Introduction
Histone methyltransferase and histone demethylase
participate in and maintain different histone methylation states
(1). Post-translational histone
modifications are involved in various cellular processes through
regulation of the chromatin structure and also participate in
recognition of histone methylation through various molecules,
thereby inducing downstream processes (2). Histone methylation serves a critical
role in numerous biological processes such as heterochromatin
formation and transcriptional regulation (3,4). Abnormal
histone methylation is closely associated with tumor development
and progression and is known to serve a role in oncogene activation
and tumor suppressor inactivation (5–7). Abnormal
histone methylation in tumors often manifests as H3K4 demethylation
and H3K27 methylation of tumor suppressor genes, which may lead to
the inactivation of these suppressor genes (8). Additionally, H3K27 demethylation and
H3K4 methylation of proto-oncogenes can lead to oncogene
activation, which ultimately promotes tumor development and
progression (9).
Histone lysine demethylase 3A (KDM3A), which
contains a Jumonji C-terminal domain (JMJC) -containing enzyme and
belongs to the JMJ domain-containing proteins (JMJD) protein
family, can specifically catalyze histone H3K9me1/2 demethylation
(10). KDM3A has a variety of
biological functions and participates in nuclear receptor
activation, energy metabolism, spermatogenesis, muscle cell
development, hypoxia-induced stress response regulation and stem
cell self-renewal (11).
Additionally, KDM3A serves an important role in tumor development
and progression. It has previously been reported that KDM3A
promotes tumor growth and invasion as well as inducing angiogenesis
in hypoxia (12,13). However, it remains unclear whether
KDM3A performs a role in tumor immune escape, an important factor
in tumor development and progression.
It has been reported that Toll-like receptor 4
(TLR4) activation induces histone methylation changes at multiple
sites (14). TLR4, as a member of the
TLR family, is mainly expressed in immune cells and performs a role
in innate and acquired immunity (15). Previous studies have reported that
TLR4 is also expressed in a variety of tumor cell lines (16–20). In
the tumor microenvironment, the TLR4 signaling pathway can be
activated to upregulate forkhead box P3 (Foxp3) expression in
regulatory T cells (Tregs) and thereby enhance the
immunosuppressive function of Tregs (21). In addition, inflammatory cytokines
release is increased to promote tumor immune escape (22). Foxp3 is a member of the
forkhead/winged helix transcription factor family and is primarily
expressed in cluster of differentiation
(CD)4+CD25+ Tregs as a critical factor of
cell development and function (23).
Tregs mainly perform immunosuppressive functions through the
following three pathways: Cell-cell contact inhibition; metabolic
disruption; and secretion of the inhibitory cytokines interleukin
(IL)-35, IL-10, and transforming growth factor-β (TGF-β) (24). It has previously been revealed that
Foxp3 is expressed not only in Tregs, but also in various tumor
tissues and cell lines, including pancreatic, breast, prostatic and
colon carcinoma (25,26). Additionally, Foxp3 is closely
associated with the development, progression and prognosis of
certain types of cancer (27).
However, the role of Foxp3 differs in various tumor subtypes. To
date, few studies have investigated the role of Foxp3 in lung
cancer, and the molecular mechanisms involved in the regulation of
Foxp3 expression in lung cancer cells have not been elucidated.
Previous studies have demonstrated that the TLR4
expression level is positively correlated with Foxp3 expression
level in human non-small cell lung tumor tissues (28,29).
Additionally, the TLR4 signaling pathway activation can induce
Foxp3 expression in the human lung adenocarcinoma A549 cell line
(29). Nonetheless, it remains
unknown whether histone methylation changes during TLR4 activation
or whether specific molecular mechanisms of TLR4 participate in the
regulation of Foxp3 expression in lung cancer cells. The present
study demonstrated that TLR4 activation in A549 cells promoted the
expression of H3K9me1/2 demethylase KDM3A. KDM3A directly activates
Foxp3 transcription through the demethylation of histone H3K9 by
binding to its promoter region. This process results in increased
secretion of downstream inhibitory cytokines and thereby
facilitates the immune escape of lung cancer cells. This is a novel
mechanism of tumor immune escape based on the deregulation of the
JMJC-domain containing histone demethylase.
Materials and methods
Cell culture
A549 cells were obtained from the Laboratory of
Immunology of Jilin University (Changchun, China) maintained in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and cultured at 37°C in 5% CO2.
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR)
Total RNA extraction from A549 cells was performed
using TRIzol® reagent (Takara Biotechnology Co., Ltd.,
Dalian, China). Reverse transcription was performed using the
Moloney murine leukemia virus and the oligo deoxy-thymidine primer
(Takara Biotechnology Co., Ltd.). The quantity of total RNA was
then determined using an Epoch multi-volume spectrophotometer
system (BioTek Corporation, Beijing, China). cDNA was synthesized
from 1.0 mg total RNA using reverse transcriptase (Takara
Biotechnology Co., Ltd.), Moloney murine leukemia virus (Takara
Biotechnology Co., Ltd.) and oligonucleotides (dT; Takara
Biotechnology Co., Ltd.) in a total volume of 20 ml, according to
the manufacturer's protocols. RT-qPCR was performed using an ABI
PRISM 7300 sequence detection system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with SYBR Premix Ex Taq™ II (Takara
Biotechnology Co., Ltd), following the manufacturer's protocol and
using the following conditions: 95°C for 30 sec, 95°C for 5 sec and
60°C for 30 sec, for 40 cycles. The results were analyzed according
to the 2−∆∆Cq formula (30). The following primers (Takara
Biotechnology Co., Ltd.) were used: Sense,
