Introduction
MicroRNAs (miRNAs or miRs) are small (19–25
nucleotides) endogenous, non-coding RNAs that regulate gene
expression via inhibiting translation and/or promoting the
degradation of target messenger RNA (mRNA) at the
post-transcriptional level (1,2). Evidence
highlights the significance of miRNAs as essential regulators of
numerous biological processes, including cell proliferation,
differentiation, apoptosis and metastasis (3). However, the precise mechanisms
underlying the function of the majority of identified miRNAs remain
unclear.
Dysregulation of miRNAs has been identified in
numerous types of human tumors (4–6),
suggesting that they serve essential roles in tumorigenesis and
tumor development. Previous studies have reported that miR-638
expression is significantly downregulated, and may serve a role as
a cancer-suppressor gene in human gastric cancer (7), breast cancer (8), basal cell carcinoma (9) and chronic lymphocytic leukemia (10). A previous study reported that the
expression of miR-638 was markedly upregulated in hepatocellular
liver cancer compared with healthy liver tissue (11). Notably, by using microarrays, miR-638
has been identified as one of the miRNAs that serve a role in the
invasive-metastatic cascade in hepatocellular carcinoma (HCC)
(12). In addition, the
downregulation of miR-638 promotes the invasion and proliferation
of human colorectal carcinoma (13)
and non-small cell lung cancer (NSCLC) (14). miR-638 has been functionally
associated with the hepatitis B virus (HBV) life cycle (15). However, the clinical significance of
miR-638 in the treatment of patients with HCC remains unclear. In
the present study, the expression of miR-638 in HCC was
investigated using reverse transcription-quantitative polymerase
chain reaction (RT-qPCR). Furthermore, the association between the
expression of miR-638 and the clinicopathological characteristics
of patients with HCC was analyzed.
Materials and methods
Human tissue specimens
Formalin-fixed paraffin-embedded (FFPE) tissue
samples, including 60 HCC and adjacent healthy liver tissue
samples, were collected from patients who underwent curative
hepatic resection for HCC between January 2008 and June 2010 at the
Department of Pathology of the First Affiliated Hospital of Xi'an
Jiaotong University (Xi'an, China). None of the patients had
received local or systemic therapy prior to surgery, and the tumor
and matched adjacent healthy tissue samples were histologically
confirmed. Written informed consent was obtained from all patients
and the study was approved by the Institute Research Ethics
Committee at the Cancer Center of Xi'an Jiaotong University (Xi'an,
China). The relevant clinicopathological characteristics of the
patients were collected from their clinical records.
Cell lines and culture conditions
Human SMMC-7721, HepG2 and Hep3B liver cancer cell
lines, and the healthy human HL-7702 liver cell line were purchased
from the Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China). These cells were cultured in Dulbecco's
modified Eagle's medium (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) and RPMI 1640 medium (GE Healthcare Life Sciences)
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) with 100 U/ml penicillin and 100 U/ml
streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at
37°C with 5% CO2.
RNA extraction
Total RNA was extracted from human FFPE tissue
samples and all cell lines using an E.Z.N.A.® FFPE RNA
kit (Omega Bio-Tek, Inc., Norcross, GA, USA) and TriPure RNA
Isolation Reagent (Roche Diagnostics, Indianapolis, IN, USA)
according to the manufacturers' protocols. The RNA concentration
and purity were determined and evaluated using the
NanoDrop® ND-1000 (NanoDrop Technologies; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). The absorbance
(A)260:A280 ratio was used to estimate the
purity of total RNA.
RT-qPCR
Complementary DNA (cDNA) was synthesized from 1 µg
of RNA following the manufacturer's protocol (Takara Biotechnology
Co., Ltd, Dalian, China). The 10-µl final reaction volume consisted
of 1 µg of total RNA, 2 µl 5X PrimeScript® Buffer, 0.5
µl PrimeScript® RT Enzyme Mix and 1 µl RT primer (Takara
Biotechnology Co., Ltd). The reaction was incubated for 15 min at
37°C followed by 5 sec at 85°C.
qPCR analyses were performed using Power
SYBR® Green PCR Master Mix (Takara Biotechnology Co.,
Ltd.) according to the manufacturer's protocol. qPCR reactions were
performed using the Applied Biosystems® 7500 PCR System
(Thermo Fisher Scientific, Inc.). The forward and reverse primers
for miR-638 and U6 are presented in Table
I. The following thermocycling conditions were performed: 95°C
for 1 min; 40 cycles of 95°C for 10 sec; and 58°C for 40 sec. The
20-µl qPCR reaction volume consisted of 10 µl SYBR Prime Ex
Taq™ II (2X) (Takara Biotechnology Co., Ltd), 1 µl
forward primer (10 mM), 1 µl reverse primer (10 mM), 2 µl cDNA
(<100 ng used per reaction) and 6 µl H2O. Results
were normalized to the expression of U6 and the relative
quantification of miRNA expression was calculated with the
2−ΔΔCq method, whereby 2−ΔΔCq = 2−[ΔCq
(HCC) - ΔCq (control)] and ΔCq = Cq miR-638 - Cq
U6 (16). A Cq value of 35
was assigned as the cut-off value for defining samples as
non-detected. All reactions were performed in triplicate.
 | Table I.RT, forward and reverse primers for
RT-quantitative polymerase chain reaction analysis of miR-638 and
U6. |
Table I.
