Introduction
Epithelial ovarian cancer (EOC) remains the most
lethal type of gynecologic malignancy in postmenopausal women in
industrialized countries (1).
Comprising a heterogeneous group of malignant tumors, EOC presents
with distinct clinicopathological and biological characteristics.
The majority (~75%) of all incidences of ovarian cancer are
classified as high-grade serous epithelial ovarian cancer (HGSOC),
which is hypothesized to originate from serous tubal
intraepithelial lesions in the fallopian tubes rather than the
ovarian epithelium (2). Less
frequently observed are endometrial and clear cell carcinomas,
associated with endometrial cells, while mucinous carcinomas are
associated with gastrointestinal tract tissue. Molecular changes in
the genes encoding tumor protein p53, breast cancer type 1
susceptibility protein (BRCA1), BRCA2, GTPase Kras and
proto-oncogene B-Raf are known to be fundamental for the
development of different subtypes of EOC. The results of
epidemiological studies have also revealed that reproductive
factors, which determine the exposure of females to steroid
hormones, particularly estrogens, serve a role in the development
of all subtypes of EOC, although their contribution varies between
subtypes (3–5). While the application of hormonal
contraceptives and a higher number of pregnancies reduce the risk
for all ovarian cancer types, estrogen-only hormone replacement
therapy (HRT) was found to increase the risk for serous and
endometrioid EOCs. A higher overall lifetime exposure to estrogens
through an early menarche and late menopause was demonstrated to be
associated with an increased risk of non-serous ovarian cancers but
it may not increase the incidence of HGSOC (6–10).
When investigating the effect of estrogens on EOC
cells, it is of note that these tumors occur predominately in
postmenopausal women, at a time when the de novo synthesis
of the most active form of estrogen, 17β-estradiol (E2), in the
ovary has ceased. However, cells in the ovary and other
estrogen-sensitive tissues, such as the endometrium and breast,
remain exposed to E2 provided from circulating estrogen precursors,
particularly estrone sulfate (E1S), taken up from the circulation
via specific transport proteins, including members of the organic
anion transporting polypeptide family (Fig. 1) (11).
A study by Sasano et al (12)
demonstrated that in postmenopausal women, the local concentration
of estrogen in breast and endometrial carcinoma are of a similar
level compared with those in premenopausal women. This local
estrogen production has been demonstrated to be important for the
progression of hormone-dependent cancer of the breast and
endometrium (13–15).
 | Figure 1.Intratumoral synthesis of E2 and its
inactivation by sulfate conjugation from circulating steroid
hormone precursors in cancer cells. E2 is synthesized from E1S in
the sulfatase pathway, and from DHEA-S in the aromatase pathway.
E1S and DHEA-S are taken up from the circulation by transporters,
such as those from the OATP family encoded by SCLO. In the
sulfatase pathway, HSD-17β1 generates E2 from E1S, while E1 and E2
are inactivated by sulfonation by SULT1E1. Sulfonated estrogens do
not bind to the ERα or ERβ. Aromatase generates E2 from androgenic
precursors via the aromatase pathway. E2, 17β-estradiol; -S,
sulfate; E1, estrone; STS, steroid sulfatase; SULT1E1, estrogen
sulfotransferase; ER, estrogen receptor; DHEA,
dehydroepiandrosterone sulfate; HSD-17β, 17β-hydroxysteroid
dehydrogenase; OATP, organic anion transporting polypeptides; SLCO,
solute carrier for organic anions. |
In breast cancer cells, the local production of E2
from E1S via the sulfatase pathway has been revealed to exceed the
production of E2 from androgenic precursors, including
dehydroepiandrosterone sulfate (DHEA-S) via the aromatase pathway
(16). In the sulfatase pathway,
subsequent to the cellular uptake of E1S, which is the most
abundant type of estrogen in the circulation of postmenopausal
women (17), estrone (E1) that has
weak estrogenic activity is formed by the enzymatic activity of the
steroid sulfatase (STS; Fig. 1).
Subsequently, E2 is produced from E1 by the activity of
17β-oxoreductase. Conversely, E2 may be reverted back to E1 via the
oxidative function of 17β-hydroxysteroid dehydrogenase. As an
inactivation pathway, E2 conjugation with sulfonate by estrogen
sulfotransferase (SULT1E1) and phenol sulfotransferase (SULT1A1)
produces estradiol sulfate (E2S), which exhibits minimal estrogenic
activity. Conversely, STS may produce active estrogens from E2S and
E1S, suggesting that an increase in the level of STS raises the
levels of active estrogens and contributes to the progression of
estrogen-sensitive types of cancer. Increased expression of STS has
previously been identified to be associated with breast cancer
progression (18). Similarly, in a
small group of patients with advanced stage ovarian cancer an
increased level of STS activity was revealed to be associated with
a worse progression-free survival (PFS) (19). Additionally, STS mRNA was detectable
in ovarian cancer cells, with similar levels detected in tissue
from pre- and postmenopausal women (20), but was not detectable in normal
ovarian surface epithelial cells (14).