5′-CTCGAACACCTTTGACAGCTCG-3′ and antisense,
5′-GGGTCATTGATGGCAACAATATC for GAPDH; sense,
5′-TCCCAGAGTTCCTCCACAAC-3′ and antisense,
5′-AGGTTGTGGCGGATGGCGTTCTTC-3′ for Foxp3; sense,
5′-CCAGGCAGAGAATGCTGAGTTC-3′ and antisense,
5′-CCAGGCAGAGAATGCTGAGTTC-3′ for heme oxygenase 1 (HO-1); sense,
5′-TACCTGAACCCGTGTTGCTCTC-3′ and antisense,
5′-GTTGCTGAGGTATCGCCAGGAA-3′ TGFβ1; sense,
5′-TCTCCGAGATGCCTTCAGCAGA-3′ and antisense,
5′-TCAGACAAGGCTTGGCAACCCA-3′ IL-10; sense,
5′-TGCCTTCACCACTCCCAAAACC-3′ and antisense,
5′-CAATCTCTTCAGAAGTGCAAGGG-3′ for IL-12A; sense,
5′-CTGGATCCGTTACAAGCGTCAG-3′ and antisense,
5′-CACTTGGACGTAGTACCTGGCT-3′ for Epstein-Barr virus induced 3
(EBI3; IL-35 is encoded by two separate genes, IL-12α and EBI3);
sense, 5′-GCCAACATTGGAGACCACTTCTG-3′ and antisense,
5′-CTCGAACACCTTTGACAGCTCG-3′ for KDM3A; sense,
5′-ACTGCTGACCATTGCTGAACGC-3′ and antisense,
5′-CCTCCTTGAGAGCCTGGATGTT-3′ for KDM3C; sense,
5′-GTGCTTTGTGGTCAGCGGAAGT-3′ and antisense,
5′-TGTGAGACAGCAACCCACGGTG-3′ for KMT2A; sense,
5′-TGTGAGACAGCAACCCACGGTG-3′ and antisense,
5′-TGCCGAATCAGCAGCTCTCGTA-3′ for KMT2D; sense,
5′-CACAGATTGTCAGTGATGCTGAAG-3′ and antisense,
5′-CTGCTGTCCAATGTGAGTCCTAC-3′ for KMT2E; sense
5′-CACCACATAGTCAGTGCTTCCTG-3′ and antisense
5′-AGTCTGACAGCGAGAGTTAGCC-3′ for enhancer of zeste 1 polycomb
repressive complex 2 subunit (EZH1); sense,
5′-GACCTCTGTCTTACTTGTGGAGC-3′ and antisense,
5′-CGTCAGATGGTGCCAGCAATAG-3′ for enhancer of zeste 2 polycomb
repressive complex 2 subunit (EZH2); sense,
5′-CCGATGACTCTTGTGAAGCAGC-3′ and antisense,
5′-GACTTCGTCTGCCAAAGTGGA-3′ for KDM4C. As aforementioned, IL-35 is
a dimeric protein composed of IL-12α and EBI3. In order to detect
the mRNA level of IL-35, IL-12α and EBI3 were required to be
detected at the same time.
Western blot analysis
Cells were lysed with radioimmunoprecipitation assay
lysis buffer, and the protein concentrations in the cell lysates
were measured using a BCA protein assay kit (Beyotime Institute of
Biotechnology, Jiangsu, China). Equal amounts of cell lysate
protein were separated by 10% SDS-PAGE, and transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with TBS and Tween 20 (TBST)
containing 5% nonfat milk for 2 h at room temperature, and then
incubated with anti-KDM3A (dilution, 1:1,000; cat. no. YM0388),
anti-Foxp3 (dilution, 1:1,000; cat no. YM0827) or β-actin mouse
monoclonal antibody (dilution, 1:4,000; cat. no. YM3039). All
antibodies were supplied by Immunoway, Jiangsu, China primary
antibodies for 2 h at room temperature. Following washing with TBST
three times at room temperature, they were then incubated with
horseradish peroxidase (HRP)-labeled goat anti-mouse secondary
antibodies (dilution, 1:4,000; cat. no., LK2003) and HPR-labeled
goat anti-rabbit secondary antibodies (dilution, 1:4,000; cat. no.,
K2001); Both were supplied by Immunoway for 45 min at room
temperature. Subsequently they were washed with TBST three times at
room temperature. Immunoreactive bands were detected using the
enhanced chemiluminescence detection kit (Thermo Fisher Scientific,
Inc.).
RNA interference
Small interfering RNAs (siRNAs) against KDM3A and
Foxp3 plasmids were designed and synthesized from RiboBio
(Guangzhou RiboBio Co., Ltd., Guangzhou, China). The A549 cells
were resuspended in serum-free DMEM, 3.5×105 cells were
seeded in 6-well plate and transfected with small interfering RNA
(siRNA)-Foxp3/siRNA-KDM3A or siRNA-negative control (NC); using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.),
then the medium was changed with DMEM containing 10% FBS 4–6 h
later. Cells were harvested at 48 h subsequent to transfection for
RT-qPCR or subsequent to 72 h for western blot analysis. A total of
3 different Foxp3/KDM3A-specific siRNAs were screened, and the most
efficient one was chosen for experiments. The specific siRNA for
Foxp3 was 5′-CAUGGACUACUUCAAGUUCdTdT-3′. The specific siRNA for
KDM3A was sense 5′-CCACCUAACCUUGGAGCAAdTdT-3′.
ELISA
A549 cells were seeded at 3.5×105 per
well in 6-well plates, incubated overnight at 37°C and transfected
with siRNA-Foxp3 as described above. After 36 h, the cells were
stimulated with lipopolysaccharide (LPS; Sigma-Aldrich; Merck KGaA;
Darmstadt, Germany) for 24 h. The concentrations of TGF-β1, IL-35
and HO-1 in the supernatants were measured with human TGF-β1, human
IL-35 and human HO-1 ELISA kits (All from Elabscience, Wuhan,
China).
Flow cytometry (FCM)
A549 cells were seeded at 3.5×105 per
well in 6-well plates, incubated overnight at 37°C and transfected
with siRNA-Foxp3 as described above. A549 cells were collected and
counted, and 1×106 cells were suspended in PBS (100 µl
total volume). The cells were fixed with 4% paraformaldehyde at 4°C
for 1 h, and then treated with 0.1% saponin (Sigma-Aldrich; Merck
KGaA) and phycoerythrin (PE) anti-mouse Foxp3 monoclonal antibody
(cat. no. M300F8-09A; 5 µl/test; Tianjin Sungene Biotech, Co.,
Ltd., Tianjin, China) at 4°C for 1 h. The cells were washed twice
with PBS and resuspended in 2% paraformaldehyde (Sigma-Aldrich;
Merck KGaA). A total of 105 events were assessed using
BD Accuri (BD Biosciences, Franklin Lakes, NJ, USA), and the data
were analyzed using the BD Accuri C6 Software version 6.0.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde and
permeabilized with 0.2% Triton X-100. Subsequent to blocking with
5% bovine serum albumin, the cells were incubated with rabbit
anti-KDM3A (1:1,000; cat. no. YM0388; Immunoway, Jiangsu, China)
antibody and mouse anti-Foxp3 antibody (1:500; cat. no. YM0827;
Immunoway, Jiangsu, China) overnight at 4°C. Subsequent to washing,
fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit
(dilution, 1:200; cat. no. GR200G-02C; Tianjin Sungene Biotech,
Co.) and PE-conjugated goat anti-mouse immunoglobulin G (dilution,
1:150; cat. no. GM200G-09C; Tianjin Sungene Biotech, Co.) were
added for 2 h at 37°C in the dark. The nuclei were stained with 1
µg/ml DAPI (Sigma-Aldrich; Merck KGaA) for 3 min. Following washing
with PBS 3 times, the cells were observed under an inverted
fluorescence microscope (IX71; Olympus Corporation, Tokyo,
Japan).