RT, forward and reverse primers for
RT-quantitative polymerase chain reaction analysis of miR-638 and
U6.
| Primer | Sequence |
|---|
| miR-638 |
| RT |
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCG |
|
|
GCAATTGCACTGGATACGACAGGCCGC-3′ |
|
Forward | 5′-ATCCAGTGCGTG
TCGTG-3′ |
|
Reverse | 5′-TGCTAGGGATCGC
GGGCGGGTG-3′ |
| U6 |
| RT |
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
|
Forward |
5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
|
Reverse |
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
miRNA target prediction
Established miRNA-target prediction tools were used
to identify potential target genes of miR-638. The following eight
prediction databases were used: DIANA TOOLS (http://diana.imis.athena-innovation.gr/), microRNA.org (http://www.microrna.org/microrna/home.do), miRDB
(http://mirdb.org/miRDB/download.html), TargetMiner
(http://www.isical.ac.in/~bioinfo_miu/mirnalist.html),
TargetScan (http://targetscan.org/), RNA22-HSA
(https://cm.jefferson.edu/rna22/), PITA
(http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html)
and RegRNA (http://regrna.mbc.nctu.edu.tw/html/tutorial.html).
The top 100 target genes in the majority of databases were recorded
and a comparison was made between them. Experimentally verified
targets and only predicted target genes in mammals that appeared
>4 times were noted in the current study.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 16.0; SPSS, Inc., Chicago, IL, USA). Student's t