Exerting the opposite effect to STS, the increased
expression of estrogen-inactivating sulfotransferases may have
beneficial effects in malignant and non-malignant diseases known to
be sensitive to estrogens. For example, in ovarian endometriosis
the expression of SULT1E1, but not of SULT1A1, was demonstrated to
be decreased compared with normal tissue (20), suggesting that SULT1E1 is important
for estrogen inactivation. The importance of SULT1E1 expression was
also revealed in breast cancer, where increased levels of the
enzyme are associated with a decreased risk of recurrence and an
improved prognosis (12). Similarly,
the overexpression of SULT1E1 was identified to reduce the growth
of hormone-sensitive breast cancer cells and block tumorigenesis in
a xenograft cancer model (21). In
endometrial cancer, higher levels of STS compared with SULT1E1 were
correlated with a poorer prognosis (22). For ovarian cancer, no studies
regarding SULT1E1 are available at present, to the best of our
knowledge.
The effects of estrogen on cell differentiation and
proliferation are largely mediated via the binding and activation
of nuclear estrogen receptor (ER) α and ERβ. Numerous studies have
revealed that in estrogen-sensitive tumors, including breast and
endometrium carcinoma, ERs and estrogen activation via the
sulfatase pathway serve a crucial role in tumor progression
(23,24). Similarly, in preclinical models of
ovarian cancer, a high expression of ERα was identified to promote
tumor progression and the development of metastasis by inducing
epithelial-mesenchymal transition. In ovarian cancer patients, ERα
was demonstrated to be important for tumor progression and a
potential target for endocrine therapy of EOC (25,26).
However, the success of endocrine therapy targeting ERα remains
limited, although the vast majority of EOC subtypes, particularly
HGSOC and endometrioid tumors, were found to express the receptor
(27). Therefore, hormonal treatment
selection based on ER, SULT1E1 and STS expression may lead to an
improved patient outcome.
The present study aimed to examine the expression of
STS, SULT1E1 and ERα protein in a well-defined cohort of patients
with advanced EOC. Additionally, the prognostic value of these
targets was determined in patients with HGSOC histology, being the
most frequently observed and lethal subtype of ovarian cancer.
Materials and methods
Patients
The levels of STS, SULT1E1 and ERα protein were
examined in paraffin-embedded tumor sections from 206 patients with
EOC. Samples were collected as part of the European Union-funded
specific targeted research project ‘Ovarian Cancer: Diagnosis of a
silent killer’. Patients with FIGO stage II–IV ovarian cancer were
included from December 2005 to November 2008 in The Department of
Gynecology at Charité, Medical University Berlin, Germany; The
Department of Obstetrics and Gynecology and Gynecologic Oncology,
University Hospital Leuven, Belgium: Department of Gynecology,
University Medical Center Hamburg-Eppendorf, Hamburg, Germany; The
Department of Obstetrics and Gynecology, Medical University of
Vienna, Austria. Patients with other malignancies were excluded.
Informed consents were obtained from all patients. The study
protocol was approved by the Ethics Committees of the participating
institutions (approval nos. EK207/2003: Berlin; ML2524: Leuven;
HEK190504: Hamburg; EK366 and EK260: Vienna), with the permission
to characterize new molecular prognostic factors for patients with
advanced EOC.
Only patients with Federation of Gynecology and
Obstetrics (FIGO) stage II–IV (27)
EOC receiving standard treatment, debulking surgery and
platinum-based chemotherapy with taxol derivatives were included in
the present study. Patients presenting with benign ovarian
diseases, low malignant potential ovarian cancer, FIGO stage I EOC
and secondary malignant diseases were excluded. All patients
provided preoperative written informed consent prior to enrollment
in the present study. Histopathological grading was done for all
tumors, grade 1-grade 3, according to tissue differentiation
(28).
PFS was defined as the time interval between the
date of primary surgery and the date of first progression or
recurrence of cancer. Disease progression subsequent to first-line
chemotherapy was diagnosed by clinical examination, tumor imaging
or by a >2-fold increase in the nadir serum cancer antigen-125
level. Overall survival (OS) was defined as the time interval
between initial cytoreductive surgery, EOC-associated mortality or
last follow-up. Clinical and histopathological evaluation was
performed under the supervision of experienced gynecologic
oncologists and pathologists in the participating institutions of
the present study.
Immunohistochemical (IHC)
staining
IHC staining was performed on tissue microarrays
(TMAs) containing 2 core sections/tumor tissue (1 mm diameter).
Subsequent to heating the sections in a tissue-drying oven for 1 h
at 60°C, antigen retrieval was performed for 10 min with
DEPP-buffer (pH 9.0; Eubio, Vienna, Austria) in a microwave.
Blocking for endogenous peroxidase and applying the Lab Vision™
Ultra V Block (UltraVision LP Detection System; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The UltraVision LP Detection
System was used according to the manufacturer's instructions.
