Introduction
Multiple myeloma (MM) is a hematologic malignancy
that is characterized by an increased number of abnormal plasma
cells in the bone marrow. Whilst curing MM is difficult, the recent
development of novel agents including bortezomib and lenalidomide
has led to considerable improvements in the outcomes of patients
with MM (1–3). The main approved treatment strategy
prior to bortezomib was to use chemotherapeutic agents with or
without autologous stem cell transplantation (ASCT). Bortezomib
became available in 2006 in Japan, and patients with MM who had
relapsed or were refractory to chemotherapy were subsequently
treated with bortezomib-based regimens. Furthermore, as bortezomib
is available as a first-line treatment at present for incident
diagnoses of MM, the treatment approach for MM has changed and
bortezomib is used as an initial treatment.
It is important to be able to predict patient
outcomes in clinical practice. Although the presence of specific
cytogenetic abnormalities within myeloma cells, such as t(4;14) and
t(11;14), is considered the most important predictor of the
prognoses of patients who have been treated with chemotherapeutic
agents, the data from several previous studies have demonstrated
that bortezomib-based treatment regimens may be able to overcome
the adverse prognostic effects of these abnormalities (4–7). The
International Staging System (ISS), which is a widely used
prognostic indicator, may also have little effect on patient
outcome predictions in this era of novel therapeutic agents
(8–10). Thus, the prognostic factors in
patients with MM who are treated using bortezomib-based regimens
remain unclear.
Immunophenotyping is widely available for the
diagnosis of, the evaluation of minimal residual disease in and the
prediction of the prognoses of patients in terms of a variety of
hematologic malignancies, including MM. Myeloma cells commonly show
the cluster of differentiation
(CD)38brightCD19−CD56+
immunophenotype and, occasionally, the
CD38brightCD19−CD56−
immunophenotype, which may be clearly distinguished from the
immunophenotype shown by normal plasma cells,
CD38brightCD19+CD56− (11,12).
Furthermore, unlike normal plasma cells, myeloma cells often do not
express the mature plasma cell (MPC)-1 antigen or the leukocyte
common antigen (CD45) (13–15). The prognostic interpretation of this
distinct immunophenotypic pattern in MM has been studied
previously. CD56 negativity in myeloma cells is considered to be an
adverse prognostic factor (16–18), and
patients with MM whose cells express CD45 survive longer compared
with those patients whose cells are negative for CD45, particularly
patients who have been treated with ASCT (14,19). A
reduction in MPC-1 expression may be associated with chemotherapy
or thalidomide resistance (13).
However, all of the previous studies that have examined the effect
of immunophenotyping on prognosis involved patients who had been
treated with chemotherapy with or without ASCT. Therefore, there
are few data concerning the clinical significance of
immunophenotyping for patients with MM who have been treated with
novel agents such as bortezomib. Thus, a comprehensive analysis of
the significance of immunophenotyping for patients with MM who have
been treated with bortezomib is required. Therefore, the present
study analyzed data from patients with MM who had been treated with
bortezomib at Nihon University School of Medicine (Tokyo, Japan).
The present study describes the significance of immunophenotyping
for patients treated with bortezomib, and discusses the molecular
diversity that may underlie the pathogenesis of MM in the context
of immunophenotypic classification.
Patients and methods
Patients and treatment
A retrospective review of data from patients between
December 2006 and August 2015 was conducted. The present study
included symptomatic patients with MM who were treated with
bortezomib plus dexamethasone as a second-line therapy or
subsequently, and excluded those who received cytotoxic agents,
including melphalan or cyclophosphamide, in combination with
bortezomib. Baseline characteristics of patients at the time of
bortezomib initiation, including age, gender, immunoglobulin
subtype, ISS (8–10), pretreatments and immunophenotypes on
myeloma cells, were investigated for the assessment of prognostic
significance. Bortezomib was administered either intravenously or
subcutaneously at a dose of 1.3 mg/m2 on days 1, 4, 8
and 11, every 3 weeks, or on days 1, 8, 15 and 22, every 5 weeks,
in combination with oral dexamethasone that was administered at a
maximum dose (40 mg/day) on days 1–4, 9–12 and 17–20 or at a
reduced dose dependent on patient background, and it was
administered intravenously (between December 2006 and December
2012) or subcutaneously (between January 2013 and August 2015). The
study was approved by the research ethics board of Nihon University
Itabashi Hospital (Identifier: RK-151208-06, approved in January
2016), and the study was conducted in accordance with the
principles of the Declaration of Helsinki.
