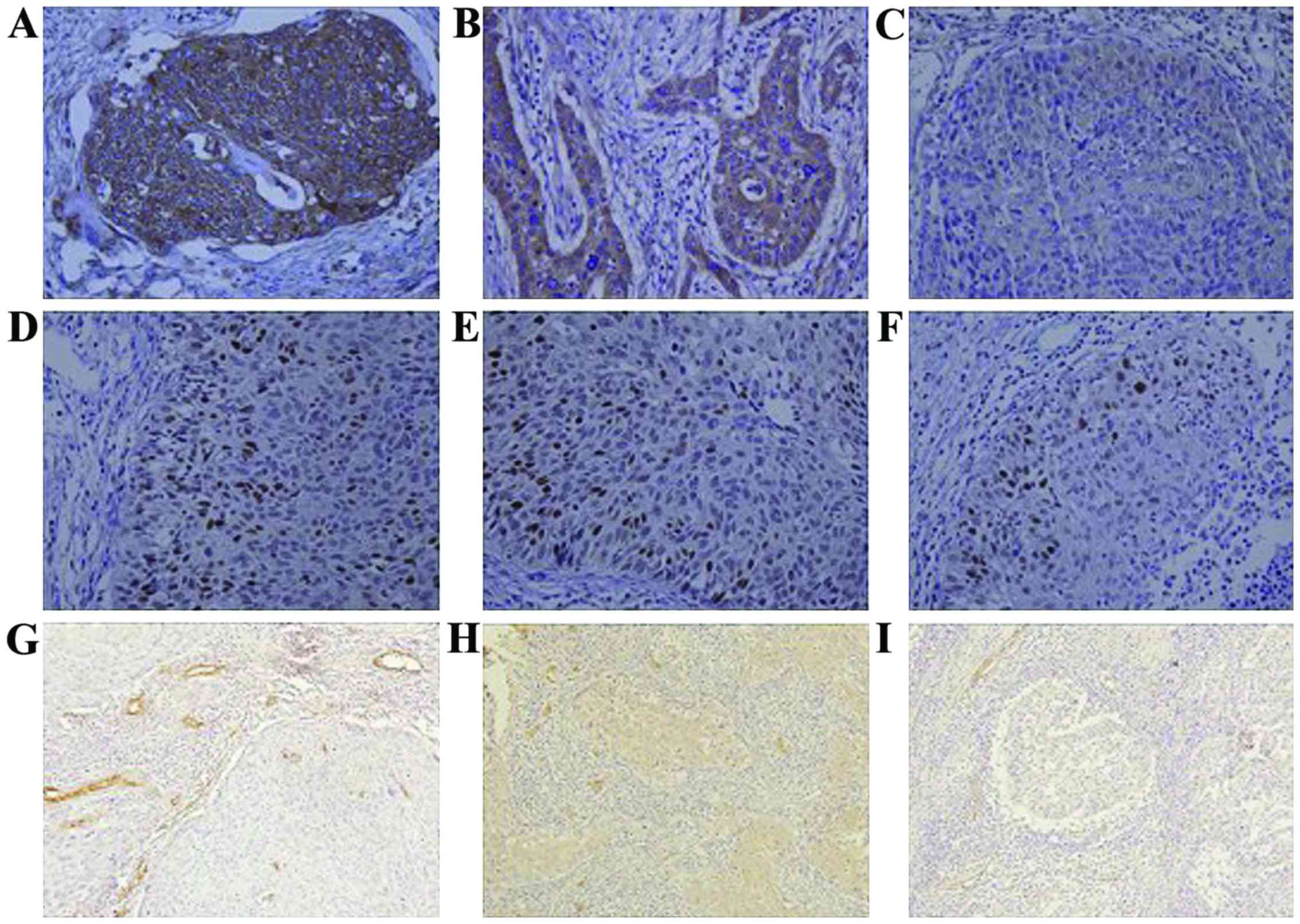
|
1
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rydzewska L, Tierney J, Vale CL and
Symonds PR: Neoadjuvant chemotherapy plus surgery versus surgery
for cervical cancer. Cochrane Database Syst Rev.
12:CD0074062012.PubMed/NCBI
|
|
3
|
Gadducci A, Sartori E, Maggino T, Zola P,
Cosio S, Zizioli V, Lapresa M, Piovano E and Landoni F:
Pathological response on surgical samples is an independent
prognostic variable for patients with Stage Ib2-IIb cervical cancer
treated with neoadjuvant chemotherapy and radical hysterectomy: An
Italian multicenter retrospective study (CTF Study). Gynecol Oncol.
