Introduction
Among malignant tumors, pancreatic cancer occurs
frequently and is one of the most severe gastrointestinal tumors
with extremely high mortality rates (1). Due to the anatomical location of the
pancreas, and the fact that tumors are found in advanced stages,
the chance of performing a curative surgery is small. Relevant data
have shown a relatively serious recurrence rate in patients within
5 years after operation, and a survival rate of <5 years for up
to ~95% of them (2). Pancreatic
cancer markedly reduces the living quality of patients, but also
causes a heavy economic burden on society. The complexity of
pancreatic cancer and its poorly understood pathogenesis, are
factors determining the difficulty in achieving a prompt diagnosis
and an effective treatment. With the development of more advanced
molecular biology techniques, the search for therapeutic targets is
currently very active with the aim of finding a specific and
effective treatment alternative.
PRR11 is a newly found gene, located at chromosome
17q22 (3). Studies have shown this
gene is an evolutionarily conserved one, which may play a role in
cell proliferation (4). Furthermore,
PRR11 is upregulated in various tumors, including brain, lung,
breast and ovarian cancer tumors; and its high expression has been
related to the development, deterioration and poor prognosis of
various tumors (5–7). Nevertheless, the relationship between
PRR11 and pancreatic cancer remains unclear. This study aimed to
investigate the effects of PRR11 on pancreatic cancer.
Materials and methods
Patients, materials and methods
In this study, paraffin-embedded tissue samples were
collected from a total of 48 patients who underwent pancreas
operation in Yantai Hospital of Traditional Chinese Medicine from
October 2012 to March 2014. The survival time of the patients was
counted from the day of enrollment in the study to the time of
death or the date of last collection of information in March, 2016.
Network telephone surveys and out-patient re-examinations were used
as means of evaluating the status of patients. This study was
approved by the Ethics Committee of Yantai Hospital of Traditional
Chinese Medicine. Signed written informed consents were obtained
from all participants and/or guardians before the study.
The essential reagents used for this study include a
rabbit polyclonal PRR11 antibody (dilution, 1:500; Sigma-Aldrich
China, Inc., Shanghai, China), rabbit streptavidin-HRP kit and
hematoxylin (both from Huaxia Yuanyang Science and Technology,
Beijing, China), neutral balsam (Zhengheng Chemical Glass
Instrument, Linyi, China), PBS solution (Huaxia Yuanyang Science
and Technology), and alcohol (Zhengheng Chemical Glass Instrument).
Instruments included an optical microscope (Beijing PDV Instrument
Co., Ltd., Beijing, China), a micropipettor (Taixing Aibo Glassware
Co. Ltd., Jiangsu, China), a fluorescence microscope, a
supercentrifuge, and an ABI7900 PCR amplifier (all from Beijing PDV
Instrument Co., Ltd.).
Cell culture
The cell strain Capan-1 of human pancreatic cancer
purchased from CoBioer Biosciences Co., Ltd. (Nanjing, China) was
cultured in 10% fetal calf serum with high glucose Dulbecco's
modified Eagle's medium (DMEM) culture solution at constant
temperature (37°C) in a cell culture incubator. The culture medium
was replaced every two days (or as needed). Serial sub-cultivation
was performed once the cells entered into the exponential growth
phase.
Extraction of RNA in pancreatic cancer
cells and PRR11 mRNA levels
mRNA expression levels were measured in tissues of
40 cases of pancreatic cancer and 8 normal pancreas controls.
Taking SYBR as fluorescent dye and β-actin (ACTB) as the internal
reference mRNA, the quantity of PRR11 mRNA expression was detected.
The PRR11 primer sequences and GAPDH primer sequences are shown in
Table I.
 | Table I.RT-PCR primer sequences and gene
silencing sequences. |
Table I.
RT-PCR primer sequences and gene
silencing sequences.
| Genes | Sequences |
|---|
| PRR11 | F:
5′-TGCGTGTGGAGTATTTGGATG-3′ |
|
| R:
5′-TGGTACAGTCAGAGCCAACCTC-3′ |
| β-actin | F:
5′-CAGCCATGTACGTTGCTATCCAGG-3′ |
|
| R:
5′-AGGTCCAGACGCAGGATGGCATG-3′ |
Expression of PRR11 protein detected
by western blot analysis
Frozen tissue samples stored in liquid nitrogen were
cut into pieces with scissors. Each sample was homogenized in lysis
buffer at a ratio of 1:20 w/v. After a centrifugation at 10,000 × g
for 20 min step the supernatant was used to measure the total
protein. The quantification of the PRR11 protein was made following
standard western blot analysis and normalizing to the internal
reference β-actin.
Expression of PRR11 protein detected
by immunohistochemistry
Stored prepared paraffin sections were dewaxed.
After washing with a 3% hydrogen peroxide aqueous solution, citric
acid buffer solution (both from Biosharp, Hefei, China) was added
and the samples were heated for renaturation. After blocking in
goat serum, the primary antibody (dilution, 1:200; cat. no.
