Introduction
Choroidal melanoma is the most common intraocular
tumor in adults, and it metastasizes mainly to the liver (1). Poor prognosis is related to various
clinical factors such as tumor size (2). Furthermore, various molecular factors
are associated with poor prognosis (3,4).
Heat shock proteins (HSPs) function as molecular
chaperones and exert cytoprotective effects. Among the HSPs,
proteins from the HSP70 family play central roles as molecular
chaperones. Bcl-2-associated athanogene 3 (BAG3) belongs to a
family of co-chaperones that interacts with the ATPase domain of
HSP70 (5). Although BAG3 is expressed
weakly in normal cells, it is overexpressed in various malignant
tumors (6–14). In melanoma cells, BAG3 is upregulated,
and exerts cell survival and anti-apoptotic effects (15–17).
However, relationships between choroidal melanoma and BAG3 are
poorly studied. Therefore, we investigated the expression of BAG3
in human choroidal melanoma as compared to normal and ocular nevus
tumor tissues.
Case report
Patients and clinical materials
A 68-year-old woman was referred to Toyama
University Hospital for further evaluation of a left intraocular
mass. Funduscopy revealed a pigmented choroidal mass in the
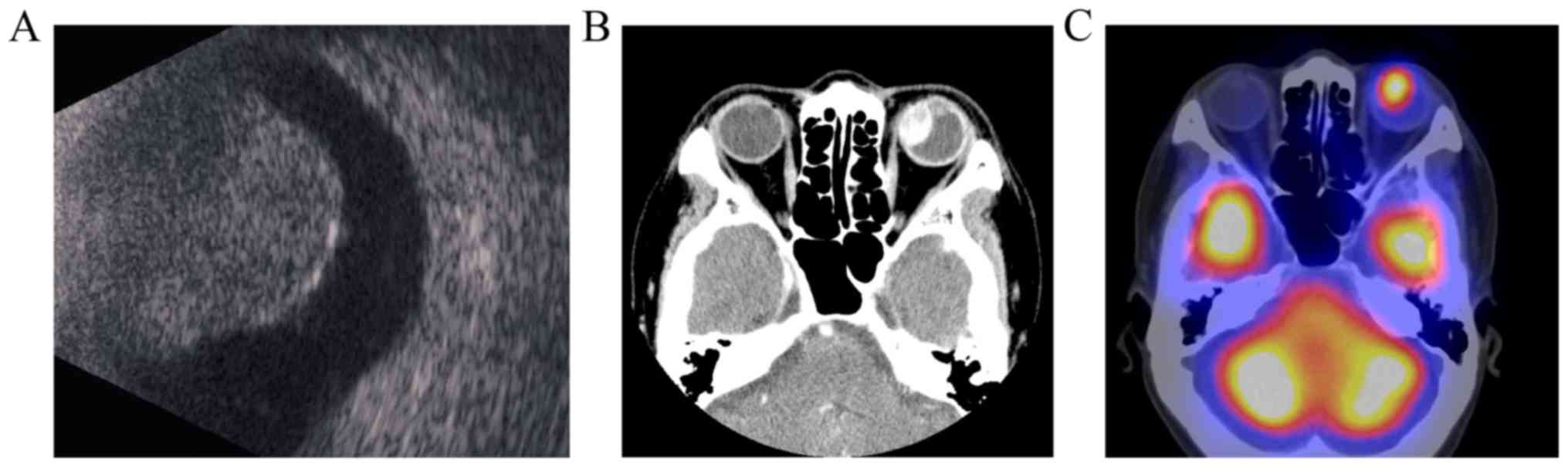
temporal fundus of her left eye. B-Mode ultrasonography revealed a
choroidal protrusion (Fig. 1A).
Computed tomography revealed an enhanced intraocular mass (Fig. 1B). Single-photon emission computed
tomography revealed a high accumulation of
N-isopropyl-p-[123I] iodoamphetamine after its
intravenous injection (Fig. 1C)
(18).