Incubation with antibodies directed against STS (cat. no. N1C3;
dilution, 1:400; GeneTex, Inc., Irvine, CA, USA) and SULT1E1 (cat.
no. 12522-1-AP; dilution, 1:200; ProteinTech Group, Inc., Chicago,
IL, USA) was performed overnight at 4°C. Antigen retrieval and
staining for ERα using mouse IgG1κ directed against recombinant ERα
protein (clone 1D5; cat. no. MA5-13191; Thermo Fisher Scientific,
Inc.) was performed as previously described by Aust et al
(29). Sections were treated with HRP
Polymer prior to the application of a DAB Plus Chromogen/DAB Plus
Substrate mixture (ratio, 1:40) (both UltraVision LP Detection
System; Thermo Fisher Scientific, Inc.). The nuclei were
counterstained with hematoxylin and the slides were then embedded
in Fluoromount (SouthernBiotech, Birmingham, AL, US).
Evaluation of IHC staining
results
Images of the TMAs were acquired with the automated
quantitative microscopy-based image analysis system TissueFAXS PLUS
(TissueGnostics GmbH, Vienna, Austria) using the 20X objective. A
minimum of 3 regions of interest were manually drawn in the tumor
areas based on the individual tissue structures and data were
acquired for these regions. HistoQuest software (version
3.0.3.0161; TissueGnostics GmbH) was used to determine the staining
intensity as gray values of between 0 and 250 arbitrary units. Gray
values were determined from the minimum (set to 0) to maximum (set
to 250) of the average optical density of the DAB stained target
using the HistoQuest software. In the histochemical analysis color
shades are picked manually in an automated color separation
produces to give a gray value channel image for each marker
(30).
The proportional expression, defined as the
percentage of positively stained tumor cells, was used for the
calculation of the, mean of mean intensity as described previously
(31). Staining intensity in the
cytoplasm (STS and SULT1E1) or in the nucleus (ERα) was assessed
subsequent to setting a cut-off associated with values from
negative control images generated by the application of
non-immunogenic IgG or phosphate-buffered saline instead of the
first antibody. To correct for false positive events, a specific
gate according to cell size and intensity staining was defined and
applied homogenously to all analyzed samples. For the assessment of
data, only samples with a sufficient tissue quality, including
structural integrity of the tissue and clear staining quality with
an appropriate negative control, were chosen for quantitative
microscopic analysis. All procedures were guided, monitored and
approved by a pathologist.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 19.0; IBM SPSS, Armonk, NY, USA). Descriptive
statistics such as the mean, median, frequency and percentage were
used to summarize the data. Data was log-transformed to reach a
normal distribution, as appropriate. The abundance values of STS,
SULT1E1 and ERα were grouped into high and low abundance groups
using the median as cut-off. Statistical correlations between low