Flow cytometry
The immunophenotyping was performed primarily on
bone marrow samples that were collected at the time of diagnosis or
prior to bortezomib treatment at Bio Medical Laboratories, Inc.
(Tokyo, Japan). The myeloma cells were analyzed using standard
immunofluorescence methods and monoclonal antibodies. Briefly,
nuclear cells isolated from patient bone marrow samples were washed
with PBS and stained with fluorescein isothiocyanate-labeled mouse
anti-CD38 (1:20; cat. no. 555459; Becton-Dickinson, San Jose, CA,
USA), phycoerythrin (PE)-labeled mouse anti-CD56 (1:20; cat. no.
347747; Becton-Dickinson), PE-labeled mouse anti-MPC-1 (1:10; cat.
no. 03524781-4; Otsuka, Tokyo, Japan), PE-labeled mouse anti-CD49e
(1:80; cat. no. 555617; Becton-Dickinson),
peridinin-chlorophyll-protein complex-labeled mouse anti-CD45
(1:20; cat. no. 347764; Becton-Dickinson), or
allophycocyanin-labeled mouse anti-CD19 (1:40; cat. no. IM2470;
Beckman Coulter, Brea, CA, USA) for 30 min at 4°C in the dark.
Cells were then washed with PBS and analyzed by flow cytometry with
a minimum acquisition of 20,000 events. Double-gating analyses were
performed with CD38/side scatter, CD19*CD56, CD45*MPC-1 and
CD45*CD49e. Values were calculated using CELLQuest, version 3.3
(Becton-Dickinson) and analyzed using original software from BML.
Non-binding mouse isotype antibodies were used as controls. The
CD38bright/side scatterlow population
represented the plasma cell fraction, and the flow cytometric data
were analyzed when abnormal plasma cell fractions, for example,
CD38bright/CD19− cells, were detected. The
samples were considered positive when at least 20% of the myeloma
cells expressed this antigen profile, as described previously
(20–22).
Statistical analysis
Time to next treatment (TNT) was defined as the
period from the date that bortezomib treatment was initiated to the
date where subsequent therapy other than bortezomib plus
dexamethasone began or the patient succumbed to any cause. The
overall survival rate (OS) was defined as the period from the date
that bortezomib treatment was initiated to the date of death. The
Kaplan-Meier method was used to estimate the TNT and OS, and the
log-rank test was used to compare the groups in association with
the TNT and OS. The factors that may affect the clinical outcomes
were analyzed using multivariate Cox proportional hazard regression
models. P<0.05 was considered to indicate a statistically
significant difference. The statistical analyses were performed
using Easy R (Saitama Medical Center, Jichi Medical University),
which is a graphical user interface for the R programming language
(The R Foundation for Statistical Computing, Vienna, Austria;
http://www.R-project.org/) (23).
Results
Characteristics and treatment of
patients
Of the patients diagnosed with symptomatic MM, 56
had been treated with bortezomib plus dexamethasone between
December 2006 and August 2015. Of these 56 patients, 46 were
available for the immunophenotyping assessment. The characteristics
of these enrolled patients are presented in Table I. The median age at diagnosis was 65
years, range 36–83 years, and the present study included 27 men and
19 women. Following the ISS assessments, 16, 10 and 15 patients
were classified as being at stages I, II and III, respectively, at
the time bortezomib treatment was initiated.
 | Table I.Baseline characteristics of patients
at the time of bortezomib initiation. |
Table I.