Dual luciferase reporter assay
A549 cells in 96-well plates were transfected with
pRL-TK and PGL3-basic or PGL3-Foxp3-promoter luciferase (all
supplied by Promega Corporation, Madison, WI, USA), using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturers protocol. The pRL-TK (Promega
Corporation) plasmid contains the Renilla reniformis luciferase
gene under the transcriptional control of constitutively expresses
low levels of Renilla luciferase. Transfected cells were lysed
using Passive Lysis Buffer (Promega Corporation), and then the
luciferase activities in the cell lysates were analyzed using a
Dual Luciferase Reporter assay kit (Promega Corporation).
Statistical analysis
Student's t-test for independent samples was used to
compare data between the experimental groups. All data were
analyzed by statistical software SPSS 17.0 (SPSS, Inc., Chicago,
IL, USA) and are presented as the mean ± standard error. P<0.05
was considered to indicate a statistically significant
difference.
Results
TLR4 activation promotes Foxp3
expression and inhibitory cytokines secretion in A549 cells
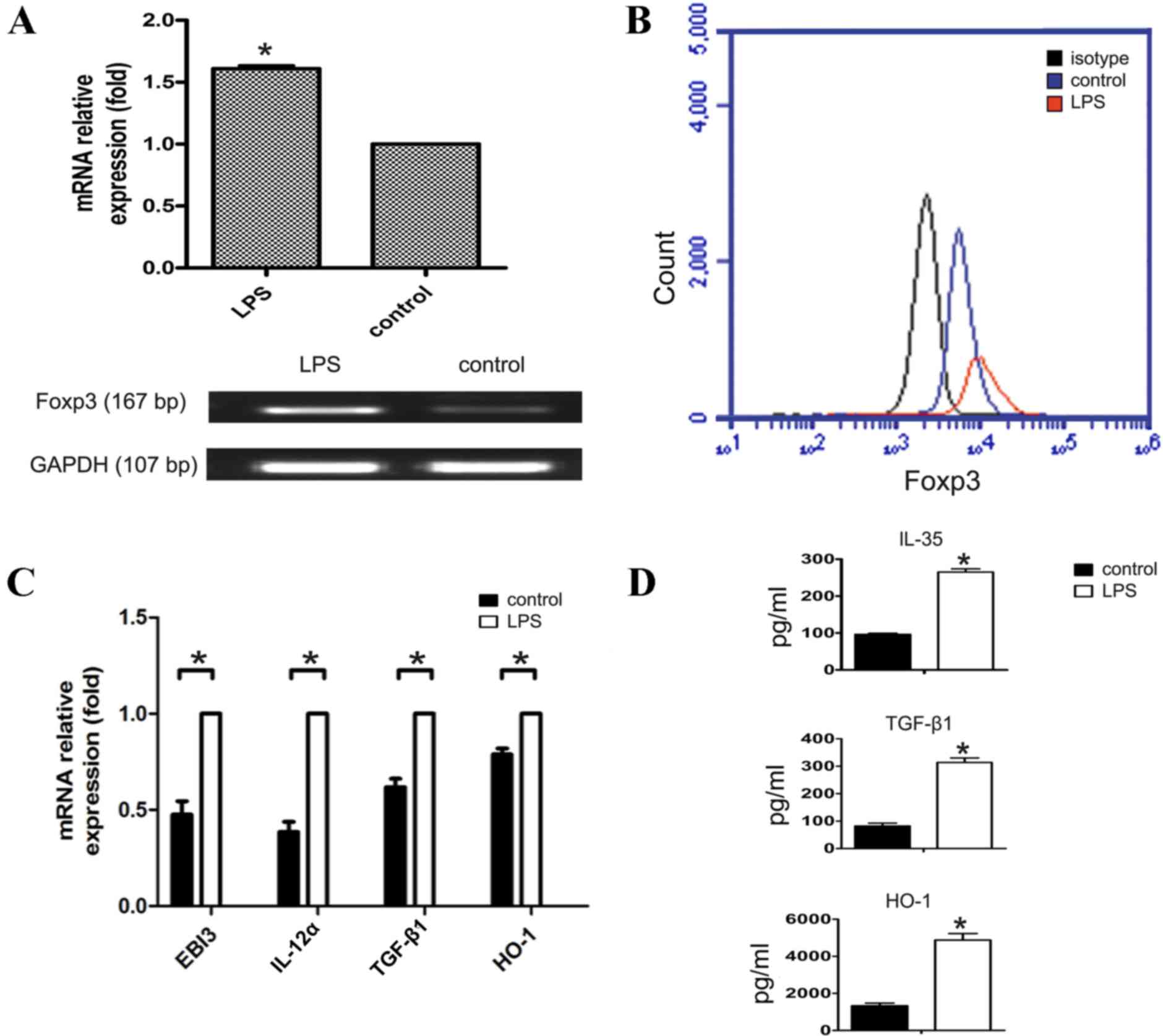
To observe the effect of TLR4 activation on Foxp3
expression and the relevant inhibitory cytokines secretion in A549
cells, the present study first determined Foxp3 expression changes
following LPS-induced activation of the TLR4 signaling pathway in
A549 cells (Fig. 1A and B).
Subsequently, the present study examined TLR4 activation-induced
expression changes of the inhibitory cytokines, TGF-β1, IL-35,
IL-10 and HO-1 that promote tumor immune escape. The mRNA level
changes were detected by RT-qPCR, and the protein expression level
changes were detected by ELISA. The results demonstrated that
except for IL-10 expression (not detected, data no shown), the
inhibitory cytokines were increased at the mRNA or protein level
following LPS activation of the TLR4 signaling pathway in A549
cells (P<0.05; Fig. 1C and D).
These results suggest that following TLR4 activation in A549 cells,
these cytokines are secreted to inhibit the functions of effector T
cells and dendritic cells (DCs) and facilitate immune escape of
lung cancer cells.