test was performed to analyze the significance of differences
between groups. To identify the association between
clinicopathological characteristics of patients with HCC and the
expression of miR-638, the c2 and Fisher's exact tests
were performed. A receiver operating characteristic (ROC) curve was
produced to evaluate the efficacy of miR-638 expression when
distinguishing between the HCC and healthy liver tissue samples.
All tests were two-tailed. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-638 expression is frequently
decreased in human HCC tissue and cell lines compared with the
healthy control groups
The expression of miR-638 was determined in 60 HCC
and corresponding healthy tissue samples using RT-qPCR and
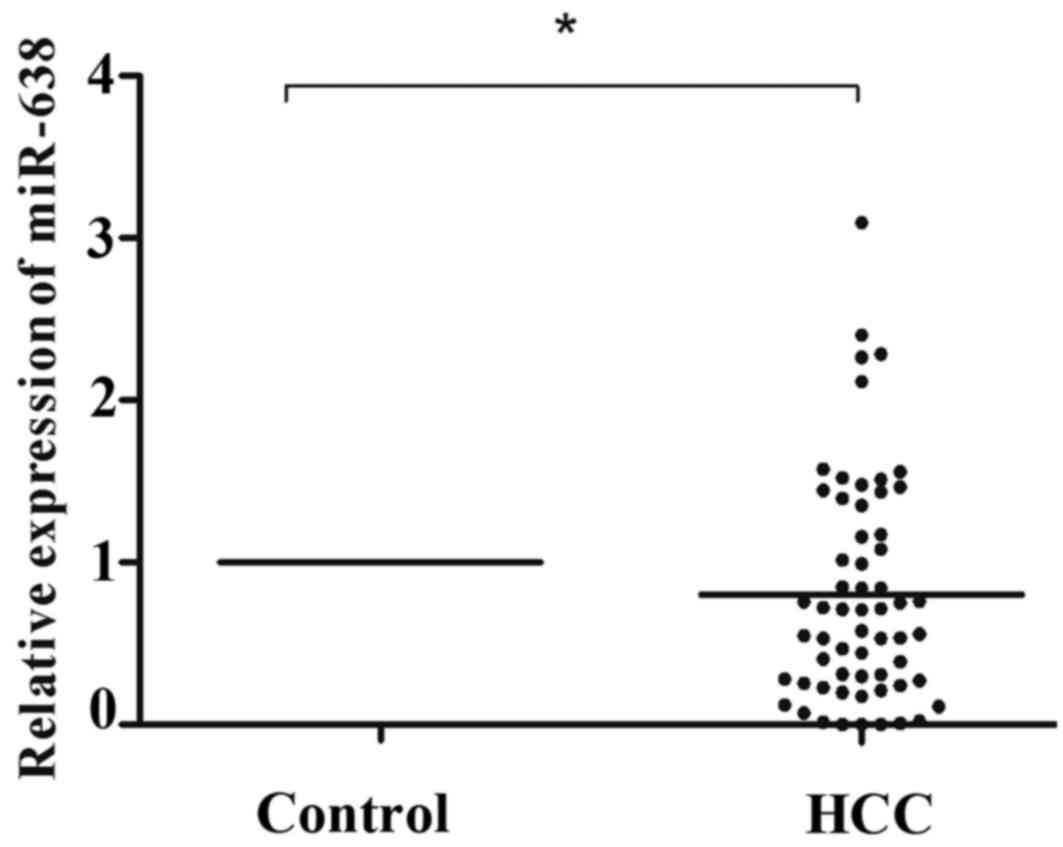
normalized to the control U6. As illustrated in Fig. 1, miR-638 expression was significantly
lower in HCC tissue compared with that in healthy tissue samples
(P=0.031). According to the median tumor (T)/non-tumor (N) tissue
ratio of miR-638 expression among the 60 HCC samples analyzed, 41
(T/N>1.0, 68.3%) cases demonstrated low expression of miR-638 in
HCC tissue compared with healthy tissue samples. To further
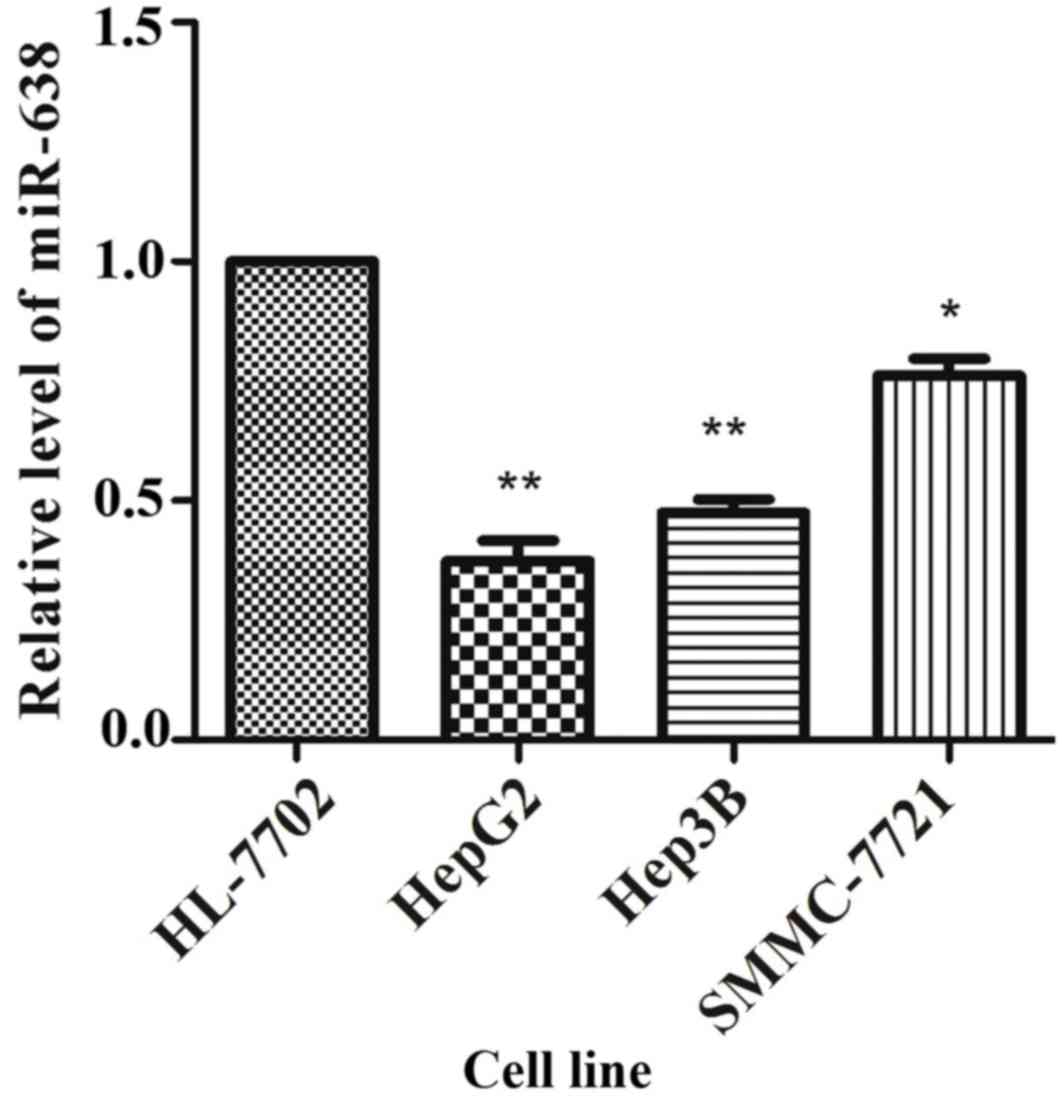
validate these results, the expression of miR-638 in cultured HCC
cells was analyzed, and it was identified that the miR-638
expression was significantly lower in SMMC-7721 (P=0.021), HepG2
(P=0.005) and Hep3B (P=0.003) cells compared with that in HL-7702
cells (Fig. 2).
