287.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang HL, Chiang CH, Hung WC and Hou MF:
Targeting of TGF-β-activated protein kinase 1 inhibits chemokine
(C-C motif) receptor 7 expression, tumor growth and metastasis in
breast cancer. Oncotarget. 6:995–1007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van den Bosch T, Koopmans AE, Vaarwater J,
van den Berg M, de Klein A and Verdijk RM: Chemokine receptor CCR7
expression predicts poor outcome in uveal melanoma and relates to
liver metastasis whereas expression of CXCR4 is not of clinical
relevance. Invest Ophthalmol Vis Sci. 54:7354–7361. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arigami T, Natsugoe S, Uenosono Y,
Yanagita S, Arima H, Hirata M, Ishigami S and Aikou T: CCR7 and
CXCR4 expression predicts lymph node status including
micrometastasis in gastric cancer. Int J Oncol. 35:19–24. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu FY, Safdar J, Li ZN, Fang QG, Zhang X,
Xu ZF and Sun CF: CCR7 regulates cell migration and invasion
through JAK2/STAT3 in metastatic squamous cell carcinoma of the
head and neck. Biomed Res Int. 2014:4153752014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu FY, Zhao ZJ, Li P, Ding X, Guo N, Yang
LL, Zong ZH and Sun CF: NF-κB participates in chemokine receptor
7-mediated cell survival in metastatic squamous cell carcinoma of
the head and neck. Oncol Rep. 25:383–391. 2011.PubMed/NCBI
|
|
36
|
Martin SK, Kamelgarn M and Kyprianou N:
Cytoskeleton targeting value in prostate cancer treatment. Am J
Clin Exp Urol. 2:15–26. 2014.PubMed/NCBI
|
|
37
|
Mashino K, Sadanaga N, Yamaguchi H, Tanaka
F, Ohta M, Shibuta K, Inoue H and Mori M: Expression of chemokine
receptor CCR7 is associated with lymph node metastasis of gastric
carcinoma. Cancer Res. 62:2937–2941. 2002.PubMed/NCBI
|
|
38
|
Baggiolini M: Chemokines and leukocyte
traffic. Nature. 392:565–568. 1998. View
Article : Google Scholar : PubMed/NCBI
|