Baseline characteristics of patients
at the time of bortezomib initiation.
| Characteristic | n=46 |
|---|
| Age, years, median
(range) | 65 (36–83) |
| Gender, n,
male/female | 27/19 |
| Immunoglobulin
subtype (n) |
|
| IgG | 30 |
| IgA | 6 |
| IgD | 1 |
| Light
chain only | 7 |
|
Non-secretory | 2 |
| International Staging
System, n |
|
| Stage
I | 16 |
| Stage
II | 10 |
| Stage
III | 15 |
|
Missing | 5 |
| Pretreatment, n |
|
| MP | 32 |
|
Multi-agent chemotherapy | 17 |
|
Thalidomide | 8 |
| High-dose
dexamethasone | 6 |
|
aPBSCT | 5 |
|
Lenalidomide | 3 |
| CP | 1 |
| Pretreatment line
number, n |
|
| 1 | 29 |
| 2 | 14 |
| ≥3 | 3 |
Immunophenotyping and prognosis
The flow cytometric data attained prior to
bortezomib treatment were available for 46 patients. Of the
patients evaluated, CD45, CD49e, CD56 and MPC-1 were positively
expressed in 16 (35%), 2 (4%), 33 (72%) and 37 (80%) patients,
respectively. Due to the low number of patients who possessed
myeloma cells that expressed CD49e, the present study focused on
the expression of CD45, CD56 and MPC-1 during the analysis of the
flow cytometric data.
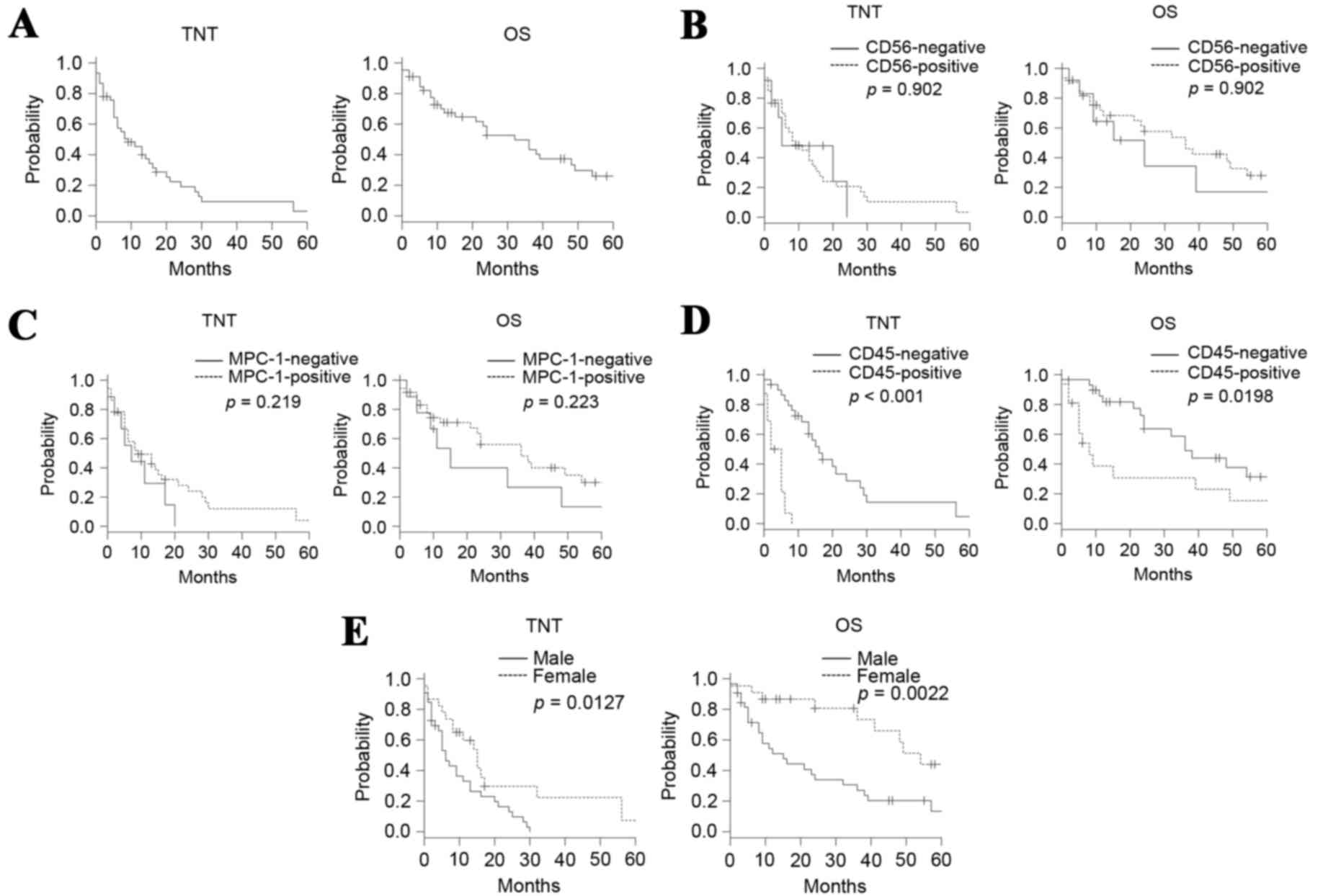
The median TNT and the median survival time (MST)
were 9 and 32 months, respectively, for the entire cohort (Fig. 1A). The TNT and OS did not differ
significantly between the patients who were positive and those who
were negative for CD56, with median TNTs of 9 and 5 months,
respectively, and MSTs of 36 and 24 months, respectively (Fig. 1B). The TNTs and OS did not differ
significantly between the patients who were positive and those who
were negative for MPC-1, with median TNTs of 9 and 7 months,
respectively, and MSTs of 36 and 15 months, respectively (Fig. 1C). Patients who were positive for CD45
expression exhibited shorter TNTs and OS compared with those who
were negative for CD45 expression, and the median TNTs were 3.5 and
16 months, respectively, and MSTs were 8 and 36 months,
respectively (Fig. 1D). Thus, among
the surface antigens evaluated, CD45 positivity was the only factor
that affected the outcomes of the patients.
Prognostic factors other than surface
antigen expression
Following the ISS assessments at the initiation of
bortezomib treatment, the median TNTs for the patients at stages I,