and high abundance groups and clinicopathological parameters were
assessed by the χ2 test, Fisher's exact test and
unpaired t-tests, as appropriate. To assess the impact of STS,
SULT1E1 and ERα on PFS and OS, linear log-transformed values were
used in univariate and multivariate Cox regression analyses.
Multivariate analyses were performed using stepwise regression
(backward elimination). P<0.05 was considered to indicate a
statistically significant difference.
Results
EOC tumors
The clinicopathological characteristics of the 206
patients with EOC are illustrated in Table I. The median observation period was 68
months (range, 1–96 months). The median age of patients at the time
of cytoreductive surgery was 56 years (range, 26–85 years), whereby
the macroscopic cytoreduction rate was 70%. Additional
clinicopathological characteristics of this cohort have previously
been described (32).
 | Table I.Epithelial ovarian cancer study
population divided into high and low ERα, STS and SULT1E1 abundance
groups using the median as cut-off. |
Table I.
Epithelial ovarian cancer study
population divided into high and low ERα, STS and SULT1E1 abundance
groups using the median as cut-off.
| Clinicopathological
characteristic | ERα low (n=106),
no. of patients (%) | ERα high (n=96),
no. of patients (%) | P-value | STS low (n=104),
no. of patients (%) | STS high (n=96),
no. of patients (%) | P-value | SULT1E1 low (n=98),
no. of patients (%) | SULT1E1 high
(n=108), no. of patients (%) | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
|
|
|
<55 | 55 (60) | 37 (40) |
| 50 (48) | 37 (39) |
| 44 (48) | 47 (52) |
|
|
≥55 | 51 (46) | 59 (54) | 0.057 | 54 (48) | 59 (52) | 0.174 | 54 (47) | 61 (56) | 0.842c |
| Histology |
|
|
|
|
|
|
|
|
|
|
Serous | 95 (52) | 88 (48) |
| 94 (52) | 87 (48) |
| 88 (48) | 97 (52) |
|
|
Non-serousa | 11 (58) | 8
(42) | 0.619 | 10 (52) | 9
(48) | 0.954 | 10 (48) | 11 (52) | 0.996 |
| FIGO stage |
|
|
|
|
|
|
|
|
|
| II | 3
(43) | 4
(57) |
| 4
(57) | 3
(43) |
| 5
(71) | 2
(29) |
|
|
III | 86 (53) | 76 (47) |
| 82 (52) | 77 (48) |
| 74 (45) | 89 (55) |
|
| IV | 17 (51) | 16 (49) | 0.914c | 18 (53) | 16 (47) | 1.000c | 19 (53) | 17 (47) | 0.329d |
| Gradeb |
|
|
|
|
|
|
|
|
|
| Grade
1/2 | 24 (44) | 31 (56) |
| 22 (42) | 31 (59) |
| 15 (28) | 38 (72) |
|
| Grade
3 | 82 (56) | 64 (44) | 0.113 | 81 (56) | 65 (45) | 0.081 | 83 (54) | 69 (46) | 0.001 |
| Residual tumor
statusb |
|
|
|
|
|
|
|
|
|
|
Absent | 73 (51) | 69 (49) |
| 66 (48) | 72 (53) |
| 66 (46) | 78 (54) |
|
|
Present | 33 (55) | 27 (45) | 0.640 | 38 (61) | 24 (39) | 0.078 | 32 (52) | 30 (48) | 0.446 |
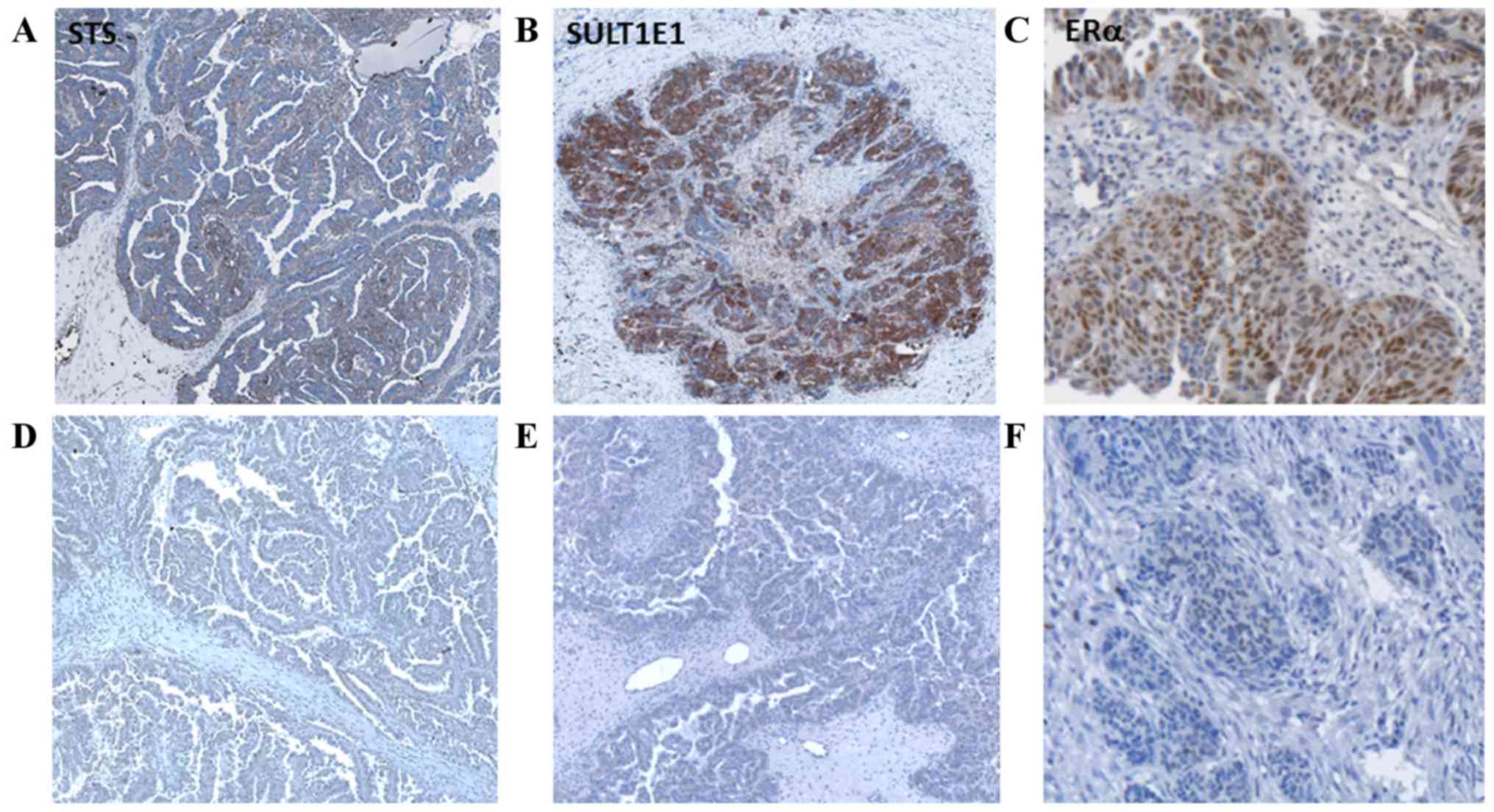
Representative images of STS, SULT1E1 and ERα
staining compared with the negative controls are illustrated in
Fig. 2. Cytoplasmic staining in the
tumor cells was visible for STS and SULT1E1. Staining of cells in
the tumor stroma was weak and infrequent in immune cells.
Immunoreactive ERα staining was observed in the tumor cell nuclei
and, occasionally, in the perinuclear region.