miR-638 expression and
clinicopathological characteristics
The associations between clinicopathological factors
and miR-638 levels were analyzed using the chi-square test and
Fisher's exact test, and the patients' clinicopathological
characteristics are illustrated in Table
II. No significant association was identified between low
miR-638 expression and age (P=0.781), gender (P=0.089), HBV
infection (P=0.114), tumor size (P=0.774), tumor node metastasis
stage (P=0.146) or distant organ hepatic metastasis (P=0.083).
However, the relative miR-638 expression levels were positively
correlated with α-fetoprotein (AFP) levels and portal vein invasion
(P=0.042, P=0.025, respectively).
 | Table II.Association between the relative
expression of miR-638 and the clinicopathological characteristics
of patients with hepatocellular carcinoma. |
Table II.
Association between the relative
expression of miR-638 and the clinicopathological characteristics
of patients with hepatocellular carcinoma.
|
|
| miR-638
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | No. of cases
(n=60) | High (19) | Low (41) | P-value |
|---|
| Age, years |
|
|
| 0.781 |
|
<50 | 26 | 9 | 17 |
|
|
≥50 | 34 | 10 | 24 |
|
| Gender |
|
|
| 0.089 |
|
Female | 13 | 7 | 6 |
|
|
Male | 47 | 12 | 35 |
|
| HBV infection
status |
|
|
| 0.114 |
| + | 44 | 11 | 33 |
|
| − | 16 | 8 | 8 |
|
| Tumor size, cm |
|
|
| 0.774 |
| ≤5 | 22 | 6 | 16 |
|
|
>5 | 38 | 13 | 25 |
|
| AFP level,
µg/l |
|
|
| 0.042 |
|
≤20 | 20 | 10 | 10 |
|
|
>20 | 40 | 9 | 31 |
|
| TNM stage |
|
|
| 0.146 |
|
I+II | 20 | 9 | 11 |
|
|
III+IV | 40 | 10 | 30 |
|
| Portal vein
invasion status |
|
|
| 0.025 |
|
Yes | 22 | 3 | 19 |
|
| No | 38 | 16 | 22 |
|
| Distant organ
hepatic metastasis status |
|
|
| 0.083 |
|
Yes | 19 | 3 | 16 |
|
| No | 41 | 16 | 27 |
|
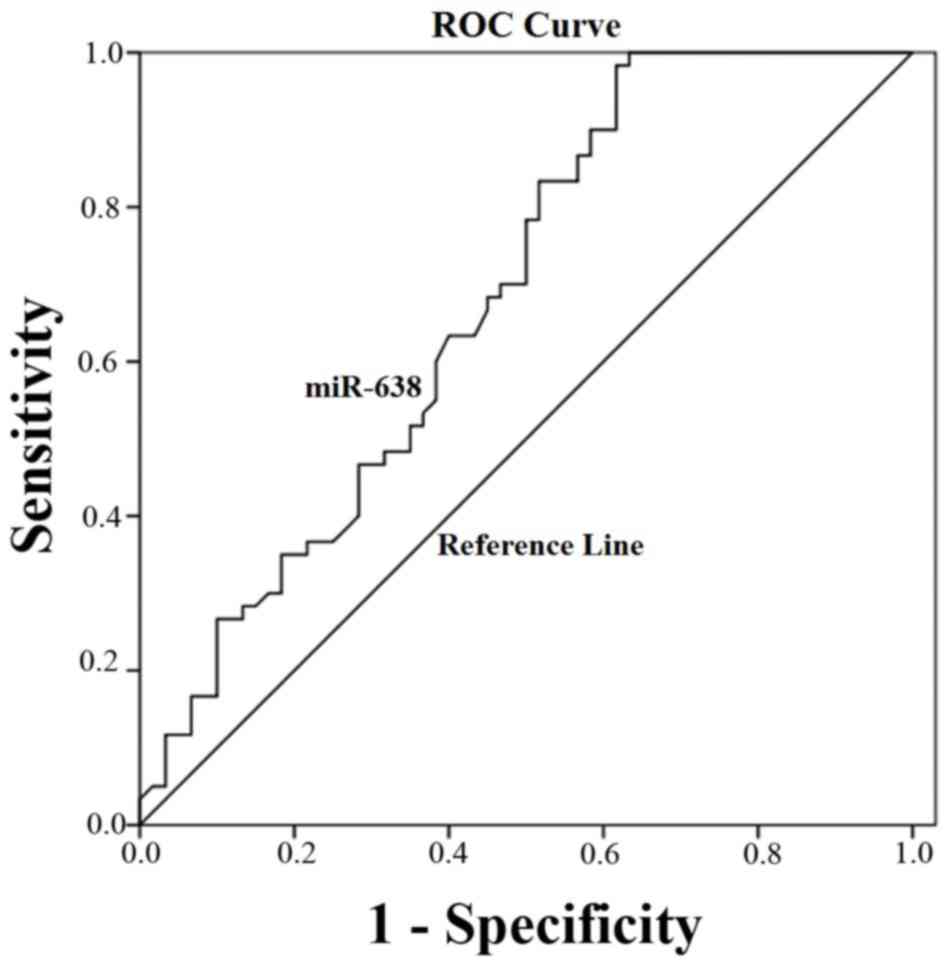
ROC curve analysis for the diagnostic
value of miRNA-638 expression in HCC tissue
ROC curve analysis was implemented to identify the
predictive value of miRNA-638 level in HCC. As illustrated in
Fig. 3, the area under the curve
(AUC) was 0.71 (95% confidence interval=0.63–0.79; P=0.001). The
cut-off value for miR-638 expression was the median
2−Δ∆Cq =0.125.
Target prediction of miR-638
Following the target prediction analysis using eight
databases, 6 validated target genes and 10 qualified target genes
were identified in ≥3 different established miRNA-target prediction