II or III were 11, 8 and 11 months, respectively (P=0.45), and the
OS for the patients at ISS stages I, II or III were 36, 49 and 24
months, respectively (P=0.084). Patient age, the pretreatment line
number and the immunoglobulin subtype did not affect the TNT or OS.
However, being male adversely affected the TNT and OS (Fig. 1E).
Univariate and multivariate
analyses
The univariate analysis revealed that compared with
the female patients, the TNT was significantly shorter for the male
patients and it also determined that CD45 positivity was associated
with a shorter TNT. The multivariate analysis revealed that CD45
positivity was an independent adverse prognostic factor for the TNT
(Table II) and OS (Table III). The multivariate analysis
demonstrated that being male was not an adverse prognostic factor
for TNT (Table II) and OS (Table III).
 | Table II.Analysis of the risk factors
associated with the time to next treatment in the study population
(n=46). |
Table II.
Analysis of the risk factors
associated with the time to next treatment in the study population
(n=46).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Male sex | 2.22
(1.08–4.08) | 0.030 | 1.89
(0.90–3.90) | 0.092 |
| CD45
positivity | 9.80
(3.71–25.71) | <0.0001 | 9.41
(3.44–25.44) | <0.0001 |
 | Table III.Analysis of the risk factors for
overall survival in the study population (n=46). |
Table III.
Analysis of the risk factors for
overall survival in the study population (n=46).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Male sex | 2.35
(1.02–5.02) | 0.044 | 2.15
(0.93–5.93) | 0.074 |
| CD45
positivity | 2.40
(1.12–5.12) | 0.025 | 2.19
(1.01–4.01) | 0.047 |
Discussion
The data from the present study demonstrate the
prognostic significance of the immunophenotype for patients with MM
who are treated with bortezomib, and they differ from the data of
previous studies of patients who underwent treatment prior to the
introduction of novel therapeutic agents (14–19).
Notably, the OS data from the present study may have been affected
by treatment that was administered subsequent to bortezomib such as
lenalidomide. Indeed, 25 patients in the present study received
lenalidomide and 7 patients received thalidomide as salvage therapy
subsequent to bortezomib administration. However, none of the
patients underwent ASCT.