For the evaluation of the abundance of STS, SULT1E1
and ERα, only tissue samples fulfilling the requirements for a
quantitative microscopy-based image analysis, aforementioned in the
methods section, were included in the present study. The mean
levels of immunoreactive STS, SULT1E1 and ERα were calculated and
patients were stratified into high and low target abundance groups
(Table I). Finally, tumor tissue
samples from 206, 200 and 202 patients were evaluated for SULT1E1,
STS and ERα abundance, respectively. No significant differences
regarding clinicopathological characteristics (tumor histology,
age, FIGO stage and residual tumor) were observed between the low
and high ERα and STS abundance groups. However, the degree of
tissue differentiation (28) was
important for the expression level as a significantly higher
SULT1E1 abundance was observed in more differentiated grade 1/2
tumors compared with less differentiated grade 3 tumors (P=0.001).
A total of 59% of the collective of grade 1/2 compared with 45%
grade 3 tumors exhibited high STS abundance (P=0.081). In addition,
STS and SULT1E1 protein levels were positively correlated with each
other (P<0.001) and with ERα (P<0.001 and P=0.001,
respectively; data not shown).
HGSOC
Different histological subtypes of EOC have
distinctive characteristics regarding the origin of the tumor, the
growth pattern, the sensitivity to chemotherapy and the prognosis.
As it is recommended to perform survival analyses for different
ovarian cancer subtypes separately (33), a homogenous group of patients with a
long observation period were used for survival analyses. The data
of 137 patients with HGSOC were used to assess the impact of
SULT1E1, STS, and ERα abundance on clinical outcome (Table II). During the median observation
period of 68 months (range, 1–96 months), a total of 84 (64%)
patients succumbed to the disease and 113 (86%) patients
experienced a tumor recurrence (data not shown).
 | Table II.Univariate and multivariate Cox
regression analyses (backward conditional) for progression-free
survival and overall survival of patients with high grade serous
ovarian carcinoma (HGSOC) with FIGO III/IV tumors (n=132). |
Table II.
Univariate and multivariate Cox
regression analyses (backward conditional) for progression-free
survival and overall survival of patients with high grade serous
ovarian carcinoma (HGSOC) with FIGO III/IV tumors (n=132).
|
| Progression-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (continuous,
per decade) | 1.18
(0.99–1.99) | 0.053 | 1.20
(1.01–1.01) | 0.040 | 1.39
(1.14–1.14) | <0.001 | 1.51
(1.22–1.22) | <0.001 |
| FIGO stage
(ordinal, III vs. IV) | 2.91
(1.86–4.86) | <0.001 | 2.87
(1.78–4.78) | <0.001 | 2.3
(1.43–3.43) | 0.001 | 2.07
(1.24–3.24) | 0.005 |
| Residual tumor
(absent vs. present) | 1.48
(0.99–2.99) | 0.053 | – |
| 2.14
(1.38–3.38) | 0.001 | 1.96
(1.23–3.23) | 0.005 |
| ERα abundance (high
vs. low)a | 1.01
(0.81–1.81) | 0.903 | – |
| 0.21
(0.67–1.67) | 0.206 | – |
|
| STS abundance (high
vs. low)a | 0.98
(0.71–1.71) | 0.926 | – |
| 0.93
(0.65–1.65) | 0.720 | – |
|
| SULT1E1 abundance
(high vs. low)a | 1.08
(0.76–1.76) | 0.676 | – |
| 0.78
(0.79–1.79) | 0.154 | 0.66
(0.45–0.45) | 0.005 |
Multivariate Cox regression analysis revealed that
SULT1E1 abundance was a significant independent predictor for OS
(hazard ratio, 0.66; 95% confidence interval, 0.45–0.94; P=0.005;
Table II). Notably, neither ERα nor
STS abundance exhibited a significant impact on OS. These results
suggest that patients with higher SULT1E1 levels have a better
prognosis, independent from the most relevant confounding
parameters demonstrated in Table II.
The abundance of ERα, STS and SULT1E1 were not significantly
associated with PFS (Table II).
Non-serous tumors
STS, SULT1E1 and ERα were also detected in
non-serous tumors (Table I). Due to
the limited number of patients (n=21; endometrioid tumors, n=11;
clear cell tumors, n=2; mixed epithelial tumors, n=7; mucinous
tumors, n=1) and the heterogeneity of the tumors, distinct survival
analyses were not performed for the non-serous tumor group.