TLR4 activation regulates inhibitory
cytokines secretion through Foxp3 in A549 cells
To additionally determine whether TLR4 performs an
immunosuppressive function by promoting inhibitory cytokines
secretion during the regulation of Foxp3 expression in A549 cells,
the present study downregulated Foxp3 expression using siRNA
(Fig. 2A and B). TLR4 expression on
A549 was activated with LPS (5 µg/ml) 48 h subsequent to
transfection with siRNA-Foxp3 or control siRNA. The results
demonstrated that TGF-β1, IL-35, and HO-1 expression levels were
significantly reduced at both the mRNA and protein levels following
Foxp3 silencing in A549 cells (P<0.05; Fig. 2C and D). This observation suggests
that Foxp3 performs a role in tumor immune escape by secreting
inhibitory cytokines in A549 cells, and this process is regulated
by TLR4.
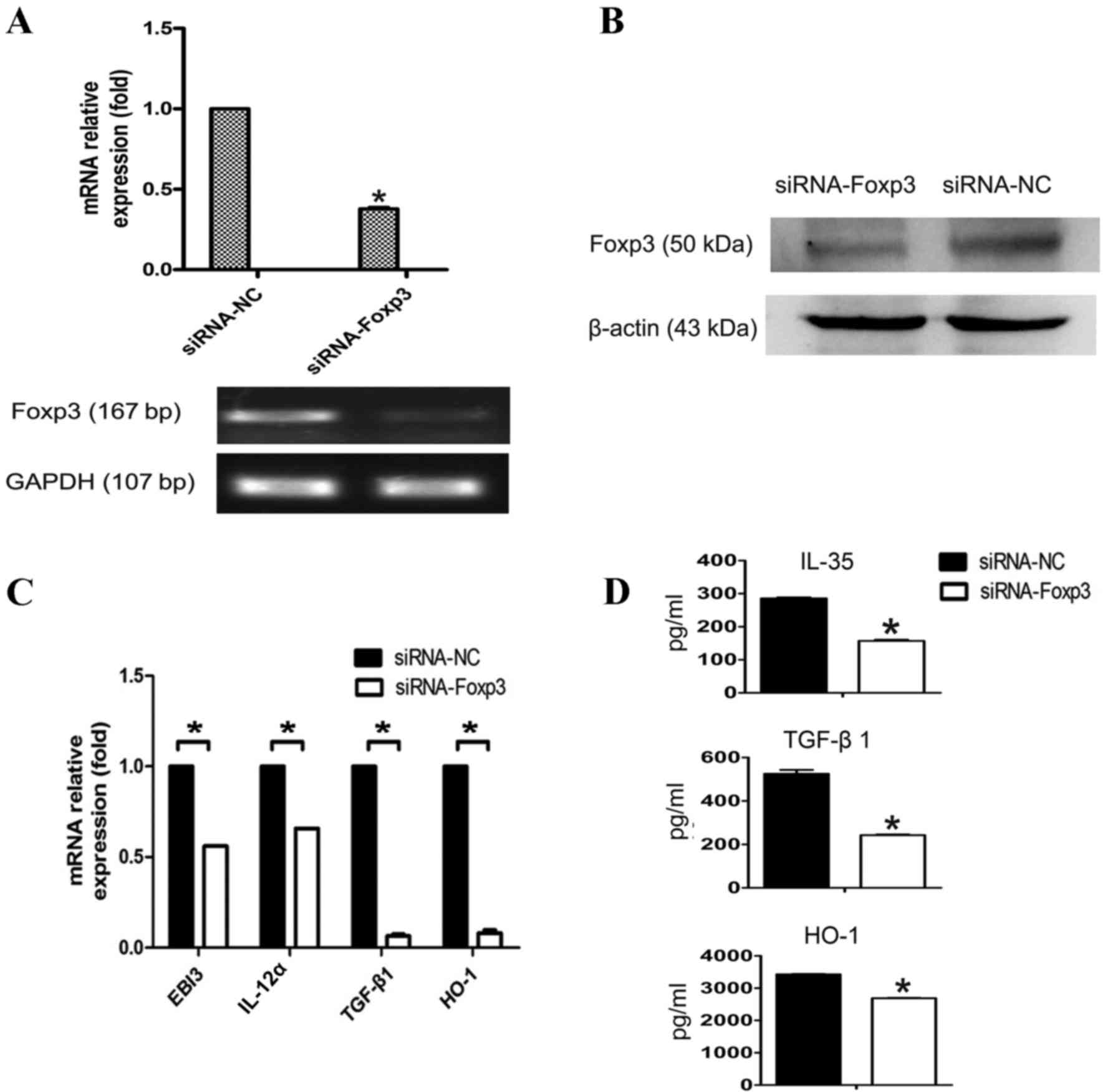 | Figure 2.Decreased inhibitory cytokines
production in Foxp3-silenced A549 cells in response to LPS
stimulation. (A) A549 cells were transfected with siRNA-NC or
siRNA-Foxp3 and mRNA expression of Foxp3 was detected by RT-qPCR.
(B) Protein amount of Foxp3 was detected using western blot
analysis. IL-35 is a dimeric protein composed of IL-12α and EBI3.
In order to detect the mRNA level of IL-35, IL-12α and EBI3 were
required to detected at the same time. (C) A549 cells were
harvested and transfected with siRNA-NC or siRNA-Foxp3, 48 h
subsequent to interference, A549 were stimulated with LPS (5 µg/ml)
for another 24 h. The mRNA expression of the inhibitory cytokines
TGF-β1, IL-35 and HO-1 were detected by RT-qPCR at the indicated
times. (D) TGF-β1, IL-35 and HO-1 in the supernatants were detected
using ELISA. Data in C and D are represented as the mean ± standard
deviation of 3 independent experiments. *P<0.05 compared with
the control group. Foxp3, forkhead box P3; LPS, lipopolysaccharide;
siRNA, small interfering RNA; NC, negative control; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction;
TGF-β1, transforming growth factor-β1; IL-35, interleukin 35; HO-1,
heme oxygenase 1. |
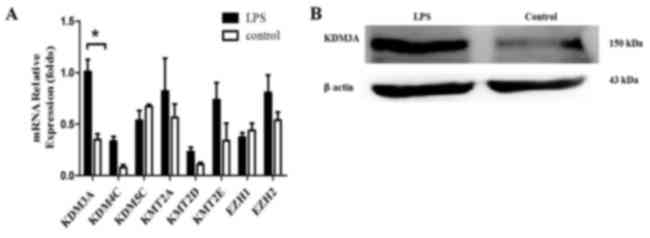
Expression of histone demethylase
KDM3A is increased following TLR4 activation
To observe the histone methylation changes of TLR4
during regulation of Foxp3 expression, the present study detected
the expression levels of associated histone methyltransferases and
demethylases (KDM3A, KDM5C, KMT2A, KMT2D, KMT2E, KDM4C, EZH1 and
EZH2) during TLR4 activation in A549 cells (14). The expression changes of these KMTs or
KDMs in A549 cells were analyzed by RT-qPCR following LPS
stimulation for 24 h. The results revealed that KDM3A was increased
significantly following TLR4 activation (P<0.05; Fig. 3). This finding suggests that the
H3K9me1/2 demethylase KDM3A is associated with TLR4 activation in
A549 cells. KDM3A likely participates in TLR4-mediated
transcriptional regulation of Foxp3 and thus performs a role in the
Foxp3-induced promotion of tumor immune escape.