programs. They were as follows: Tetraspanin 1 (TSPAN1),
cyclin-dependent kinase 2 (CDK2), Sp2 transcription factor (Sp2),
tumor protein p53 inducible nuclear protein 2 (TP53INP2), SRY-box 2
(SOX2), breast cancer 1 (BRCA1), dishevelled binding antagonist of
beta catenin 3 (DACT3), StAR-related lipid transfer (START),
StAR-related lipid transfer domain containing 10 (STARD10), protein
O-linked mannose N-acetylglucosaminyltransferase 1 (β 1,2-)
(POMGNT1), transcription elongation regulator 1 like (TCERG1L),
zinc finger protein 281 (ZNF281), vascular endothelial growth
factor A (VEGFA), hepatic leukemia factor (HLF), basic,
immunoglobulin-like variable motif-containing (BIVM), neuronal PAS
domain protein 4 (NPAS4) and muskelin 1 (MKLN1) (Table III).
 | Table III.Target prediction of microRNA-638
following searching on eight different databases. |
Table III.
Target prediction of microRNA-638
following searching on eight different databases.
|
| Database |
|---|
|
|
|
|---|
| Target gene | RegRNA | miRDB | TargetScan | RNA22-HSA | Target Miner | DIANA TOOLS | microRNA. org | PITA |
|---|
| TSPAN1 | + |
| + |
|
|
| + |
|
| CDK2 | + |
| + |
|
|
|
|
|
| Sp2 | + |
| + |
|
| + |
| + |
| TP53INP2 | + |
| + |
|
|
|
| + |
| SOX2 | + | + | + |
|
| + | + | + |
| BRCA1 | + |
| + |
|
|
| + |
|
| DACT3 | + | + | + |
|
|
| + |
|
| STARD10 | + |
| + |
|
|
| + | + |
| POMGNT1 | + |
| + |
|
| + | + | + |
| TCERG1L | + | + | + |
|
| + | + | + |
| ZNF281 | + |
|
| + | + |
| + |
|
| VEGF | + |
| + |
|
|
| + | + |
| HLF | + |
| + |
| + | + |
| + |
| BIVM | + | + | + |
|
|
| + | + |
| NPAS4 | + |
| + | + | + |
| + | + |
| MKLN1 | + |
| + | + | + |
| + |
|
Discussion
miRNA alterations have been identified in numerous
human cancer types (4), which can act
as oncogenes and/or tumor suppressors (2). Each miRNA has hundreds or thousands of
mRNA targets and target genetic regions, such as 3′-untranslated
regions (UTRs), 5′-UTRs and coding regions at the transcriptional
level, subsequently affecting protein expression (17,18). It
has been demonstrated that miRNAs are well preserved in FFPE tissue
due to their short length, thus underscoring the suitability of
FFPE tissue specimens as appropriate resources for miRNA expression
analyses (19–21). Currently, multiple methods are used to
identify and quantify miRNAs in tumor samples, including microarray
(22), RT-qPCR (23), RNA sequencing (24) and in situ hybridization
(25).