Discussion
The intratumoral production of E2 from circulating
E1S via the sulfatase pathway, including the enzymes STS for
estrogen activation and SULT1E1 for estrogen inactivation, serves
an important role in estrogen-associated cancer of the breast and
endometrium (34). Therefore, the
present study determined the levels of STS, SULT1E1 and ERα in a
well-defined cohort of 205 EOC patients. This revealed that SULT1E1
abundance exhibited a significant independent prognostic value for
OS in 137 patients with HGSOC treated with debulking surgery and
standard platinum-based adjuvant chemotherapy. A high level of
SULT1E1 was associated with a longer OS in patients with HGSOC,
which is concordant with results from previous studies of breast
and endometrial cancer. In these tumors, high SULT1E1 levels were
found to be associated with a better prognosis for patients, as
demonstrated by a decreased risk of recurrence and an increased OS
(13,22). This beneficial role of SULT1E1 is
further supported by the finding that SULT1E1 is typically
downregulated in malignant tissue (34,35). In
addition, preclinical studies have demonstrated that the
inactivation of E2 by sulfonation reduces the proliferative effects
of estrogens on hormone-sensitive tumor cells (36). Furthermore, the conjugation of
estrogens by SULT1E1 creates water-soluble estrogen sulfates, which
may be rapidly excreted from the cells. Effective sulfonation and
rapid excretion of the sulfonated E2 may prevent the metabolic
activation of the hormone into potential mutagenic catechol
metabolites, which increase the mutation rate of DNA and lead to
chromosomal instability (37,38). Since the present study did not
identify a significant association between STS and OS/PFS, in HGSOC
SULT1E1 may be of a higher relevance compared with STS for estrogen
inactivation and estrogen homeostasis. However, Chura et al
(19) reported that a decreased level
of STS activity was associated with a higher OS, based on a smaller
cohort of patients with ovarian cancer (n=37) with no additional
characterization of the tumor subtypes. Therefore, the
clinicopathological characteristics and number of patients included
in the present study may explain the diverging data in regards to
STS, particularly as a large number of patients with HGSOC were
included. However, data on the association between STS expression
and tumor progression remain controversial even in otherwise
well-studied endometrial carcinomas. This may be partly explained
by different approaches to investigating estrogen-modifying
enzymes. For example, Abulafia et al (39) identified that there was higher STS
activity in endometrial carcinoma compared with normal endometrial
tissues using DHEA-sulfate as a substrate, but Tanaka et al
(40) reported a decreased level of
STS activity in endometrial carcinoma compared with normal
endometrium as the lining of the uterus and endometrial
adenocarcinoma-derived cells using E1S as a substrate.
Despite the uncertainty with respect to the role of
STS in the progression of gynecological cancers and the limited
data from clinical trials, STS has been proposed to be a potential
hormonal therapeutic target for the treatment of
estrogen-associated cancer (41). It
must be considered that the regulation of STS expression is subject
to various feedback mechanisms, including the levels of active
estrogens (42). Therefore, in EOC
the increased inactivation of E2 by low levels of SULT1E1 may cause
an upregulation of STS. Indeed, a significant association between
the expression of SULT1E1/STS and ERα was identified in the present
study, similarly to the results of a previous study investigating
breast cancer (43). In the present
study, although STS and SULT1E1 abundance was significantly
associated and the abundance of these enzymes was significantly
associated with the level of ERα, the receptor itself exhibited no
significant impact on PFS or OS. In a study with a large cohort of
patients with serous EOC, the expression levels of ERα and ERβ did
not provide any prognostic information (14,44).
From the data obtained, the present study concludes
that targeting estrogen-modifying enzymes in the sulfatation
pathway is a potential strategy for the endocrine therapy of
estrogen-sensitive ovarian cancer. Targeting the sulfatase pathway
was previously suggested as an endocrine therapy option for breast
cancer (45). In a mouse xenograft
model, blocking aromatase together with the application of the STS
inhibitor STX64 reduced the estrogen level in tumors (46). This is important as the application of
the estrogen-depleting aromatase inhibitor exemestane can cause an
upregulation of intratumoral STS, leading to therapy resistance
(47). Whether SULT1E1 may also be
downregulated through feedback mechanisms remains unknown. However,
STX64 was found to inhibit the growth of the ER+ ovarian
carcinoma OVCAR-3 cell line and thus may be considered a target for
ovarian cancer therapy (48). The
importance of SULT1E1 was also identified in the use of the prodrug
tibolone, which improves bone structure in postmenopausal women as
it is metabolized into 2 products with antagonistic effects on ERα.
In the endometrium, SULT1E1 effectively converts these 2 metabolic
products to inactive sulfate conjugates, preventing ERα activation
and reducing the risk of endometrial cancer induction (49).
In conclusion, the results of the present study
suggest that estrogen-modifying enzymes in the sulfatase pathway,
such as STS and SULT1E1, are potential endocrine therapy targets
for the treatment of patients with EOC.
Acknowledgements
The present study was supported by the European
Union-funded Sixth Framework Programme Specific Targeted Research
Project Ovarian Cancer: Diagnosis of a silent killer (grant no.
018698).
References
|
1
|
American Cancer Society, American Cancer
Society, . 2016 Ovarian epithelial, fallopian tube and primary
peritoneal cancer treatment (PDQ®). https://www.cancer.gov/types/ovarian/hp/ovarian-epithelial-treatment-pdqJanuary
17–2017.
|
|
2
|
Kessler M, Fotopoulou C and Meyer T: The
molecular fingerprint of high grade serous ovarian cancer reflects
its fallopian tube origin. Int J Mol Sci. 14:6571–6596. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown J and Frumovitz M: Mucinous tumors
of the ovary: Current thoughts on diagnosis and management. Curr
Oncol Rep. 16:3892014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sapiezynski J, Taratula O,
Rodriguez-Rodriguez L and Minko T: Precision targeted therapy of
ovarian cancer. J Control Release. 243:250–268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chuffa LG, Lupi-Junior LA, Costa AB,
Amorim JP and Seiva FR: The role of sex hormones and steroid
receptors on female reproductive cancers. Steroids. 118:93–108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beral V; Million Women Study
Collaborators, ; Bull D, Green J and Reeves G: Ovarian cancer and
hormone replacement therapy in the Million Women Study. Lancet.