SAB1102068; Sigma-Aldrich China, Inc.) was added and the samples
incubated at 4°C overnight. Next day the samples were washed, and
then the secondary goat anti-rabbit (HRP) IgG antibody (dilution,
1/2,000; cat. no. ab6721) was added and incubation at 37°C for 1 h.
Color development and re-dyeing were conducted after washing.
Finally, observations were conducted under a microscope (Nikon,
Tokyo, Japan).
Scoring of tissue samples was done by calculating
the number of positive cells in each field of view. Scores were
divided into four levels. The first level (scored as 0) contained
<5% of positive cells. The second level (scored as 1) referred
to <40% of positive cells. The third level (scored as 2)
referred to >40% of positive cells. The fourth and highest level
(scored as 3) referred to >60% positive cells. After statistical
analysis, the above scoring results were divided into two groups. A
low expression group of PRR11 for levels I and II, and a high
expression group of PRR11 for levels III and IV. The final results
were used in the statistical analyses.
Cell wound healing assay
The cell strains pPRR11-sh (inhibiting the
expression of PRR11) and pControl-sh (expressing an empty control
vector) were constructed and used in a wound healing assay.
Firstly, the stable cell strains were inoculated on a 6-well plate,
respectively. Cultivation was conducted under standard conditions
for 12 h. A separation glass needle was used to draw a thin line
through the cell layer in each one of the plates. Dislodged cells
were washed away, and fresh culture medium without serum was added,
before placing the plates back in the incubator for a total of 48
h.
Statistical analysis
Experimental data processing was tested using the
SPSS 19.0 statistical software (IBM, Armonk, NY, USA). The
difference in the expression level of PRR11 protein in the
pancreatic cancer group and the normal group was calculated.
Survival analysis was conducted for clinical prognosis data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Changes of PRR11 mRNA in pancreatic
cancer tissues
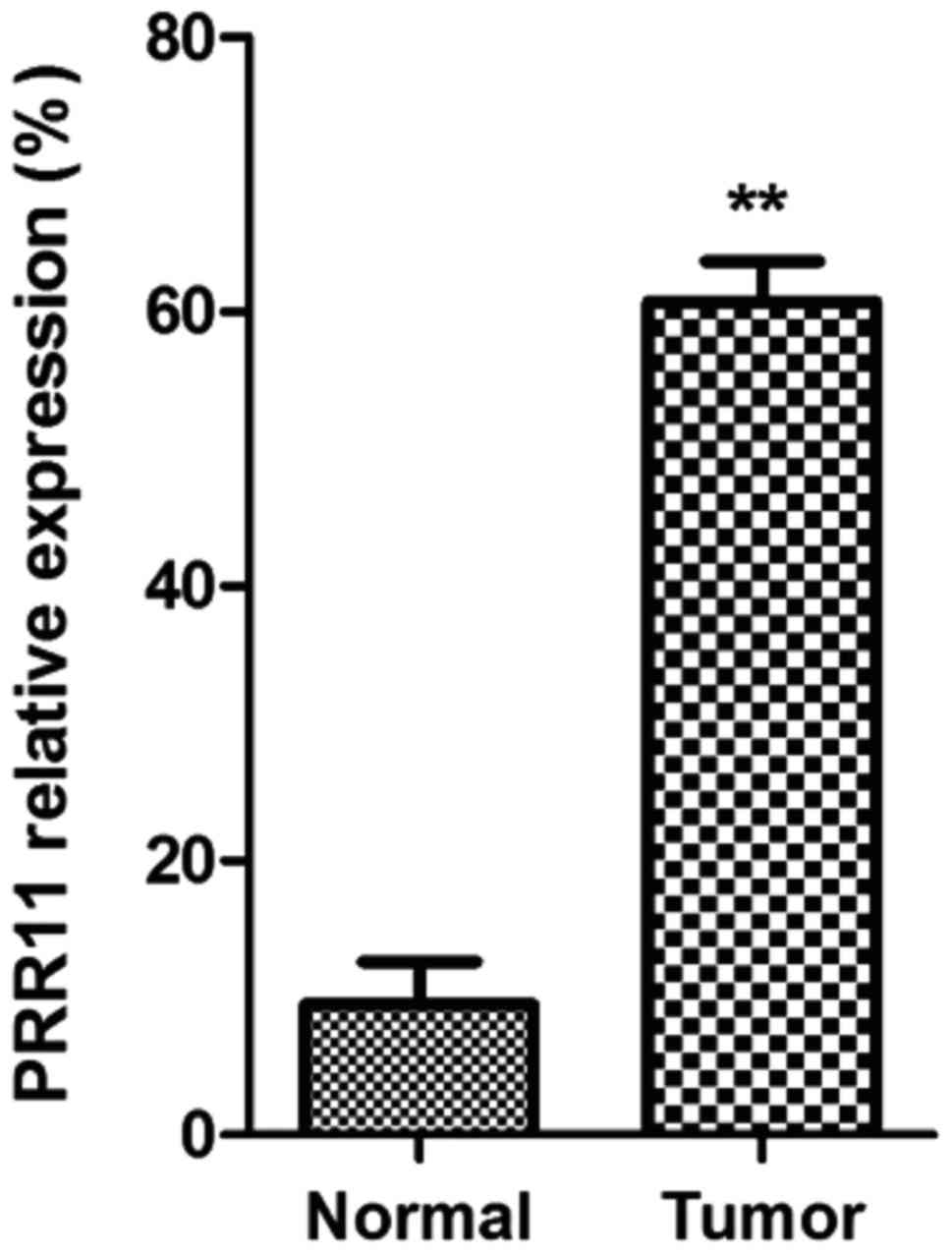
As shown in Fig. 1,
mRNA expression of PRR11 in pancreatic cancer tissues was
significantly higher than that in normal pancreatic tissues
(P<0.01).