In the present study, RT-qPCR was performed to
assess the expression of miR-638 in FFPE tissue samples from
patients with HCC. miRNAs that have been validated and possess the
potential to affect cellular function are available at the miRNA
database miRBase (www.mirbase.org/) (26).
The primers for miR-638 were designed using the above miRNA
database in the present study. Previous studies have demonstrated
that miR-638 is downregulated in certain human tumor types, such as
gastric cancer (7), breast cancer
(8), NSCLC (14) and colorectal carcinoma (13). A previous study revealed that the
downregulation of miR-638 was present in 68% (41/60) of primary
human NSCLC tissue samples compared with that in the paired healthy
samples (14).
Lin et al (11)
detected the expression of miR-638 in high-density multiple organ
tumor and healthy tissue microarrays using in situ
hybridization. This study reported that the expression of miR-638
was markedly upregulated in hepatocellular liver cancer tissue
(n=20) compared with healthy liver tissue (n=5) samples. However,
in the present study, it was suggested that miR-638 serves a role
as tumor suppressor in HCC. The proportion of low expression
miR-638 was 68.3% (41/60) among the 60 patients with HCC and the
relative expression of miR-638 in HCC tissue samples was
significantly lower compared with the expression in the healthy
control group. The conflicting data on miR-638 expression in HCC
may be explained by various factors, such as tissue specificity,
different populations and small sample sizes. Notably, miR-638
expression in HCC was detected in a small cohort and the
clinicopathological characteristics were not evaluated in the
previous study (11).
In the current study, miR-638 expression was
detected in a relatively larger sample size, which minimized the
effect of individual differences, and a full-panel analysis was
performed between miR-638 expression and the clinicopathological
characteristics of patients with HCC. In addition, a lower
expression of miR-638 was identified in several HCC cell lines
(HepG2, SMMC-7721 and Hep3B) compared with the healthy human
hepatic HL-7702 cell line. Furthermore, the results of the present
study demonstrated that serum miR-638 expression was significantly
lower in patients with HCC compared with the healthy control group
(P<0.001; data not shown).
To the best of our knowledge, the present study is
the first to investigate the association between miR-638 expression
and the clinicopathological characteristics of patients with HCC.
The results of the current study suggest that low miR-638
expression correlates with AFP levels and portal vein invasion
status. Although statistically significant, the correlation between
AFP and low miR-638 expression was weak. AFP was predicted as one
of the potential target genes of miR-638 following the use of
established miRNA-target prediction tools. Further studies into
whether AFP expression is regulated by miR-638 are warranted. The
results of ROC analysis indicate that miR-638 possesses a moderate
diagnostic value in HCC, with an AUC of 0.71. Several studies have
demonstrated the potential of miRNAs as predictors of therapeutic
response and overall survival rate in patients with cancer. A study
performed by Parasramka et al (27) identified miR-638 as one of the
garcinol-specific miRNA biomarkers that sensitize human pancreatic
adenocarcinoma cells to the combination treatment of garcinol and
gemcitabine. Furthermore, miR-638 was identified as a potential
predictor of early virological response to interferon treatment in
patients with chronic hepatitis B (15). In addition, miR-638 was one of the
four miRNAs identified through genome-wide serum miRNA profiling
that predict the survival rate of patients with nasopharyngeal
carcinoma (28). A previous study
demonstrated that the downregulation of miR-638 in colorectal
cancer predicts poor survival (13).
As the function and role of miR-638 in HCC remain
unclear, the present study aimed to identify the potential target
genes of miR-638. Following searching in eight different
established miRNA-target prediction programs, the following 16
genes were identified: TSPAN1, CDK2, Sp2, TP53INP2, SOX2, BRCA1,
DACT3, STARD10, POMGNT1, TCERG1L, ZNF281, VEGF, HLF, BIVM, NPAS4
and MKLN1. Among these possible target genes, CDK2, Sp2, TP53INP2,
SOX2, TSPAN1 and BRCA1 are verified target genes of miR-638.
Previous studies have demonstrated that miR-638 inhibits cell
proliferation by targeting Sp2 in gastric cancer (7); inhibits cell proliferation and invasion;
and regulates cell cycle by targeting TSPAN1 in human colorectal
carcinoma (13). The results of a
previous study revealed that the downregulation of miR-638 promotes
cell proliferation and invasion, and induces mesenchymal-like
transition in NSCLC by directly targeting SOX2, whereas the
upregulation of miR-638 can reverse the effect (14). These results suggested that miR-638
may serve as a novel tumor suppressor. However, the studies
mentioned above were performed in vitro or on animal models,
and there are no in vivo studies on miR-638 use as an
anticancer therapy at present. In addition to the verified targets
of miR-638, the potential target genes of miR-638, including DACT3,
STARD10, TSPAN1, POMGNT1, TCERG1L, ZNF281 and VEGF, have been
demonstrated to serve important roles in certain types of cancer.