To definitively treat this strongly suspected case
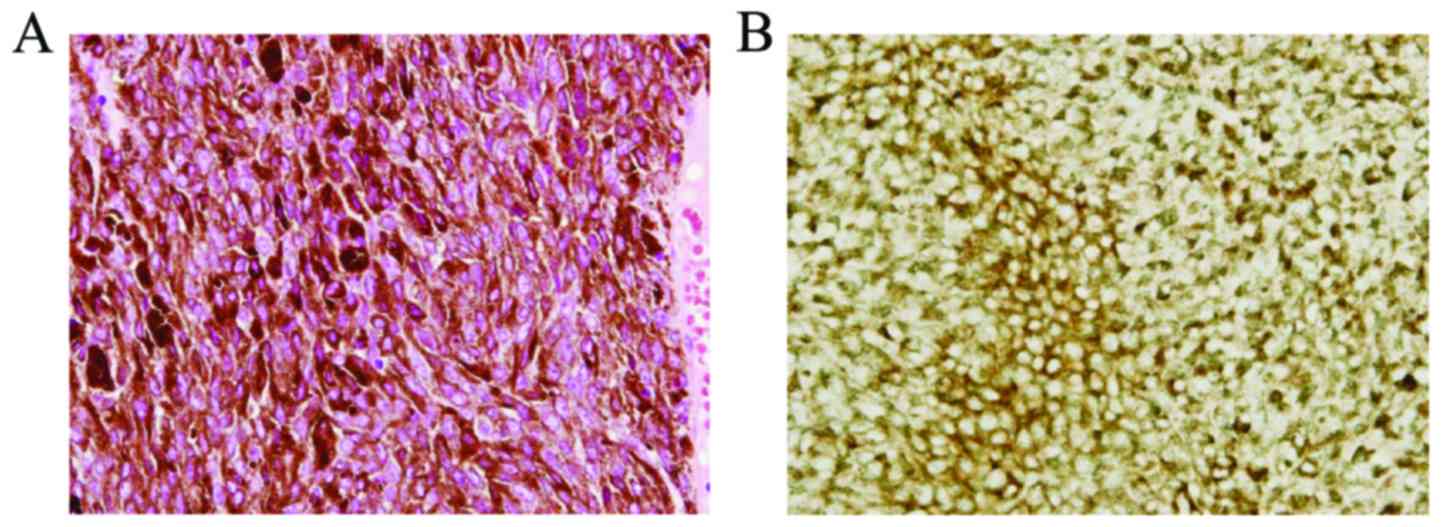
of choroidal melanoma, we enucleated the eye. Immunohistochemical
stains were positive for melan-A (Fig.
2), HMB-45, and S-100 (not shown) (19). Histopathology confirmed choroidal
melanoma without vascular or optic nerve invasion. Additionally, we
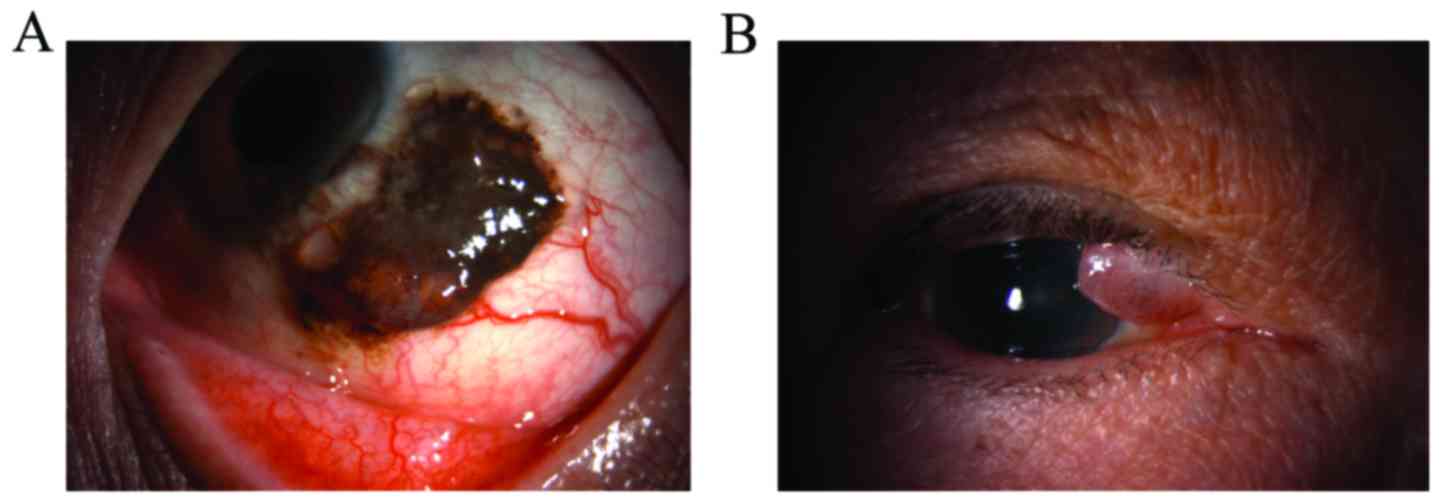
surgically resected a conjunctival tumor from a 44-year-old man
(Fig. 3A) and a lid tumor from a
74-year-old man (Fig. 3B); these
tumors were diagnosed as conjunctival nevus and lid nevus,
respectively.