Expression level of PRR11 protein in
pancreatic cancer tissues
The expression of PRR11 protein was measured in 38
cases of pancreatic cancer tissues and in 10 normal pancreatic
tissues. Within the 38 cases of pancreatic cancer tissues, there
were 30 positive cases by immunohistochemistry. In the 10 cases of
normal tissues, the negative rate was 100%, giving a P<0.05 for
a statistical significant difference. The results are shown in
Table II.
 | Table II.Expression of PRR11 protein in
pancreatic cancer and in normal pancreatic tissues. |
Table II.
Expression of PRR11 protein in
pancreatic cancer and in normal pancreatic tissues.
| Tissues | Cases | Positive
immunohistochemistry | Negative
immunohistochemistry | Positive rate
(%) | χ2 | P-value |
|---|
| Pancreatic cancer
tissues | 38 | 30 | 8 | 78.9 | 32.047 | P<0.05 |
| Normal tissues | 10 | 0 | 10 | 0 |
|
|
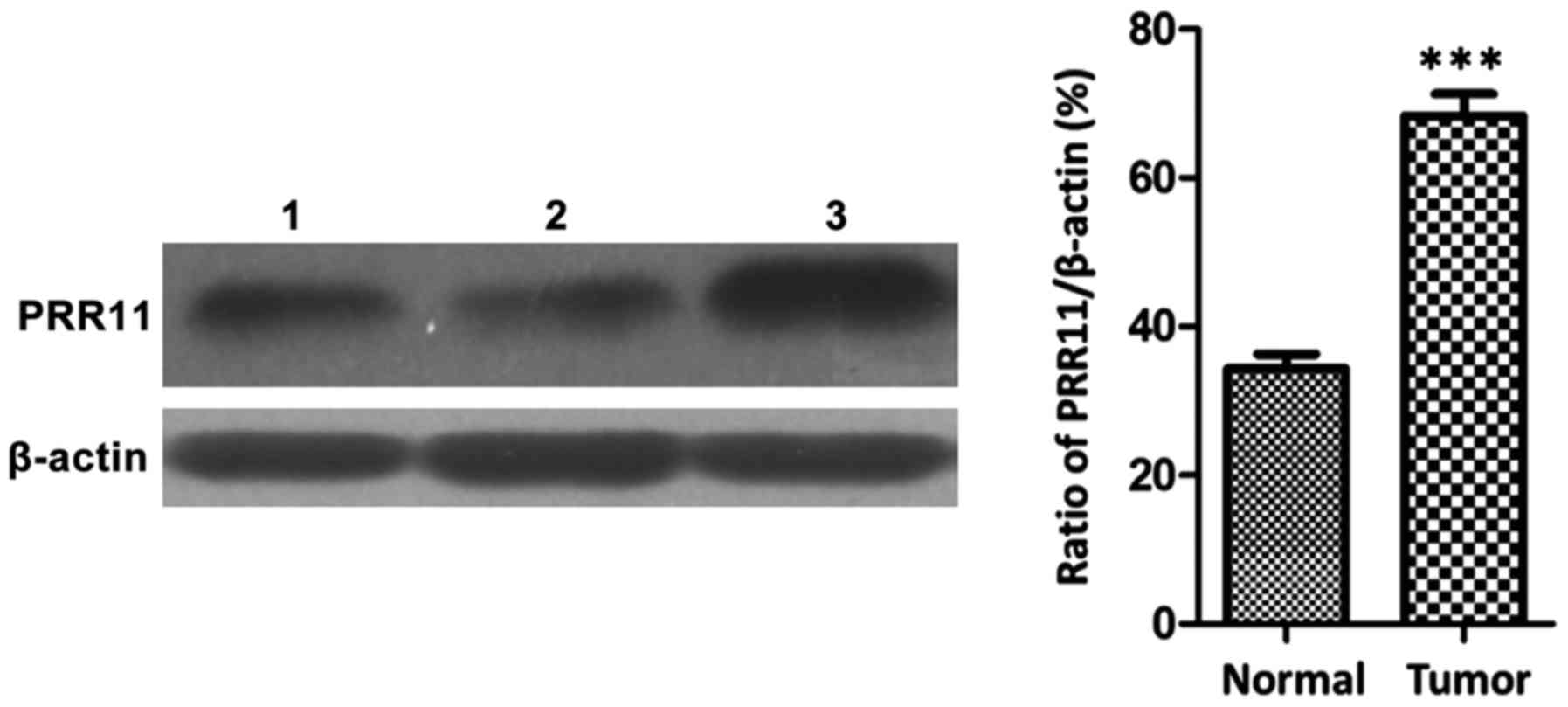
Western blot results showed that the protein
expression of PRR11 in pancreatic cancer tissues was significantly
higher than that in non-cancerous tissues (P<0.001), as shown in
Fig. 2.