369:1703–1710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kurian AW, Balise RR, McGuire V and
Whittemore AS: Histologic types of epithelial ovarian cancer: Have
they different risk factors? Gynecol Oncol. 96:520–530. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rice LW: Hormone prevention strategies for
breast, endometrial and ovarian cancers. Gynecol Oncol.
118:202–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schock H, Surcel HM, Zeleniuch-Jacquotte
A, Grankvist K, Lakso HÅ, Fortner RT, Kaaks R, Pukkala E, Lehtinen
M, Toniolo P and Lundin E: Early pregnancy sex steroids and
maternal risk of epithelial ovarian cancer. Endocr Relat Cancer.
21:831–844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trabert B, Brinton LA, Anderson GL,
Pfeiffer RM, Falk RT, Strickler HD, Sliesoraitis S, Kuller LH, Gass
ML, Fuhrman BJ, et al: Circulating estrogens and postmenopausal
ovarian cancer risk in the Women's Health initiative observational
study. Cancer Epidemiol Biomarkers Prev. 25:648–656. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Svoboda M, Wlcek K, Taferner B, Hering S,
Stieger B, Tong D, Zeillinger R, Thalhammer T and Jäger W:
Expression of organic anion-transporting polypeptides 1B1 and 1B3
in ovarian cancer cells: Relevance for paclitaxel transport. Biomed
Pharmacother. 65:417–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sasano H, Miki Y, Nagasaki S and Suzuki T:
In situ estrogen production and its regulation in human breast
carcinoma: From endocrinology to intracrinology. Pathol Int.
59:777–789. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suzuki T, Miki Y, Nakamura Y, Ito K and
Sasano H: Steroid sulfatase and estrogen sulfotransferase in human
carcinomas. Mol Cell Endocrinol. 340:148–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren X, Wu X, Hillier SG, Fegan KS,
Critchley HO, Mason JI, Sarvi S and Harlow CR: Local estrogen
metabolism in epithelial ovarian cancer suggests novel targets for
therapy. J Steroid Biochem Mol Biol. 150:54–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rizner TL: Estrogen biosynthesis, phase I
and phase II metabolism, and action in endometrial cancer. Mol Cell
Endocrinol. 381:124–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McNamara KM and Sasano H: The
intracrinology of breast cancer. J Steroid Biochem Mol Biol.
145:172–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Müller P, Rothschild SI, Arnold W,
Hirschmann P, Horvath L, Bubendorf L, Savic S and Zippelius A:
Metastatic spread in patients with non-small cell lung cancer is
associated with a reduced density of tumor-infiltrating T cells.
Cancer Immunol Immunother. 65:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanamura T, Niwa T, Gohno T, Kurosumi M,
Takei H, Yamaguchi Y, Ito K and Hayashi S: Possible role of the
aromatase-independent steroid metabolism pathways in hormone
responsive primary breast cancers. Breast Cancer Res Treat.
143:69–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chura JC, Blomquist CH, Ryu HS and Argenta
PA: Estrone sulfatase activity in patients with advanced ovarian
cancer. Gynecol Oncol. 112:205–209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hevir N, Ribič-Pucelj M and Lanišnik
Rižner T: Disturbed balance between phase I and II metabolizing
enzymes in ovarian endometriosis: A source of excessive
hydroxy-estrogens and ROS? Mol Cell Endocrinol. 367:74–84. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Y, Liu X, Guo F, Ning Y, Zhi X, Wang X,
Chen S, Yin L and Li X: Effect of estrogen sulfation by SULT1E1 and
PAPSS on the development of estrogen-dependent cancers. Cancer Sci.
103:1000–1009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Utsunomiya H, Ito K, Suzuki T, Kitamura T,
Kaneko C, Nakata T, Niikura H, Okamura K, Yaegashi N and Sasano H:
Steroid sulfatase and estrogen sulfotransferase in human
endometrial carcinoma. Clin Cancer Res. 10:5850–5856. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Purohit A, Woo LW and Potter BV: Steroid
sulfatase: A pivotal player in estrogen synthesis and metabolism.
Mol Cell Endocrinol. 340:154–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mungenast F and Thalhammer T: Estrogen
biosynthesis and action in ovarian cancer. Front Endocrinol
(Lausanne). 5:1922014.PubMed/NCBI
|
|
25
|
Park SH, Cheung LW, Wong AS and Leung PC:
Estrogen regulates Snail and Slug in the down-regulation of
E-cadherin and induces metastatic potential of ovarian cancer cells
through estrogen receptor alpha. Mol Endocrinol. 22:2085–2098.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haring J, Schuler S, Lattrich C, Ortmann O
and Treeck O: Role of estrogen receptor β in gynecological cancer.
Gynecol Oncol. 127:673–676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Voutsadakis IA: Hormone receptors in
serous ovarian carcinoma: Prognosis, pathogenesis, and treatment
considerations. Clin Med Insights Oncol. 10:17–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Silverberg SG: Histopathologic grading of
ovarian carcinoma: A review and proposal. Int J Gynecol Pathol.