Normal retinochoroidal and melanoma tissue samples
were obtained from the enucleated eye (Fig. 4), and nevus tissue samples were
obtained from the resected tumor tissues (Fig. 3A and B). Our procedures conformed to
the tenets of the World Medical Association's Declaration of
Helsinki. Written informed consent was obtained from the patients
after provision of sufficient information about the procedures.
Western blotting
Protein extracts were prepared by homogenizing
tissue samples in a lysis buffer (150 mM NaCl, 1% Nonidet P-40, and
50 mM Tris-HCl, pH 8.0) containing a protease inhibitor cocktail
(Nacalai Tesque, Kyoto, Japan). After electrophoresis on sodium
dodecyl sulfate-polyacrylamide gels, proteins were transferred
electrophoretically onto polyvinylidene fluoride membranes. The
following primary antibodies were used: rabbit monoclonal anti-BAG3
(GTX62327; GeneTex Inc., Irvine, CA, USA); mouse monoclonal
anti-HSP70 (SR-B810; MBL, Nagoya, Japan); rabbit monoclonal anti
anti-HSF1 (GTX62022; GeneTex Inc.) and mouse monoclonal
anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (MAB374;
Millipore, Temecula, CA, USA). The immunoreactive proteins were
visualized using a luminescence image analyzer (LAS 4000mini; GE
Healthcare, Tokyo, Japan) with an enhanced chemiluminescence
detection system. GAPDH served as the loading control.
RNA isolation
Using an RNeasy Total RNA Extraction kit (Qiagen
K.K., Tokyo, Japan), total RNA was extracted from tissue samples
and treated with on-column DNase I (RNase-free DNase kit, Qiagen
K.K.) (20).
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed on a Real-Time PCR Mx3005P system
(Agilent Technologies, Santa Clara, CA, USA) using a SYBR PreMix
ExTaq kit (Takara Bio, Inc., Shiga, Japan). The relevant primer
sequences are listed in Table I. mRNA
expression levels for each protein were normalized to the mRNA
expression level for GAPDH (20).
 | Table I.Nucleotide sequences of primers for
target genes. |
Table I.
Nucleotide sequences of primers for
target genes.
| Genes | Nucleotide sequence
(5′-3′) | GenBank accession
no. |
|---|
| Bcl-2-associated
athanogene 3 |
|
|
|
Sense |
CGACCAGGCTACATTCCCAT | NM_004281 |
|
Antisense |
TCTGGCTGAGTGGTTTCTGG |
|
| Glyceraldehyde
3-phosphate dehydrogenase |
|
|
|
Sense |
AAGGCTGGGGCTCATTTGCA | NM_002046 |
|
Antisense |
ATGACCTTGCCCACAGCCTT |
|
Statistical analysis
Measurements are reported as means ± standard
deviations. Student's t-test was used for statistical analysis, and
P<0.05 was considered statistically significant.