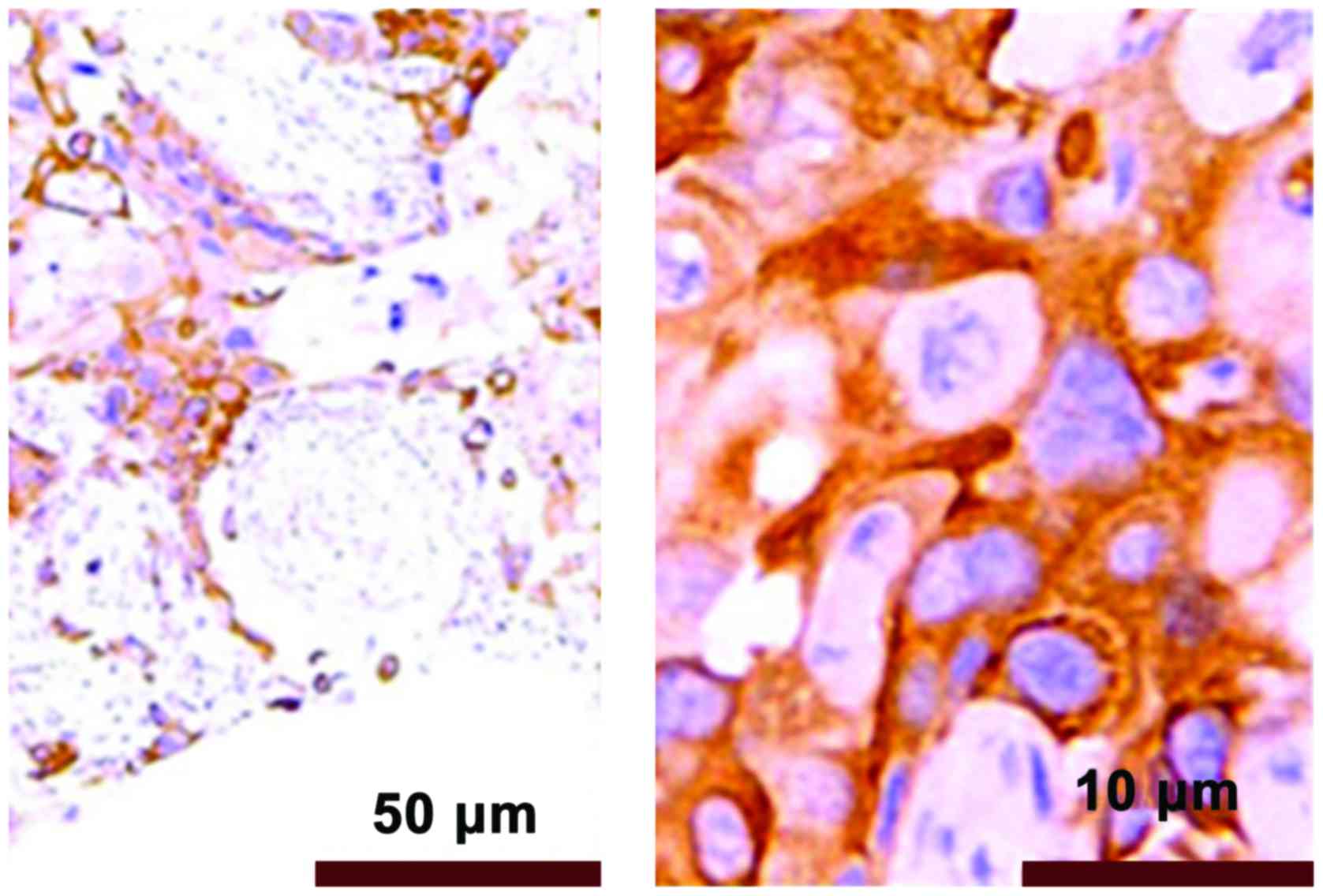
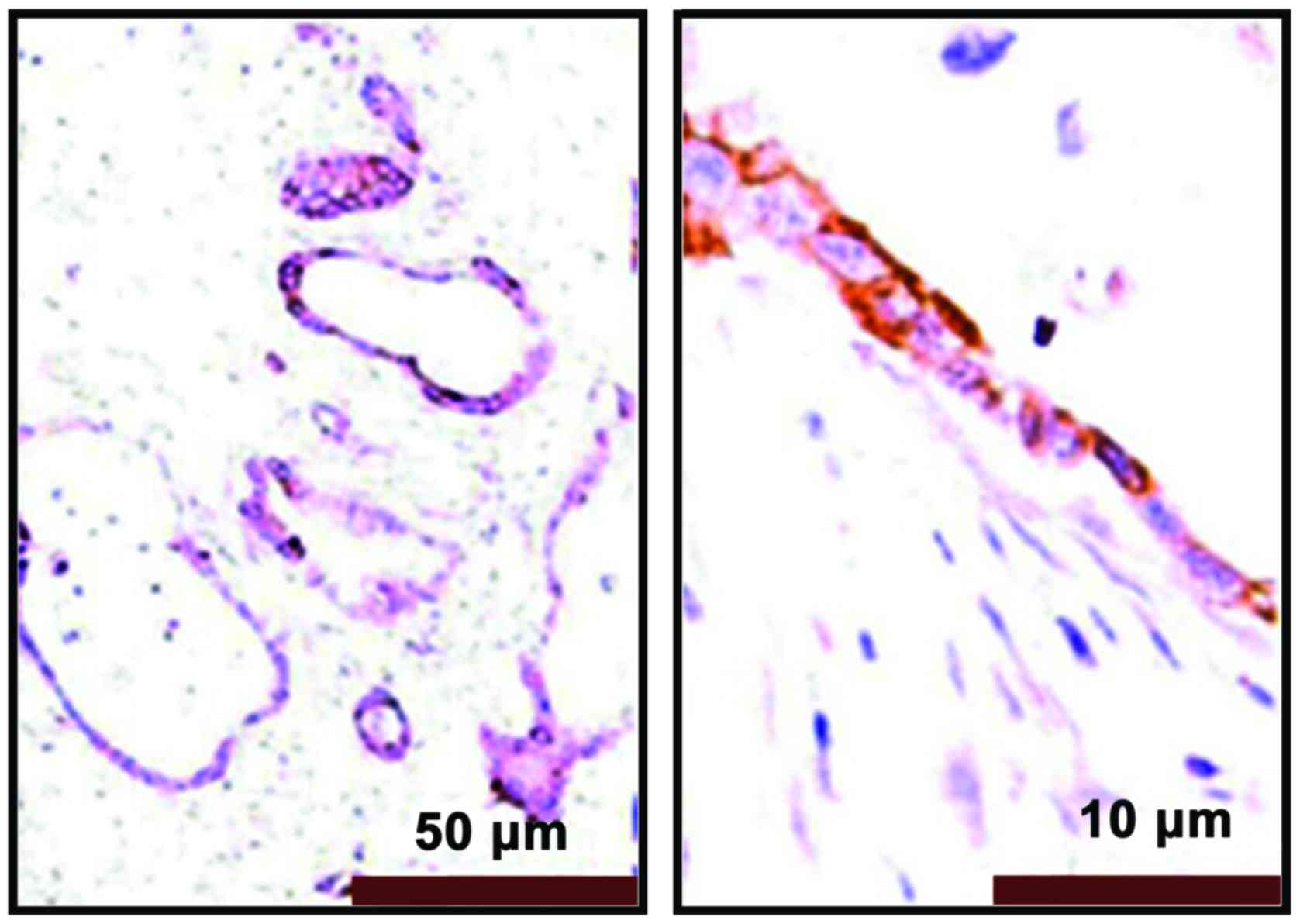
The immunohistochemical results showed that PRR11
positive staining was pale yellow or brown yellow, as shown in
Fig. 3. Non-tumor tissues were
non-stained or took on weak staining, as shown in Fig. 4.
Relationship between the expression of
PRR11 protein and cell migration by the wound healing assay
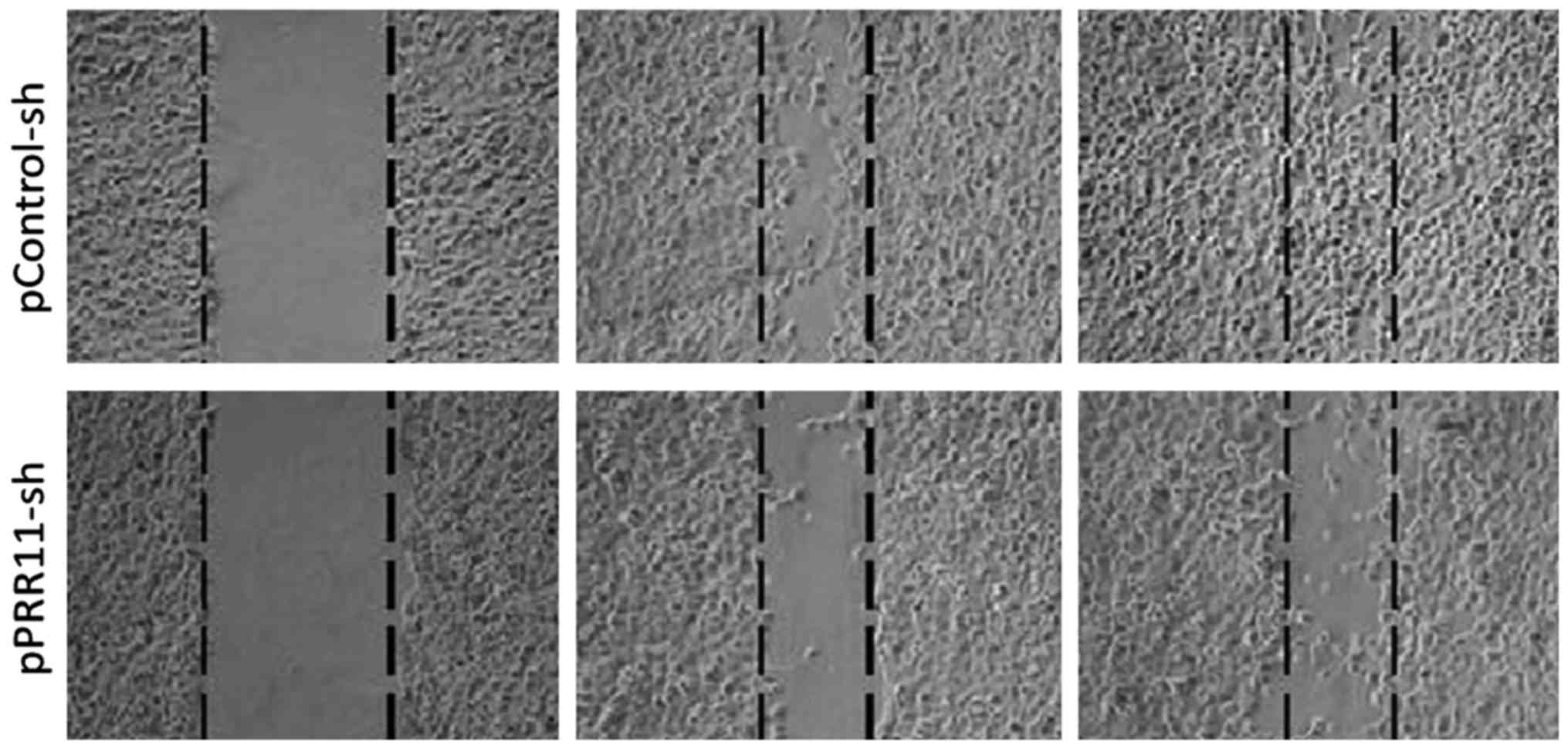
After pPRR11-sh and pControl-sh cell strains were
inoculated onto wells of a 6-well plate, photos were taken at 0, 24
and 48 h to record the changes in cell migration rate. The results
showed that the migration efficiency of the cancer cells with a
PRR11 knockdown was obviously lower than that of the cancer cells
expressing an empty control vector, indicating that inhibition of
the expression of PRR11 may significantly reduce the cell
migration. The results can be seen in Fig. 5.
Relationship between the expression of
PRR11 and clinical parameters
The differences in age and gender between patients
with pancreatic cancer had no effects on the expression of PRR11
protein (P>0.05), while the differentiation degree, clinical TNM
stages and lymphatic metastasis of pancreatic cancer cases were
related to the expression of PRR11 protein (P<0.05). The results
are shown in Table III.
 | Table III.Relationship between the expression of
PRR11 in pancreatic cancer and clinical parameters. |
Table III.
Relationship between the expression of