19:7–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aust S, Bachmayr-Heyda A, Pateisky P, Tong
D, Darb-Esfahani S, Denkert C, Chekerov R, Sehouli J, Mahner S, van
Gorp T, et al: Role of TRAP1 and estrogen receptor alpha in
patients with ovarian cancer-a study of the OVCAD consortium. Mol
Cancer. 11:692012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kounnis V, Ioachim E, Svoboda M, Tzakos A,
Sainis I, Thalhammer T, Steiner G and Briasoulis E: Expression of
organic anion-transporting polypeptides 1B3, 1B1, and 1A2 in human
pancreatic cancer reveals a new class of potential therapeutic
targets. Onco Targets Ther. 4:27–32. 2011.PubMed/NCBI
|
|
31
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
32
|
Chekerov R, Braicu I, Castillo-Tong DC,
Richter R, Cadron I, Mahner S, Woelber L, Marth C, van Gorp T,
Speiser P, et al: Outcome and clinical management of 275 patients
with advanced ovarian cancer International Federation of Obstetrics
and Gynecology II to IV inside the European Ovarian Cancer
Translational Research Consortium-OVCAD. Int J Gynecol Cancer.
23:268–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kobel M, Kalloger SE, Boyd N, McKinney S,
Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, et al:
Ovarian carcinoma subtypes are different diseases: Implications for
biomarker studies. PLoS Med. 5:e2322008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rizner TL: The important roles of steroid
sulfatase and sulfotransferases in gynecological diseases. Front
Pharmacol. 7:302016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smuc T and Rizner TL: Aberrant
pre-receptor regulation of estrogen and progesterone action in
endometrial cancer. Mol Cell Endocrinol. 301:74–82. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li L, He D, Wilborn TW, Falany JL and
Falany CN: Increased SULT1E1 activity in HepG2 hepatocytes
decreases growth hormone stimulation of STAT5b phosphorylation.
Steroids. 74:20–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Falany CN, Comer KA, Dooley TP and Glatt
H: Human dehydroepiandrosterone sulfotransferase. Purification,
molecular cloning, and characterization. Ann N Y Acad Sci.
774:59–72. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Adjei AA and Weinshilboum RM:
Catecholestrogen sulfation: Possible role in carcinogenesis.
Biochem Biophys Res Commun. 292:402–408. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Abulafia O, Lee YC, Wagreich A, Economos
K, Serur E and Nacharaju VL: Sulfatase activity in normal and
neoplastic endometrium. Gynecol Obstet Invest. 67:57–60. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanaka K, Kubushiro K, Iwamori Y, Okairi
Y, Kiguchi K, Ishiwata I, Tsukazaki K, Nozawa S and Iwamori M:
Estrogen sulfotransferase and sulfatase: Roles in the regulation of
estrogen activity in human uterine endometrial carcinomas. Cancer
Sci. 94:871–876. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Purohit A, Fusi L, Brosens J, Woo LW,
Potter BV and Reed MJ: Inhibition of steroid sulphatase activity in
endometriotic implants by 667 COUMATE: A potential new therapy. Hum
Reprod. 23:290–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reed MJ, Purohit A, Woo LW, Newman SP and
Potter BV: Steroid sulfatase: Molecular biology, regulation, and
inhibition. Endocr Rev. 26:171–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zaichuk T, Ivancic D, Scholtens D,
Schiller C and Khan SA: Tissue-specific transcripts of human
steroid sulfatase are under control of estrogen signaling pathways
in breast carcinoma. J Steroid Biochem Mol Biol. 105:76–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jonsson JM, Arildsen N Skovbjerg, Malander
S, Måsbäck A, Hartman L, Nilbert M and Hedenfalk I: Sex steroid
hormone receptor expression affects ovarian cancer survival. Transl
Oncol. 8:424–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin SX, Chen J, Mazumdar M, Poirier D,
Wang C, Azzi A and Zhou M: Molecular therapy of breast cancer:
Progress and future directions. Nat Rev Endocrinol. 6:485–493.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Foster PA, Woo LW, Potter BV, Reed MJ and
Purohit A: The use of steroid sulfatase inhibitors as a novel
therapeutic strategy against hormone-dependent endometrial cancer.
Endocrinology. 149:4035–4042. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chanplakorn N, Chanplakorn P, Suzuki T,
Ono K, Chan MS, Miki Y, Saji S, Ueno T, Toi M and Sasano H:
Increased estrogen sulfatase (STS) and 17beta-hydroxysteroid
dehydrogenase type 1 (17beta-HSD1) following neoadjuvant aromatase
inhibitor therapy in breast cancer patients. Breast Cancer Res
Treat. 120:639–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Day JM, Purohit A, Tutill HJ, Foster PA,
Woo LW, Potter BV and Reed MJ: The development of steroid sulfatase
inhibitors for hormone-dependent cancer therapy. Ann N Y Acad Sci.
1155:80–87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Falany JL and Falany CN: Regulation of
SULT1E1 expression in Ishikawa adenocarcinoma cells by tibolone.
Steroids. 71:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hormone Health Network: Menopause, .
http://www.hormone.org/diseases-and-conditions/womens-health/menopauseJanuary
17–2017.
|