Notably, data from the present study demonstrated
that the expression of CD45 by myeloma cells was a critical adverse
prognostic factor that affected the TNT and OS, which conflicts
with previously published data that suggested favorable outcomes
for patients with MM who were CD45 positive and were treated with
chemotherapy and ASCT, but were not administered bortezomib
(14,19). The levels of CD45 expression are
increased subsequent to bortezomib treatment (24), which suggests an association between
the CD45 molecule and bortezomib resistance. Therefore, the present
study hypothesized that the contradictory nature of these data with
respect to the findings of previous studies is important for
understanding the critical role of a treatment strategy that
involves combination therapy, for example, bortezomib-based
treatment followed by ASCT for patients with MM, and that it may
assist in explaining the different drug resistance mechanisms that
occur between bortezomib and chemotherapeutic agents. Differences
in the characteristics of myeloma cells in terms of the expression
of CD45 have been studied comprehensively. The CD45-positive
fraction of myeloma cells is a growth component that responds to
interleukin-6 (IL-6) (25–27), which is a critical factor that
regulates cell growth and survival through the autocrine or
paracrine systems of the myeloma cells. In addition, IL-6 serves a
major role in the activation of the Janus family tyrosine
kinase-signal transducer and activator of transcription (JAK-STAT)
signaling pathway, mainly via signal STAT3 and STAT 5 (28). Collectively, these data suggest that
the CD45-positive component of myeloma cells exhibits a high level
of activity that is associated with the JAK-STAT pathway, and that
the activation of the CD45 component may be associated with poor
responses to bortezomib. The findings from a recent study that used
phospho-flow cytometry demonstrated that CD45-positive myeloma
cells were associated with higher levels of STAT3 and/or STAT5
activity (29). Furthermore, patients
with MM who exhibited higher levels of STAT3 and STAT5
phosphorylation exhibited favorable outcomes when they were treated
with chemotherapy with or without ASCT (29). These results support the hypothesis
that the critical pathways for the pathogenesis and/or development
of MM differ according to the CD45 status of the patient.
Therefore, it may be suggested that the outcomes of patients
treated with bortezomib may depend on the underlying mechanisms
that promote myeloma development in each patient.
ASCT is considered to be a valid treatment option
for younger patients with MM. The data of the present study
demonstrated that patients with MM who were CD45 positive exhibited
poorer outcomes. When the different prognostic interpretations of
CD45 expression are considered in association with the treatment
strategy, ASCT may compensate for bortezomib treatment. Therefore,
the present study proposed that patients with MM who are
administered bortezomib-based treatment should undergo ASCT,
particularly when the myeloma cells are positive for CD45 and the
patient is able to tolerate ASCT.
CD56 expression is common in myeloma cells, but not
in normal plasma cells. A lack of CD56 expression has been
demonstrated to be associated with poorer outcomes when ASCT is not
undertaken, but the negative effect of an absence of CD56
expression is overcome when patients undergo ASCT (16–18,30).
Although the present study did not investigate the effect of ASCT
on OS, the expression of CD56 did not affect OS, which suggests
that the bortezomib-based regimen may overcome the prognostic
significance of CD56 negativity. Additionally, the maturity of the
myeloma cells, which was indicated by the expression of MPC-1, was
not a predictor of the TNT or OS in the present study. However, a
reduction in the expression of MPC-1 in some patients was
identified, including those who were administered bortezomib as
first-line therapy, who were refractory to or had relapsed
subsequent to bortezomib treatment, suggesting that MPC-1-negative
clones may be associated with bortezomib resistance. Additional
studies are required to clarify the association between MPC-1
expression and the treatment outcomes.
In the present study, cytogenetic analyses using
fluorescence in situ hybridization to detect specific
cytogenetic abnormalities were not performed in the majority of
cases, which is a limitation of the present study. Another
limitation is associated with possible selection bias, as not all
of the samples of the patients underwent flow cytometric analysis
at the time of diagnosis or prior to bortezomib treatment.
Furthermore, the treatment strategy was not uniform at Nihon
University School of Medicine, due to the comorbidities of the
patients and/or the discretion of the physicians.
In conclusion, patients with MM may be stratified
according to their immunophenotypic classifications to predict
outcomes, and this may assist to plan treatment. Consecutive
evaluations of surface antigen expression by myeloma cells may
serve a role in treatment optimization.
Acknowledgements
Dr Katisuhiro Miura and Dr Yohihiro Hatta received
lecture fees and honoraria from Celgene K.K. and Janssen
Pharmaceutical K.K. Professor Masami Takei received honoraria from
Janssen Pharmaceutical K.K.