PRR11 in pancreatic cancer and clinical parameters.
| Parameters | Cases | Positive | Negative | Positive rate
(%) | χ2 | P-value |
|---|
| Age (years) |
| ≥60 | 25 | 20 | 5 | 80.0 |
|
|
|
<60 | 13 | 10 | 3 | 76.9 | 1.013 | P=0.315 |
| Gender |
| Male | 20 | 16 | 4 | 80.0 |
|
|
|
Female | 18 | 14 | 4 | 77.8 | 0.058 | P=0.809 |
| Differentiation
degree |
| High | 8 | 4 | 4 | 50.0 |
|
|
Middle | 18 | 14 | 4 |
77.78 |
| Low | 12 | 12 | 0 | 100.0 | 8.881 | P=0.0118 |
| TNM stages |
| Stage
1 | 10 | 5 | 5 | 50.0 |
|
|
| Stage
2 | 23 | 20 | 3 | 87.0 |
|
|
| Stage
3 | 5 | 5 | 0 | 100.0 | 10.441 | P=0.0054 |
| Presence of lymphatic
metastasis |
| Yes | 10 | 10 | 0 | 100.0 |
|
|
| No | 28 | 20 | 8 | 71.4 | 9.305 | P=0.032 |
Relationship between expression of
PRR11 and prognosis
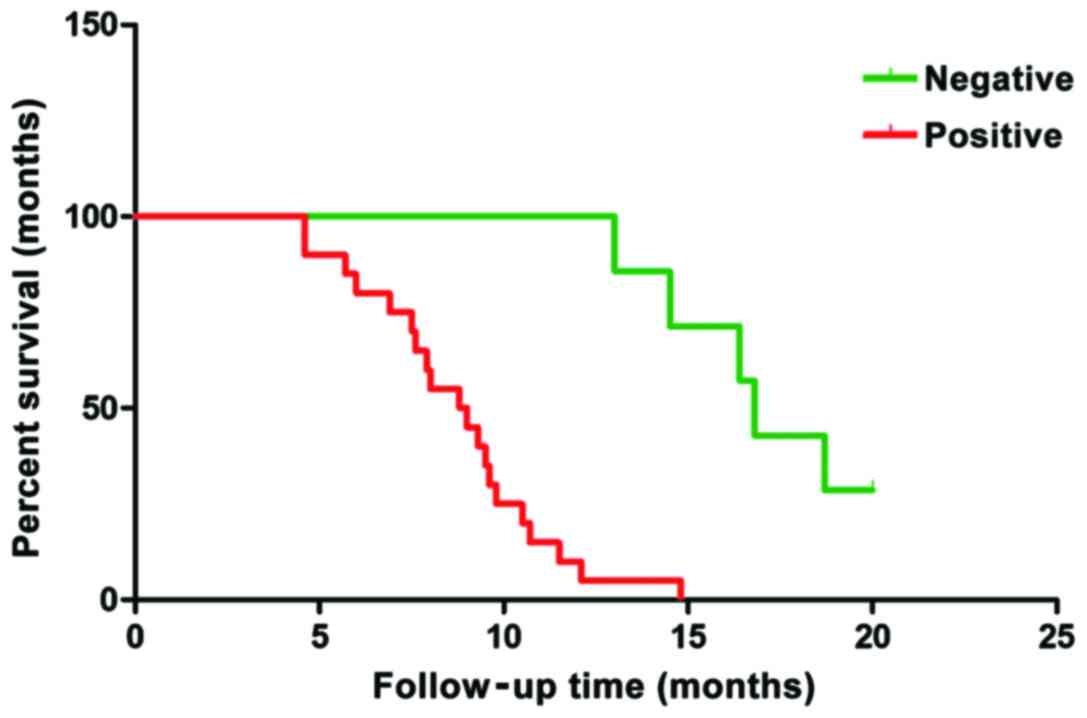
Follow-up was conducted for all the patients with
pancreatic cancer. Their survival time was calculated from the
completion of the operation. The clinical data of the selected
cases were comprehensive and the cut-off time was March 2016. The
analysis of survival data showed that there were 3 cases of failure
to follow-up, 22 cases of death and 5 cases of survival. Among the
death cases, 3 had negative expression and 19 had positive
expression of PRR11 protein. Among the survival cases, 4 cases had
negative expression of PRR11 protein and 1 case had positive
expression of PRR11 protein. A one-way analysis of variance was