KDM3A facilitates TLR4
activation-induced Foxp3 expression and inhibitory cytokines
secretion in A549 cells
To examine the role of KDM3A in the TLR4-mediated
regulation of Foxp3, the present study designed a specific siRNA
for the human KDM3A gene (siRNA-KDM3A) and transfected it into A549
cells. The silencing efficiency was determined by RT-qPCR and
western blot assays 48 h following transfection. The results showed
that the silencing effect of KDM3A was evident in A549 cells
transfected with siRNA-KDM3A (Fig. 4A and
B), which can be used to study cellular signaling in the
absence of KDM3A.
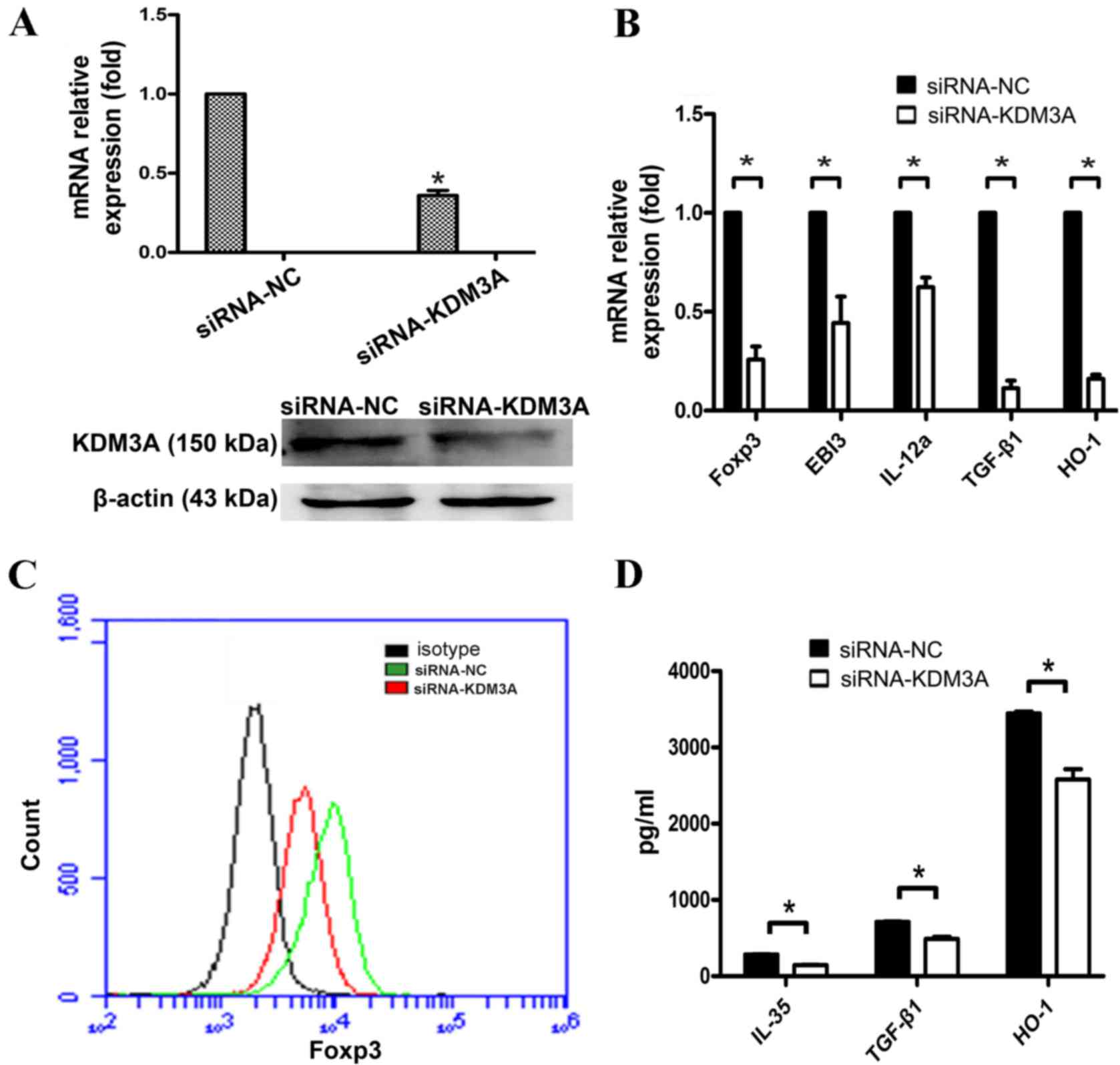 | Figure 4.Decreased inhibitory cytokines
production in KDM3A-silenced A549 cells in response to LPS
stimulation. (A) A549 cells were transfected with siRNA-NC or
siRNA-KDM3A. The mRNA expression of KDM3A was detected by RT-qPCR.
The protein amount of KDM3A was detected using western blot
analysis. (B) A549 cells were harvested and transfected with
control siRNA or siRNA-KDM3A. Subsequently, 48 h following
interference, A549 were stimulated with LPS (5 µg/ml) for another
24 h. The mRNA expression of Foxp3 and inhibitory cytokines TGF-β1,
EBI3, IL-12α and HO-1 were detected by RT-qPCR at the indicated
times. IL-35 is a dimeric protein composed of IL-12α and EBI3. In
order to detect the mRNA level of IL-35, IL-12α and EBI3 were
required to detected at the same time. (C) Protein level of Foxp3
was detected by flow cytometry. (D) TGF-β1, IL-35 and HO-1
expression levels in the supernatants were detected using ELISA.