Previous studies have revealed that DACT3 serves a role as an
epigenetic regulator in colorectal cancer (29), and the loss of STARD10 expression
identifies a group of breast cancer patients with poor prognosis,
independently of erb-b2 receptor tyrosine kinase 2 and triple
negative expression status (30).
Furthermore, previous studies have suggested that POMGNT1 serves as
a prognostic factor for glioma patient survival (31) and that TCERG1L is a risk marker for
colon cancer in patients with ulcerative colitis (32). ZNF281 has been identified to be
involved in epithelial-mesenchymal transition and cancer (33). Previous studies have demonstrated that
VEGF has various effects on several types of cancer, including the
promotion angiogenesis, invasion and migration (34,35).
Presently, to the best of our knowledge, there are no data that
associate cancer with BIVM, NPAS4 or MKLN1. The predicted and
experimentally verified targets of miR-638 may serve an essential
role in tumorigenesis and progression. A limitation of the present
study is the relatively small sample size. Thus, a larger cohort
study is required to establish the diagnostic value of miR-638 in
HCC. Furthermore, in vitro and in vivo experiments
are required to define the role and underlying mechanism of miR-638
involvement in the development and progression of HCC.
In conclusion, the results of the present study have
demonstrated for the first time that the expression of miR-638 is
frequently decreased in HCC, and is correlated with AFP levels and
portal vein invasion status. This suggests that miR-638 serves a
significant role in the development and progression of HCC. The
findings of the present study and the predicted target genes
identified suggest that miR-638 acts as an oncomiR in HCC
tumorigenesis and progression. Furthermore, it has been suggested
that miR-638 has a moderate diagnostic value in HCC. Therefore,
miR-638 may be considered as a potential novel predictor of HCC and
a target for promising alternatives of specific therapeutic
treatment for patients with HCC.
References
|
1
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berindan-Neagoe I, Pdel Monroig C,
Pasculli B and Calin GA: MicroRNAome genome: A treasure for cancer
diagnosis and therapy. CA Cancer J Clin. 64:311–336. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sen R, Ghosal S, Das S, Balti S and
Chakrabarti J: Competing endogenous RNA: The key to
posttranscriptional regulation. ScientificWorldJournal.
2014:8962062014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao LY, Yao Y, Han J, Yang J, Wang XF,
Tong DD, Song TS, Huang C and Shao Y: miR-638 suppresses cell
proliferation in gastric cancer by targeting Sp2. Dig Dis Sci.
59:1743–1753. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan X, Peng J, Fu Y, An S, Rezaei K,
Tabbara S, Teal CB, Man YG, Brem RF and Fu SW: miR-638 mediated
regulation of BRCA1 affects DNA repair and sensitivity to UV and
cisplatin in triple-negative breast cancer. Breast Cancer Res.
16:4352014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sand M, Skrygan M, Sand D, Georgas D, Hahn
SA, Gambichler T, Altmeyer P and Bechara FG: Expression of
microRNAs in basal cell carcinoma. Br J Dermatol. 167:847–855.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu DX, Zhu W, Fang C, Fan L, Zou ZJ, Wang
YH, Liu P, Hong M, Miao KR, Liu P, et al: miR-181a/b significantly
enhances drug sensitivity in chronic lymphocytic leukemia cells via
targeting multiple anti-apoptosis genes. Carcinogenesis.
33:1294–1301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin Y, Zeng Y, Zhang F, Xue L, Huang Z, Li
W and Guo M: Characterization of microRNA expression profiles and
the discovery of novel microRNAs involved in cancer during human
embryonic development. PLoS One. 8:e692302013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tao ZH, Wan JL, Zeng LY, Xie L, Sun HC,
Qin LX, Wang L, Zhou J, Ren ZG, Li YX, et al: miR-612 suppresses
the invasive-metastatic cascade in hepatocellular carcinoma. J Exp
Med. 210:789–803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Fei B, Wang Q, Song M, Yin Y,
Zhang B, Ni S, Guo W, Bian Z, Quan C, et al: MicroRNA-638 inhibits
cell proliferation, invasion and regulates cell cycle by targeting
tetraspanin 1 in human colorectal carcinoma. Oncotarget.