Results
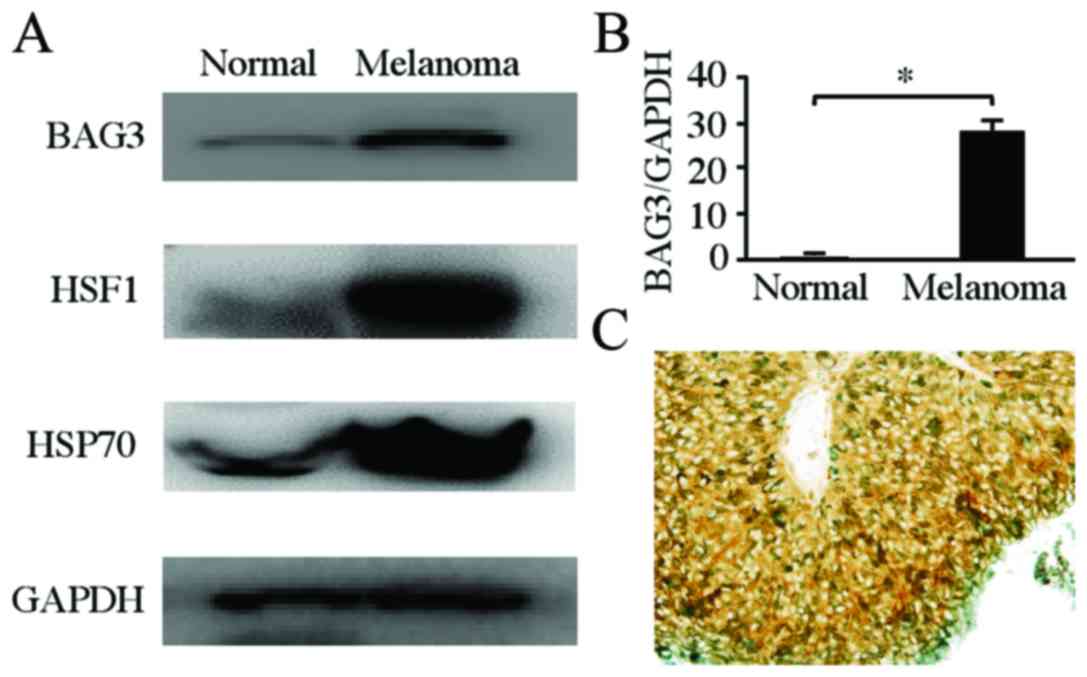
To analyze the involvement of BAG3 within a
choroidal melanoma, we examined its protein and mRNA expression
levels using western blotting and qPCR, respectively. The BAG3
protein level in the human choroidal melanoma tissue was
upregulated compared to that in normal retinochoroidal tissue
(Fig. 5A). Furthermore, as observed
using Western blotting, the expression levels of heat shock factor
1 (HSF1) and HSP70 were upregulated in human choroidal melanoma
relative to expression levels in normal retinochoroidal tissues
(Fig. 5A). Similarly, qPCR indicated
that the BAG3 mRNA level in the human choroidal melanoma tissue was
significantly higher than that in normal retinochoroidal tissue
(n=4, P=0.000291) (Fig. 5B).
Additionally, we confirmed BAG3 expression in choroidal melanoma
using immunohistochemical analysis (Fig.
5C).
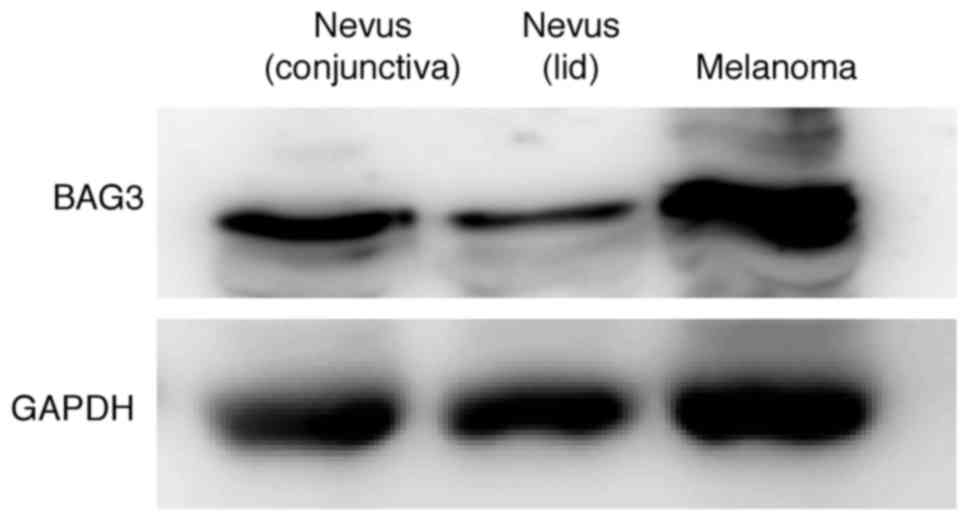
Western blots also indicated that the BAG3 level in
the human choroidal melanoma tissue was upregulated compared to
those in nevus tissue samples from other patients. Moreover, BAG3
levels in the conjunctival nevus were higher than those in the lid
nevus (Fig. 6). These findings
suggest that BAG3 was upregulated in the human choroidal melanoma
relative to normal retinochoroidal and nevus tissues.