Data in C and D are represented as the mean ± standard deviation of
3 independent experiments. *P<0.05 compared with the control
group. KDM3A, lysine demethylase 3A; siRNA, small interfering RNA;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; LPS, lipopolysaccharide; IL-12α, interleukin 12α; TGF-β1,
transforming growth factor-β1; HO-1, heme oxygenase 1. |
siRNA-KDM3A and siRNA-NC were
transfected into A549 cells
Subsequent to 24 h of LPS stimulation, the mRNA
level changes of Foxp3 and its downstream cytokines, TGF-β1, IL-35
and HO-1, were examined by RT-qPCR. The expression level changes of
associated proteins were assayed by FCM and ELISA. The results
showed that Foxp3 expression was significantly reduced in A549
cells following TLR4 activation when KDM3A was silenced. Meanwhile,
TGF-β1, HO-1 and IL-35 secretion was significantly reduced
(P<0.05; Fig. 4C and D). These
findings indicate that KDM3A performs a positive regulatory role
during TLR4 regulation of Foxp3 and inhibitory cytokines secretion
in A549 cells.
KDM3A regulates Foxp3 by activating
its promoter activity in A549 cells
To additionally study the role of KDM3A in the
TLR4-mediated regulation of Foxp3 transcription, A549 cells were
stained by rabbit anti-KDM3A and mouse anti-Foxp3 primary antibody,
and FITC-conjugated goat anti-rabbit and PE-conjugated goat
anti-mouse secondary antibody. Certain overlying yellow
fluorescence illustrated that KDM3A and Foxp3 were co-localized in
A549 cells (Fig. 5A). The present
study then generated a construct in which luciferase expression was
driven by regulatory regions of Foxp3 (Fig. 5B). The results demonstrated that KDM3A
silencing significantly suppressed luciferase activity driven by
the Foxp3 promoter (Fig. 5C). This
finding elucidates that KDM3A regulates Foxp3 transcription by
binding its promoter directly.
 | Figure 5.KDM3A facilitates Foxp3 expression in
A549 cells. (A) A549 cells were detected by immunofluorescence
staining with the rabbit anti-KDM3A and mouse anti-Foxp3 primary
antibodies and fluorescein isothiocyanate-conjugated goat
anti-rabbit (green) and phycoerythrin-conjugated goat anti-mouse
(red) secondary antibodies. The nuclei were stained with DAPI
(blue), and the cells were observed by inverted fluorescence
microscopy. The merged image indicates the overlap of KDM3A
(green), Foxp3 (red) and DAPI (blue), and the yellow in the merged
image indicates overlap of green and red labels. (B) The construct
containing luciferase expression was driven by promoters of Foxp3.
(C) A549 cells were co-transfected with siRNA-NC or siRNA-KDM3A
with luciferase reporter plasmids containing either PGL3-basic or
PGL3-Foxp3-promoter. Luciferase was measured. The fold changes of
relative luciferase activity in siRNA-KDM3A with indicated plasmids
transfected cells were normalized to siRNA-NC with corresponding
indicated plasmids transfected cells, respectively. Data are
represented as the mean ± standard deviation of 3 independent
experiments. *P<0.05 compared with the control group. KDM3A,
lysine demethylase 3A; Foxp3, forkhead box P3; siRNA, small
interfering RNA; NC, negative control; UTR, untranslated region;
siRNA, small interfering RNA; NC, negative control. |
Together, the findings of the present
study have demonstrated that TLR4 activation can promote the
expression of the H3K9me1/2 demethylase KDM3A in A549 cells
KDM3A binds directly to the Foxp3 promoter and
promotes Foxp3 transcription, thereby increasing the secretion of
Foxp3-related downstream inhibitory cytokines such as TGF-β1,
IL-35, and HO-1. These cytokines can suppress effector T cells and
DCs, ultimately facilitating the immune escape of lung cancer
cells.
Discussion
TLR4 is a member of the type I transmembrane
glycoprotein receptor family and serves as a pattern recognition
receptor. TLR4 was originally identified in immune cells, and it
performs a vital role in the primary and secondary immune
responses. LPS as an exogenous ligand of TLR4, once recognized and
specifically bound by TLR4, can activate the TLR4 signaling pathway
(31). High expression levels of TLR4
have recently been implicated in colorectal, stomach and ovarian
tumor tissues and cell lines (32–34). In
the tumor microenvironment, TLR4 activation promotes tumor immune
escape by releasing increased amounts of inflammatory cytokines and
is closely associated with the prognosis of tumors (35,36). Once
activated, the TLR4 pathway can also induce activation and enhance
the immunosuppressive function of Tregs (37). Foxp3 is the key driver of Tregs
differentiation and immunosuppressive function (38). One of the major immunosuppressive
pathways of Tregs is the secretion of inhibitory cytokines (e.g.
IL-35, IL-10, TGF-β1 and HO-1) and the suppression of the function
of effector T cells and DCs.
The present study demonstrated that TLR4 activation
in A549 cells can promote Foxp3 expression and IL-35, TGF-β1 and
HO-1 secretion (IL-10 expression was not detected; data not shown).
The secretion of these inhibitory cytokines was significantly
reduced subsequent to silencing Foxp3 expression. The results
indicate that, similar to its expression in Tregs, the Foxp3
expressed in lung adenocarcinoma cells serves a role in promoting
tumor immune escape, and this process is regulated by TLR4.
Additionally, histone methylation performs a critical role in the
activation of the TLR4 signaling pathway. As previously reported,
JMJD3 can participate in LPS-induced transcription of various genes
(39,40). The H3K4me3 level of transcriptional
genes induced by TLR4 activation is relatively high, and MLL
performs a key role in this process. EZH1 catalyzes the TLR4
signaling pathway to negatively regulate the H3K427 methylation
level at the transcription start site (TSS) of the Tollip gene.
This mechanism results in a lower transcription level of the Tollip
gene and thereby promotes TLR4 pathway activation and downstream
cytokines secretion (14).
Histone H3 methylation at sites K4, K9, K27, K36,
K79 and H4K20 changes the looseness of chromatin and the
accessibility of gene loci, and thus regulating gene transcription
activity. Demethylation of histone H3K9 is a sign of
transcriptional activation. Lysine demethylase 1A that contains
JMJC is capable of specifically removing methyl groups on the
lysine residue of histones. KDM3A, as the first member of the JMJD
family to be discovered, mainly demethylates mono- and
dimethyl-H3K9, resulting in H3K9 demethylation and transcriptional
activation (41). KDM3A regulates the
expression of various genes and extensively participates in
physiopathological processes by catalyzing histone H3K9
demethylation.