131:640–644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nemunaitis J, Clayman G, Agarwala SS,
Hrushesky W, Wells JR, Moore C, Hamm J, Yoo G, Baselga J, Murphy
BA, et al: Biomarkers predict p53 gene therapy efficacy in
recurrent squamous cell carcinoma of the head and neck. Clin Cancer
Res. 15:7719–7725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lehmann BD and Pietenpol JA: Targeting
mutant p53 in human tumors. J Clin Oncol. 30:3648–3650. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng XL, Wang TT, Fu M, Zhou J and Liu
FM: Inhibitory effect of recombinant human adenovirus-P53 combined
with paclitaxel on human cervical cancer HeLa cells and its
mechanism. Chin J Cancer Biother. 20:192–196. 2013.(In
Chinese).
|
|
7
|
Lane DP, Cheok CF and Lain S: p53-based
cancer therapy. Cold Spring Harb Perspect Biol. 2:a0012222010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burdak-Rothkamm S, Rothkamm K, McClelland
K, Al Rashid ST and Prise KM: BRCA1, FANCD2 and Chk1 are potential
molecular targets for the modulation of a radiation-induced DNA
damage response in bystander cells. Cancer Lett. 356(Pt B 2):
454–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang G, Xin Y, Zheng JN and Liu YQ:
Combining conditionally replicating adenovirus-mediated gene
therapy with chemotherapy: A novel antitumor approach. Int J
Cancer. 129:263–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prados J, Alvarez PJ, Melguizo C,
Rodriguez-Serrano F, Carrillo E, Boulaiz H, Vélez C, Marchal JA,
Caba O, Ortiz R, et al: How is gene transfection able to improve
current chemotherapy? The role of combined therapy in cancer
treatment. Curr Med Chem. 19:1870–1888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wirth T, Parker N and Ylä-Herttuala S:
History of gene therapy. Gene. 525:162–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tassone P, Old M, Teknos TN and Pan Q:
p53-based therapeutics for head and neck squamous cell carcinoma.
Oral Oncol. 49:733–737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li D, Zhang Y, Xie Y, Xiang J, Zhu Y and
Yang J: Enhanced tumor suppression by adenoviral PTEN gene therapy
combined with cisplatin chemotherapy in small-cell lung cancer.
Cancer Gene Ther. 20:251–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen GX, Zheng LH, Liu SY and He XH:
rAd-p53 enhances the sensitivity of human gastric cancer cells to
chemotherapy. World J Gastroenterol. 17:4289–4297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao Y, Guangqi E, Wang E, Pal K, Dutta SK,
Bar-Sagi D and Mukhopadhyay D: VEGF exerts an
angiogenesis-independent function in cancer cells to promote their
malignant progression. Cancer Res. 72:3912–3918. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tomao F, Papa A, Rossi L, Zaccarelli E,
Caruso D, Zoratto F, Panici P Benedetti and Tomao S: Angiogenesis
and antiangiogenic agents in cervical cancer. Onco Targets Ther.
7:2237–2248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Perrot-Applanat M and Di Benedetto M:
Autocrine functions of VEGF in breast tumor cells: Adhesion,
survival, migration and invasion. Cell Adhes Migr. 6:547–553. 2012.
View Article : Google Scholar
|
|
18
|
Holmgren L, Jackson G and Arbiser J: p53
induces angiogenesis-restricted dormancy in a mouse fibrosarcoma.
Oncogene. 17:819–824. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Xiao S, Li Y and Zhang S: Clinical
antiangiogenic effect of recombinant adenovirus-p53 combined with
hyperthermia for advanced cancer. Chin J Cancer Res. 25:749–755.
2013.PubMed/NCBI
|
|
20
|
Crinelli R, Bianchi M, Menotta M, Carloni
E, Giacomini E, Pennati M and Magnani M: Ubiquitin over-expression
promotes E6AP autodegradation and reactivation of the p53/MDM2
pathway in HeLa cells. Mol Cell Biochem. 318:129–145. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang X and Lu L: Expression of HPV-16 E6
protein and p53 inactivation increases the uterine cervical cancer
invasion. Drug Res (Stuttg). 65:70–73. 2015.PubMed/NCBI
|
|
22
|
Yu YF, Zhang Y, Shen N, Zhang RY and Lu
XQ: Effect of VEGF, P53 and telomerase on angiogenesis of gastric
carcinoma tissue. Asian Pac J Trop Med. 7:293–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding S, Li C, Lin S, Yang Y, Liu D, Han Y,
Zhang Y, Li L, Zhou L and Kumar S: Comparative evaluation of
microvessel density determined by CD34 or CD105 in benign and
malignant gastric lesions. Hum Pathol. 37:861–866. 2006. View Article : Google Scholar : PubMed/NCBI
|