Discussion
The mechanisms of choroidal melanoma progression and
metastasis remain poorly understood, and treatment options are
limited. Regardless of the progress of diagnostic technology,
choroidal melanoma causes death due to liver metastasis (21). Accordingly, the study of choroidal
melanoma-specific biomarkers is important for improving prognosis
accuracy.
It is thought that an association between the heat
shock response and melanoma is important. HSF1 is required for
melanoma invasion and metastasis (22). BAG3, a co-chaperone of HSP70, is
overexpressed in multiple malignant tumors and exerts
anti-apoptotic effects (15–17). Observations in vitro and in
vivo indicate that the induction of BAG3 is at least partly
mediated by the activation of HSF1 (23). In this study, BAG3 levels were
upregulated via HSF1 activation in human choroidal melanoma
relative to its expression levels in normal retinochoroidal
tissues. However, little is known about the anti-apoptotic role of
BAG3 in human choroidal melanoma. To our knowledge, we are the
first to report overexpression of BAG3 protein and mRNA in human
choroidal melanoma relative to expression levels in normal
retinochoroidal and ocular nevus tissues. Franco et al
reported that BAG3 levels in eye melanoma are relatively low, but
are related to metastasis at other sites (15). It is possible that BAG3-positive
choroidal melanoma is associated with a poor prognosis. We think
that careful follow-up of patients is necessary in BAG3-positive
choroidal melanoma.
Similar to its effects in other malignant tumors,
BAG3 may contribute to survival through anti-apoptotic activity in
choroidal melanoma. We believe that BAG3 may be a prognostic marker
and therapeutic target. Further investigation is necessary to
understand the relationships between choroidal melanoma and
BAG3.
In conclusion, BAG3 is overexpressed in human
choroidal melanoma relative to other related tissues. Our findings
suggest that BAG3 may offer a therapeutic target for patients with
choroidal melanoma.
Acknowledgements
This study was supported in part by a Grant-in-Aid
for Scientific Research (16K20309) from the Japan Society for the
Promotion of Science.
Glossary
Abbreviations
Abbreviations:
|
BAG3
|
Bcl-2-associated athanogene 3
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
HSF1
|
heat shock factor 1
|
|
HSP70
|
heat shock protein 70
|
|
HSPs
|
heat shock proteins
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
SD
|
standard deviation
|
References
|
1
|
Wöll E, Bedikian A and Legha SS: Uveal
melanoma: Natural history and treatment options for metastatic
disease. Melanoma Res. 9:575–581. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shields CL, Furuta M, Thangappan A, Nagori
S, Mashayekhi A, Lally DR, Kelly CC, Rudich DS, Nagori AV, Wakade
OA, et al: Metastasis of uveal melanoma millimeter-by-millimeter in
8033 consecutive eyes. Arch Ophthalmol. 127:989–998. 1999.
View Article : Google Scholar
|
|
3
|
Mooy CM and De Jong PT: Prognostic
parameters in uveal melanoma: A review. Surv Ophthalmol.
41:215–228. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scholes AG, Damato BE, Nunn J, Hiscott P,
Grierson I and Field JK: Monosomy 3 in uveal melanoma: Correlation
with clinical and histologic predictors of survival. Invest
Ophthalmol Vis Sci. 44:1008–1011. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takayama S, Xie Z and Reed JC: An
evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone
regulators. J Biol Chem. 274:781–786. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liao Q, Ozawa F, Friess H, Zimmermann A,
Takayama S, Reed JC, Kleeff J and Büchler MW: The anti-apoptotic
protein BAG-3 is overexpressed in pancreatic cancer and induced by
heat stress in pancreatic cancer cell lines. FEBS Lett.