Furthermore, KDM3A serves an important role in tumor
development and progression. For example, KDM3A is expressed in
non-small cell lung and bladder cancer tissues and cell lines.
KDM3A can directly activate transcription of homeobox A1 (HOXA1)
through the demethylation of histone H3K9 by binding to its
promoter region. In addition, KDM3A regulates the expression of
HOXA1-dependent cyclin D1, activates G1/S phase
transformation of tumor cells and promotes tumor growth (18). KDM3A is also expressed in hepatoma
cell lines to promote cellular proliferation and invasion
capabilities. KDM3A facilitates the inter-epithelial-mesenchymal
transition of tumor cells, which is closely related to the
metastasis of the hepatoma (19). In
Ewing's sarcoma (EWS), overexpressed KDM3A inhibits the expression
of microRNA (miR) −122, an EWS/friend leukemia integration 1
transcription factor-repressed miR, and thereby promotes cancer
development and progression (42).
Furthermore, hypoxia-inducible factor 1α (HIF-1α) is an upstream
regulatory gene of KDM3A; when the tumor volume exceeds 1–2
mm3, the hypoxic microenvironment in the tumor center
stimulates HIF-1α activation, induces pro-angiogenesis factors
(e.g. vascular endothelial growth factor and platelet-derived
growth factor), and maintains continuous tumor growth (43). The KDM3A promoter region, HRE, binds
to HIF-1α to upregulate KDM3A expression and thereby changes the
methylation level of histone H3K9 in downstream target genes,
regulates the expression of hypoxia-related genes and promotes
tumor cell growth. The present study revealed that the expression
of the H3K9me1/2 demethylase KDM3A was significantly increased
following LPS-induced TLR activation in A549 cells Therefore, it
may be inferred that KDM3A is associated with TLR4 activation in
A549 cells and likely participates in TLR4 regulation
transcriptional of Foxp3. In subsequent assays, the present study
revealed that TLR4 activation-induced Foxp3 expression was reduced
by specific interference with KDM3A expression, which affected
TGF-β1, IL-35 and HO-1 secretion. The dual luciferase reporter
assay demonstrated that KDM3A directly binds to the Foxp3 promoter
to activate its transcription, leading to increased inhibitory
cytokines secretion under Foxp3 regulation. However, the
KDM3A-binding site of Foxp3 requires further confirmation.
Based on the aforementioned findings, the present
study has demonstrated that during TLR4 activation-induced nuclear
transport of Foxp3, the H3K9me1/2 demethylase KDM3A directly binds
to the Foxp3 promoter and thereby stimulates Foxp3 transcription
through the demethylation of histone H3K9 in lung carcinoma cells.
Additionally, KDM3A facilitates the secretion of TGF-β1, IL-35 and
HO-1 that is regulated by Foxp3. These cytokines suppress the
function of effector T cells and DCs and ultimately promote immune
escape of lung carcinoma cells. The findings of the present study
reveal an important role of epigenetic histone methylation in
regulating the immune escape of lung carcinoma cells. The present
data also suggest that KDM3A can provide new clues for the
treatment of lung cancer and serve as a useful therapeutic target
in patients with lung adenocarcinoma.
References
|
1
|
Alam H, Gu B and Lee MG: Histone
methylation modifiers in cellular signaling pathways. Cell Mol Life
Sci. 72:4577–4592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herzog M, Josseaux E, Dedeurwaerder S,
Calonne E, Volkmar M and Fuks F: The histone demethylase Kdm3a is
essential to progression through differentiation. Nucleic Acids
Research. 40:7219–7232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rea S, Eisenhaber F, OCarroll D, Strahl
BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD
and Jenuwein T: Regulation of chormatin structure by site-specific
histone H3 methyltransferase. Nature. 406:593–599. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gol IMG and Bestor TH: Histone
modification and replacement in chromatin activation. Genes Dev.
16:1739–1742. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suikki HE, Kujala PM, Tammela TL, van
Weerden WM, Vessella RL and Visakorpi T: Genetic alterations and
changes in expression of histone demethylases in prostate cancer.
Prostate. 70:889–898. 2010.PubMed/NCBI
|
|
6
|
Yang J, Ledaki I, Turley H, Gatter KC,
Montero JC, Li JL and Harris AL: Role of hypoxia-inducible factors
in epigenetic regulation via histone demethylases. Ann N Y Acad
Sci. 1177:185–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang GG, Allis CD and Chi P: Chromatin
remodeling and cancer, part I: Covalent histone modification.
Trends Mol Med. 13:363–372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiang Y, Zhu Z, Han G, Lin H, Xu L and
Chen CD: JMJD is a histone H3K27 demethylase. Cell Res. 17:850–857.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nichol N, Dupéré-Richer D, Ezponda T,
Licht JD and Miller WH Jr: H3K27 methylation: A focal point of
epigenetic deregulation in cancer. Adv Cancer Res. 131:59–95. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamane K, Toumazou C, Tsukada Y,
Erdjument-Bromage H, Tempst P, Wong J and Zhang Y: JHDM2A, a
Jmjc-containing H3K9 demethylase, facilitates transcription
activation by androgen receptor. Cell. 125:483–495. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Zhang Q, Ding Y, Li X, Zhao D, Zhao
K, Guo Z and Cao X: Histone lysine methyltransferase Ezh1 promotes
TLR-triggered inflammatory cytokines production by suppressing
tollip. J Immunol. 194:2838–2846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takeda K and Akira S: Toll-like receptors
in innate immunity. Int Immunol. 17:1–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li TT, Ogiono S and Qian ZR: Toll-like
receptor signaling in colorectal cancer: Carcinogenesis to cancer
therapy. Word J Gastroenterol. 20:17699–17708. 2014.
|
|
14
|
Wang L, Zhao Y, Qian J, Sun L, Lu Y, Li H,
Li Y, Yang J, Cai Z and Yi Q: Toll-like receptor-4 signaling in
mantle cell lymphoma: Effects on tumor growth and immune evasion.