applied and a survival curve was drawn (Fig. 6). The survival time of negative
expressors of PRR11 protein was clearly longer than that of
positive expressors. The difference had statistical significance
(P=0.0023) and the results are shown in Table IV and Fig.
6.
 | Table IV.Expression of PRR11 protein and its
relation to the survival time of patients. |
Table IV.
Expression of PRR11 protein and its
relation to the survival time of patients.
| Groups | Cases | Average survival time
(months) | P-value |
|---|
| Negative PRR11 | 7 | 17.06 |
|
| Positive PRR11 | 20 | 7.91 | P=0.0023 |
Discussion
Carrying a high mortality rate, pancreatic cancer is
difficult to cure (8,9). Moreover, its clinical manifestations are
roughly similar to, and even the same as, those of other diseases
of the pancreas, which causes confusion amongst clinicians who
cannot diagnose it accurately. At the same time, since the tumor
deteriorates easily and the metastasis rate is high, the prognosis
of the operation is not good. Researchers have hoped to obtain
progress in the treatment methods by studying a large amount of
cases and treatment protocols in the clinic. However, with the
rapid development of molecular biology in recent years, that
studies on pathological mechanisms at the molecular level are
gaining more knowledge continuously. Some important genes and
proteins influencing the occurrence and development of pancreatic
cancer have been found (10–15). At present, PRR11 is a favorite marker
in cancer studies as it has been proven to affect transcription of
key players, which inhibit cancers and affect progress of various
tumors. Studies have shown the PRR11 protein is mainly distributed
in the cytoplasm and its gene is located in chromosome 17q22
(5). This gene has the highest
expression level in ovaries and the thyroid gland and low
expression levels in brain, heart and other organs. Its expression
level in prostate, cervix uteri and lung tissues is slightly higher
than that of other well-differentiated tissues. Based on this, many
researchers have speculated that this gene may take part in the
process of cell proliferation. Moreover, relevant studies on cancer
tissues also have shown that PRR11 may cause a continuous
deterioration of cancer, as its expression in malignant tumors is
significantly higher than that in normal tissues (16,17).
This study aimed at studying the expression of PRR11
protein in pancreatic cancer. Through analysis of our results, it
was primarily observed that the prognosis of patients was related
to the presence of absence of expression by immunohistochemistry.
The expression of PRR11 protein in the 38 cases of human pancreatic
cancer was higher than that in tissues from normal pancreas. The
inhibition of the expression of PRR11 protein in pancreatic cancer
cells reduced the cell migration capability of the cells,
suggesting that the expression of PRR11 is related with the
invasiveness of the pancreatic cancer.