503:151–157. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kassis JN, Virador VM, Guancial EA, Kimm
D, Ho AS, Mishra M, Chuang EY, Cook J, Gius D and Kohn EC: Genomic
and phenotypic analysis reveals a key role for CCN1 (CYR61) in
BAG3-modulated adhesion and invasion. J Pathol. 218:495–504. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiappetta G, Ammirante M, Basile A,
Rosati A, Festa M, Monaco M, Vuttariello E, Pasquinelli R, Arra C,
Zerilli M, et al: The antiapoptotic protein BAG3 is expressed in
thyroid carcinomas and modulates apoptosis mediated by tumor
necrosis factor-related apoptosis-inducing ligand. J Clin
Endocrinol Metab. 92:1159–1163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang HQ, Liu BQ, Gao YY, Meng X, Guan Y,
Zhang HY and Du ZX: Inhibition of the JNK signalling pathway
enhances proteasome inhibitor-induced apoptosis of kidney cancer
cells by suppression of BAG3 expression. Br J Pharmacol.
158:1405–1412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki M, Iwasaki M, Sugio A, Hishiya A,
Tanaka R, Endo T, Takayama S and Saito T: BAG3 (BCL2-associated
athanogene 3) interacts with MMP-2 to positively regulate invasion
by ovarian carcinoma cells. Cancer Lett. 303:65–71. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang JT, Wang JL, Du W, Hong J, Zhao SL,
Wang YC, Xiong H, Chen HM and Fang JY: MicroRNA 345, a
methylation-sensitive microRNA is involved in cell proliferation
and invasion in human colorectal cancer. Carcinogenesis.
32:1207–1215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Festa M, Del Valle L, Khalili K, Franco R,
Scognamiglio G, Graziano V, de Laurenzi V, Turco MC and Rosati A:
BAG3 protein is overexpressed in human glioblastoma and is a
potential target for therapy. Am J Pathol. 178:2504–2512. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu P, Xu B, Li J and Lu H: BAG3 gene
silencing sensitizes leukemic cells to Bortezomib-induced
apoptosis. FEBS Lett. 583:401–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Romano MF, Festa M, Pagliuca G, Lerose R,
Bisogni R, Chiurazzi F, Storti G, Volpe S, Venuta S, Turco MC and
Leone A: BAG3 protein controls B-chronic lymphocytic leukaemia cell
apoptosis. Cell Death Differ. 10:383–385. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franco R, Scognamiglio G, Salerno V,
Sebastiani A, Cennamo G, Ascierto PA, Botti G, Turco MC and Rosati
A: Expression of the anti-apoptotic protein BAG3 in human
melanomas. J Invest Dermatol. 132:252–254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Basile A, Zeppa R, Pasquino N, Arra C,
Ammirante M, Festa M, Barbieri A, Giudice A, Pascale M, Turco MC
and Rosati A: Exposure to 50 Hz electromagnetic field raises the
levels of the anti-apoptotic protein BAG3 in melanoma cells. J Cell
Physiol. 226:2901–2907. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ammirante M, Rosati A, Arra C, Basile A,
Falco A, Festa M, Pascale M, d'Avenia M, Marzullo L, Belisario MA,
et al: IKK{gamma} protein is a target of BAG3 regulatory activity
in human tumor growth. Proc Natl Acad Sci USA. 107:7497–7502. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goto H: Clinical efficacy of 123I–IMP
SPECT for the diagnosis of malignant uveal melanoma. Int J Clin
Oncol. 9:74–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levasseur SD, Paton KE, van Raamsdonk CD,
Heran MK and White VA: Mutation of GNAQ in a cytologically unusual
choroidal melanoma in an 18-month-old child. JAMA Ophthalmol.
131:810–812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tabuchi Y, Yunoki T, Hoshi N, Suzuki N and
Kondo T: Genes and gene networks involved in sodium
fluoride-elicited cell death accompanying endoplasmic reticulum
stress in oral epithelial cells. Int J Mol Sci. 15:8959–8978. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spanknebel K, Coit DG, Bieligk SC, Gonen
M, Rosai J and Klimstra DS: Characterization of micrometastatic
disease in melanoma sentinel lymph nodes by enhanced pathology:
Recommendations for standardizing pathologic analysis. Am J Surg
Pathol. 29:305–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamura Y, Fujimoto M, Fukushima S,
Nakamura A, Hayashida N, Takii R, Takaki E, Nakai A and Muto M:
Heat shock factor 1 is required for migration and invasion of human
melanoma in vitro and in vivo. Cancer Lett. 354:329–335. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Franceschelli S, Rosati A, Lerose R, de
Nicola S, Turco MC and Pascale M: Bag3 gene expression is regulated
by heat shock factor 1. J Cell Physiol. 215:575–577. 2008.
View Article : Google Scholar : PubMed/NCBI
|