Cancer. 119:782–791. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmausser B, Andrulis M, Endrich S,
Mȕller-Hermelink HK and Eck M: Toll-like receptors TLR4, TLR5 and
TLR9 on gastric carcinoma cells: An implication for interaction
with Helicobacter pylori. Int J Med Microbiol. 295:179–185. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou M, McFarland-Mancini MM, Funk HM,
Husseinzadeh N, Mounajjed T and Drew AF: Toll-like receptor
expression in normal ovary and ovarian tumors. Cancer Immunol
Immunother. 58:1375–2385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang EL, Qian ZR, Nakasono M, Tanahashi T,
Yoshimoto K, Bando Y, Kudo E, Shimada M and Sano T: High expression
of Toll-like receptor 4/myeloid differentiation factor 88 signals
correlates with poor prognosis in colorectal cancer. Br J Cancer.
102:908–915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Milkova L, Voelcker V, Forstreuter I, Sack
U, Andereqq U, Simon JC and Maier-Simon C: The NF-kappaB signaling
pathway is involved in the LPS/IL-2-induced upregulation of Foxp3
expression in human CD4+CD25high regulatory T cells. Exp Dermatol.
19:29–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li B, Samanta A, Song X, Furuuchi K,
Iacono KT, Kennedy S, Katsumata M, Saouaf SJ and Greene MI: FOXP3
ensembles in T-cell regulation. Immunol Rev. 212:99–131. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmitt EG and Williams CB: Generation and
function of induced regulatory T cells. Front Immunol. 4:1522013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karanikas V, Speletas M, Zamanakou M,
Kalala F, Loules G, Kerenidi T, Barda AK, Gourgoulianis KI and
Germenis AE: Foxp3 expression in human cancer cells. J Transl Med.
6:192008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He W, Liu Q, Wang L, Chen W, Li N and Cao
X: TLR4 signaling promotes immune escape of human lung cancer cells
by inducing immunosuppressive cytokines and apoptosis resistance.
Mol Immunol. 44:2850–2859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hinz S, Pagerols-Raluy L, Oberg HH,
Ammerpohl O, Grȕssel S, Sipos B, Grȕtzmann R, Pilarsky C,
Unqefroren H, Saeqer HD, et al: Foxp3 expression in pancreatic
carcinoma cells as a novel mechanism of immune evasion in cancer.
Cancer Res. 67:8344–8350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Merlo A, Casalini P, Carcangiu ML,
Malventano C, Triulzi T, Mènard S, Tagliabue E and Balsari A: FOXP3
expression and overall survival in breast cancer. J Clin Oncol.
27:1746–1752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Liu R, Li W, Chen C, Katoh H, Chen
GY, McNally B, Lin L, Zhou P, Zuo T, et al: Somatic single hits
inactivate the X-linked tumor suppressor FOXP3 in the prostate.
Cancer Cell. 16:336–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Triulzi T, Tagliabue E, Balsari A and
Casalini P: FOXP3 expression in tumor cells and implications for
cancer progression. J Cell Physiol. 228:30–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu HY, Li C, Yang W, Gai XD, Jia T, Lei YM
and Li Y: FOXP3 and TLR4 protein expression are correlated in
non-small cell lung cancer: Implications for tumor progression and
escape. Acta Histochem. 115:151–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia T, Fu H, Sun J, Zhang Y, Yang W and Li
Y: Foxp3 expression in A549 cells is regulated by Toll-like
receptor 4 through nuclear factor-κB. Mol Med Rep. 6:167–172.
2012.PubMed/NCBI
|
|
29
|
Κawai T and Akira S: The role of pattern
recognition receptors in innate immunity: Update on Toll like
receptors. Nat Immunol. 11:373–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim KH, Jo MS, Suh DS, Yoon MS, Shin DH,
Lee JH and Choi KU: Expression and signification of the TLR4/MyD88
signaling pathway in ovarian epithelial cancers. Word J Surg Oncol.
10:1932012. View Article : Google Scholar
|
|
32
|
Huang B, Zhao J, Li H, He KL, Chen Y, Chen
SH, Mayer L, Unkeless JC and Xiong H: Toll-like receptors on tumor
cells facilitate evasion of immune surveillance. Cancer Res.
65:5009–5014. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Zhao Y, Qian J, Sun L, Lu Y, Li H,
Li Y, Yanq J, Cai Z and Yi Q: Toll-like receptor-4 signaling in
mantle cell lymphoma: Effects on tumor growth and immune evasion.
Cancer. 119:782–791. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goto Y, Arigami T, Kitago M, Nquyen SL,
Narita N, Ferrone S, Morton DL, Irie RF and Hoon DS: Activation of
Toll-like receptors 2, 3 and 4 on human melanoma cells induces
inflammatory factors. Mol Cancer Ther. 7:3642–3653. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Laouar Y, Welte T, Fu XY and Flavell RA:
STAT3 is required for Flt3L-dependent dendritic cell
differentiation. Immunity. 19:903–912. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Welte T, Koch F, Schuler G, Lechner J,
Doppler W and Heufler C: Granulocyte-macrophage colony-stimulating
factor induces a unique set of STAT factors in murine dendritic
cells. Eur J Immunol. 27:2737–2740. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsukada Y, Fang J, Erdjument-Bromage H,
Warren ME, Borchers CH, Tempst P and Zhang Y: Histone demethylation
by a family of Jmjc domain-containing protein. Nature. 439:811–816.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Okada Y, Scott G, Ray MK, Mishina Y and
Zhang Y: Histone demethylase JHDM2A is critical for Tnp1 and Prm1
transcription and spermatogenesis. Nature. 450:119–123. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Z, Zhou S, Liao L, Chen X, Meisrrich M
and Xu J: Jmjd1a demethylase-regulated histone modification is
essential for cAMP-response element modulator-regulated gene
expression and spermatogenesis. J Biol Chem. 285:2758–2770. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Parrish JK, Sechler M, Winn RA and Jedicka
P: The histone demethylase KDM3A is a microRNA-22-regulated tumor
promoter in ewing sarcoma. Oncogene. 34:257–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Uemura M, Yamamoto H, Takemasa I, Mimori
K, Hemmi H, Mizushima T, Ikeda M, Sekimoto M, Matsuura N, Doki Y
and Mori M: Jumonji domain containing 1A is a novel prognostic
marker for colorectal cancer: In vivo identification from hypoxic
tumor cells. Clin Cancer Res. 16:4636–4646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mahajan K, Lawrence HR, Lawrence NJ and
Mahajan NP: ACK1 tyrosine kinase interacts with histone demethylase
KDM3A to regulate the mammary tumor oncogene HOXA1. J Biol Chem.
289:28179–28191. 2014. View Article : Google Scholar : PubMed/NCBI
|