References
|
1
|
Richardson PG, Barlogie B, Berenson J,
Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina
M, Alexanian R, et al: A phase 2 study of bortezomib in relapsed,
refractory myeloma. N Engl J Med. 348:2609–2617. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richardson PG, Sonneveld P, Schuster MW,
Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D,
Lonial S, Goldschmidt H, et al: Bortezomib or high-dose
dexamethasone for relapsed multiple myeloma. N Engl J Med.
352:2487–2498. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dimopoulos M, Spencer A, Attal M, Prince
HM, Harousseau JL, Dmoszynska A, Miguel San J, Hellmann A, Facon T,
Foà R, et al: Lenalidomide plus dexamethasone for relapsed or
refractory multiple myeloma. N Engl J Med. 357:2123–2132. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Avet-Loiseau H, Leleu X, Roussel M, Moreau
P, Guerin-Charbonnel C, Caillot D, Marit G, Benboubker L, Voillat
L, Mathiot C, et al: Bortezomib plus dexamethasone induction
improves outcome of patients with t(4;14) myeloma but not outcome
of patients with del(17p). J Clin Oncol. 28:4630–4634. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang H, Trieu Y, Qi X, Xu W, Stewart KA
and Reece D: Bortezomib therapy response is independent of
cytogenetic abnormalities in relapsed/refractory multiple myeloma.
Leuk Res. 31:779–782. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jagannath S, Richardson PG, Sonneveld P,
Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Cowan
JM and Anderson KC: Bortezomib appears to overcome the poor
prognosis conferred by chromosome 13 deletion in phase 2 and 3
trials. Leukemia. 21:151–157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sagaster V, Ludwig H, Kaufmann H, Odelga
V, Zojer N, Ackermann J, Küenburg E, Wieser R, Zielinski C and
Drach J: Bortezomib in relapsed multiple myeloma: Response rates
and duration of response are independent of a chromosome
13q-deletion. Leukemia. 21:164–168. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuroda J, Shimura Y, Ohta K, Tanaka H,
Shibayama H, Kosugi S, Fuchida S, Kobayashi M, Kaneko H and Uoshima
N: Limited value of the international staging system for predicting
long-term outcome of transplant-ineligible, newly diagnosed,
symptomatic multiple myeloma in the era of novel agents. Int J
Hematol. 99:441–449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan D, Kim K, Kim JS, Eom HS, Teoh G, Ong
KH, Goh YT, Durie BG, Chng WJ and Lee JH: The impact of upfront
versus sequential use of bortezomib among patients with newly
diagnosed multiple myeloma (MM): A joint analysis of the Singapore
MM Study Group and the Korean MM Working Party for the Asian
myeloma network. Leuk Res. 37:1070–1076. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maltezas D, Dimopoulos MA, Katodritou I,
Repousis P, Pouli A, Terpos E, Panayiotidis P, Delimpasi S,
Michalis E and Anargyrou K: Re-evaluation of prognostic markers
including staging, serum free light chains or their ratio and serum
lactate dehydrogenase in multiple myeloma patients receiving novel
agents. Hematol Oncol. 31:96–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harada H, Kawano MM, Huang N, Harada Y,
Iwato K, Tanabe O, Tanaka H, Sakai A, Asaoku H and Kuramoto A:
Phenotypic difference of normal plasma cells from mature myeloma
cells. Blood. 81:2658–2663. 1993.PubMed/NCBI
|
|
12
|
Kawano MM, Mihara K, Tsujimoto T, Huang N
and Kuramoto A: A new phenotypic classification of bone marrow
plasmacytosis. Int J Hematol. 61:179–188. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuroda Y, Sakai A, Okikawa Y, Munemasa S,
Katayama Y, Hyodo H, Imagawa J, Takimoto Y, Okita H, Ohtaki M and
Kimura A: The maturation of myeloma cells correlates with
sensitivity to chemotherapeutic agents. Int J Hematol. 81:335–341.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar S, Rajkumar SV, Kimlinger T, Greipp
PR and Witzig TE: CD45 expression by bone marrow plasma cells in
multiple myeloma: Clinical and biological correlations. Leukemia.