5:12083–12096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia Y, Wu Y, Liu B, Wang P and Chen Y:
Downregulation of miR-638 promotes invasion and proliferation by
regulating SOX2 and induces EMT in NSCLC. FEBS Lett. 588:2238–2245.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Wang T, Wakita T and Yang W:
Systematic identification of microRNA and messenger RNA profiles in
hepatitis C virus-infected human hepatoma cells. Virology.
398:57–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tay Y, Zhang J, Thomson AM, Lim B and
Rigoutsos I: MicroRNAs to Nanog, Oct4 and Sox2 coding regions
modulate embryonic stem cell differentiation. Nature.
455:1124–1128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lytle JR, Yario TA and Steitz JA: Target
mRNAs are repressed as efficiently by microRNA-binding sites in the
5′UTR as in the 3′UTR. Proc Natl Acad Sci USA. 104:9667–9672. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peiró-Chova L, Peña-Chilet M,
López-Guerrero JA, García-Giménez JL, Alonso-Yuste E, Burgues O,
Lluch A, Ferrer-Lozano J and Ribas G: High stability of microRNAs
in tissue samples of compromised quality. Virchows Arch.
463:765–774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang X, Chen J, Radcliffe T, Lebrun DP,
Tron VA and Feilotter H: An array-based analysis of microRNA
expression comparing matched frozen and formalin-fixed
paraffin-embedded human tissue samples. J Mol Diagn. 10:513–519.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siebolts U, Varnholt H, Drebber U, Dienes
HP, Wickenhauser C and Odenthal M: Tissues from routine pathology
archives are suitable for microRNA analyses by quantitative PCR. J
Clin Pathol. 62:84–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu CG, Calin GA, Volinia S and Croce CM:
MicroRNA expression profiling using microarrays. Nat Protoc.
3:563–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Benes V and Castoldi M: Expression
profiling of microRNA using real-time quantitative PCR, how to use
it and what is available. Methods. 50:244–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin W, Grant J, Stothard P, Moore SS and
Guan LL: Characterization of bovine miRNAs by sequencing and
bioinformatics analysis. BMC Mol Biol. 10:902009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sempere LF and Korc M: A method for
conducting highly sensitive microRNA in situ hybridization and
immunohistochemical analysis in pancreatic cancer. Methods Mol
Biol. 980:43–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Griffiths-Jones S, Saini HK, Van Dongen S
and Enright AJ: Mirbase: Tools for microRNA genomics. Nucleic Acids
Res. 36:D154–D158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parasramka MA, Ali S, Banerjee S,
Deryavoush T, Sarkar FH and Gupta S: Garcinol sensitizes human
pancreatic adenocarcinoma cells to gemcitabine in association with
microRNA signatures. Mol Nutr Food Res. 57:235–248. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu N, Cui RX, Sun Y, Guo R, Mao YP, Tang
LL, Jiang W, Liu X, Cheng YK, He QM, et al: A four-miRNA signature
identified from genome-wide serum miRNA profiling predicts survival
in patients with nasopharyngeal carcinoma. Int J Cancer.
134:1359–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang X, Tan J, Li J, Kivimäe S, Yang X,
Zhuang L, Lee PL, Chan MT, Stanton LW, Liu ET, et al: DACT3 is an
epigenetic regulator of Wnt/beta-catenin signaling in colorectal
cancer and is a therapeutic target of histone modifications. Cancer
Cell. 13:529–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murphy NC, Biankin AV, Millar EK, McNeil
CM, O'Toole SA, Segara D, Crea P, Olayioye MA, Lee CS, Fox SB, et
al: Loss of STARD10 expression identifies a group of poor prognosis
breast cancers independent of HER2/Neu and triple negative status.
Int J Cancer. 126:1445–1453. 2010.PubMed/NCBI
|
|
31
|
Lan J, Guo P, Lin Y, Mao Q, Guo L, Ge J,
Li X, Jiang J, Lin X and Qiu Y: Role of glycosyltransferase PomGnT1
in glioblastoma progression. Neuro Oncol. 17:211–222. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim TO, Park J, Kang MJ, Lee SH, Jee SR,
Ryu DY, Yang K and Yi JM: DNA hypermethylation of a selective gene
panel as a risk marker for colon cancer in patients with ulcerative
colitis. Int J Mol Med. 31:1255–1261. 2013.PubMed/NCBI
|
|
33
|
Hahn S and Hermeking H: ZNF281/ZBP-99: A
new player in epithelial-mesenchymal transition, stemness, and
cancer. J Mol Med (Berl). 92:571–581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fernández M, Semela D, Bruix J, Colle I,
Pinzani M and Bosch J: Angiogenesis in liver disease. J Hepatol.
50:604–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sampat KR and O'Neil B: Antiangiogenic
therapies for advanced hepatocellular carcinoma. Oncologist.
18:430–438. 2013. View Article : Google Scholar : PubMed/NCBI
|