19:1466–1470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Otsuyama K, Asaoku H and Kawano MM: An
increase in MPC-1− and MPC-1-CD45+ immature
myeloma cells in the progressive states of bone marrow
plasmacytosis: The revised phenotypic classification of monoclonal
marrow plasmacytosis (MOMP-2005). Int J Hematol. 83:39–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sahara N, Takeshita A, Shigeno K, Fujisawa
S, Takeshita K, Naito K, Ihara M, Ono T, Tamashima S, Nara K, et
al: Clinicopathological and prognostic characteristics of
CD56-negative multiple myeloma. Br J Haematol. 117:882–885. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sahara N and Takeshita A: Prognostic
significance of surface markers expressed in multiple myeloma: CD56
and other antigens. Leuk Lymphoma. 45:61–65. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang H, Samiee S and Yi QL: Prognostic
relevance of CD56 expression in multiple myeloma: A study including
107 cases treated with high-dose melphalan-based chemotherapy and
autologous stem cell transplant. Leuk Lymphoma. 47:43–47. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moreau P, Robillard N, Avet-Loiseau H,
Pineau D, Morineau N, Milpied N, Harousseau JL and Bataille R:
Patients with CD45 negative multiple myeloma receiving high-dose
therapy have a shorter survival than those with CD45 positive
multiple myeloma. Haematologica. 89:547–551. 2004.PubMed/NCBI
|
|
20
|
Bene MC, Castoldi G, Knapp W, Ludwig WD,
Matutes E, Orfao A and van't Veer MB: Proposals for the
immunological classification of acute leukemias. European group for
the immunological characterization of leukemias (EGIL). Leukemia.
9:1783–1786. 1995.PubMed/NCBI
|
|
21
|
Iriyama N, Asou N, Miyazaki Y, Yamaguchi
S, Sato S, Sakura T, Maeda T, Handa H, Takahashi M, Ohtake S, et
al: Normal karyotype acute myeloid leukemia with the
CD7+ CD15+ CD34+ HLA-DR
+ immunophenotype is a clinically distinct entity with a
favorable outcome. Ann Hematol. 93:957–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iriyama N, Hatta Y, Takeuchi J, Ogawa Y,
Ohtake S, Sakura T, Mitani K, Ishida F, Takahashi M, Maeda T, et
al: CD56 expression is an independent prognostic factor for relapse
in acute myeloid leukemia with t(8;21). Leuk Res. 37:1021–1026.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tagoug I, Plesa A and Dumontet C:
Bortezomib influences the expression of malignant plasma cells
membrane antigens. Eur J Pharmacol. 706:11–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mahmoud MS, Ishikawa H, Fujii R and Kawano
MM: Induction of CD45 expression and proliferation in U-266 myeloma
cell line by interleukin-6. Blood. 92:3887–3897. 1998.PubMed/NCBI
|
|
26
|
Fujii R, Ishikawa H, Mahmoud MS, Asaoku H
and Kawano MM: MPC-1−CD49e− immature myeloma
cells include CD45+ subpopulations that can proliferate
in response to IL-6 in human myelomas. Br J Haematol. 105:131–140.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ishikawa H, Tsuyama N, Abroun S, Liu S, Li
FJ, Taniguchi O and Kawano MM: Requirements of src family kinase
activity associated with CD45 for myeloma cell proliferation by
interleukin-6. Blood. 99:2172–2181. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leonard WJ: Role of Jak kinases and STATs
in cytokine signal transduction. Int J Hematol. 73:271–277. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brown R, Yang S, Weatherburn C, Gibson J,
Ho PJ, Suen H, Hart D and Joshua D: Phospho-flow detection of
constitutive and cytokine-induced pSTAT3/5, pAKT and pERK
expression highlights novel prognostic biomarkers for patients with
multiple myeloma. Leukemia. 29:483–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hundemer M, Klein U, Hose D, Raab MS,
Cremer FW, Jauch A, Benner A, Heiss C, Moos M, Ho AD and
Goldschmidt H: Lack of CD56 expression on myeloma cells is not a
marker for poor prognosis in patients treated by high-dose
chemotherapy and is associated with translocation t(11;14). Bone
Marrow Transplant. 40:1033–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|