The analysis of the follow-up data for the
pancreatic cancer patients allowed us to conclude that the 5-year
survival time for those with a PRR11 protein negative
immunohistochemistry was higher than for those with high PRR11
expression. Moreover, it was found that the level of expression of
the PRR11 protein in pancreatic cancer was positively correlated
with invasion, disease stage and tissue differentiation of the
tumor. This result was consistent with that reported in the
literature. In our study, the average survival time of patients
with positive expression of PRR11 protein was 8.52 months, while
the average survival time of patients with negative expression was
16.23 months, suggesting a link between the level of PRR11
expression and the prognosis.
Collectively, this study showed that the levels of
mRNA and protein from the PRR11 gene in the tissues of patients
with pancreatic cancer were significantly higher than those in the
tissues of normal patients. At least in theory, inhibiting the
expression of the PRR11 gene could significantly inhibit the
growth, proliferation, migration and tumorigenic ability of
pancreatic cancer cells. Further studies should focus on the
mechanism of action of PRR11 in the development of pancreatic
cancer and other tumors, as well as on finding levels for diagnosis
and treatment follow-up using PRR11 as a molecular target.
References
|
1
|
Luo J, Adami HO, Reilly M, Ekbom A,
Nordenvall C and Ye W: Interpreting trends of pancreatic cancer
incidence and mortality: a nation-wide study in Sweden (1960–2003).
Cancer Causes Control. 19:89–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beger HG, Rau B, Gansauge F, Leder G,
Schwarz M and Poch B: Pancreatic cancer - low survival rates. Dtsch
Arztebl Int. 105:255–262. 2008.PubMed/NCBI
|
|
3
|
Chen Y, Cha Z, Fang W, Qian B, Yu W, Li W,
Yu G and Gao Y: The prognostic potential and oncogenic effects of
PRR11 expression in hilar cholangiocarcinoma. Oncotarget.
6:20419–20433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ji Y, Xie M, Lan H, Zhang Y, Long Y, Weng
H, Li D, Cai W, Zhu H, Niu Y, et al: PRR11 is a novel gene
implicated in cell cycle progression and lung cancer. Int J Biochem
Cell Biol. 45:645–656. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song Z, Liu W, Xiao Y, Zhang M, Luo Y,
Yuan W, Xu Y, Yu G and Hu Y: PRR11 is a prognostic marker and
potential oncogene in patients with gastric cancer. PLoS One.
10:e01289432015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Zhang Y, Zhang C, Weng H, Li Y,
Cai W, Xie M, Long Y, Ai Q, Liu Z, et al: The gene pair PRR11 and
SKA2 shares a NF-Y-regulated bidirectional promoter and contributes
to lung cancer development. Biochim Biophys Acta. 1849:1133–1144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang C, Zhang Y, Li Y, Zhu H, Wang Y, Cai
W, Zhu J, Ozaki T and Bu Y: PRR11 regulates late-S to G2/M phase
progression and induces premature chromatin condensation (PCC).
Biochem Biophys Res Commun. 458:501–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saif MW: Research in pancreatic cancer: an
update after ASCO 2012. JOP. 13:330–331. 2012.PubMed/NCBI
|
|
9
|
Maisonneuve P and Lowenfels AB:
Epidemiology of pancreatic cancer: an update. Dig Dis. 28:645–656.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grønborg M, Kristiansen TZ, Iwahori A,
Chang R, Reddy R, Sato N, Molina H, Jensen ON, Hruban RH, Goggins
MG, et al: Biomarker discovery from pancreatic cancer secretome
using a differential proteomic approach. Mol Cell Proteomics.
5:157–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stevens L, Pathak S, Nunes QM,
Pandanaboyana S, Macutkiewicz C, Smart N and Smith AM: Prognostic
significance of pre-operative C-reactive protein and the
neutrophil-lymphocyte ratio in resectable pancreatic cancer: a
systematic review. HPB Oxf. 17:285–291. 2015. View Article : Google Scholar
|
|
12
|
Kou T, Kanai M, Yamamoto M, Xue P, Mori Y,
Kudo Y, Kurita A, Uza N, Kodama Y, Asada M, et al: Prognostic model
for survival based on readily available pretreatment factors in
patients with advanced pancreatic cancer receiving palliative
chemotherapy. Int J Clin Oncol. 21:118–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Earl J, Garcia-Nieto S, Martinez-Avila JC,
Montans J, Sanjuanbenito A, Rodríguez-Garrote M, Lisa E, Mendía E,
Lobo E, Malats N, et al: Circulating tumor cells (Ctc) and kras
mutant circulating free DNA (cfDNA) detection in peripheral blood
as biomarkers in patients diagnosed with exocrine pancreatic
cancer. BMC Cancer. 15:7972015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kishikawa T, Otsuka M, Ohno M, Yoshikawa
T, Takata A and Koike K: Circulating RNAs as new biomarkers for
detecting pancreatic cancer. World J Gastroenterol. 21:8527–8540.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu L, Xiang J, Chen R, Fu D, Hong D, Hao
J, Li Y, Li J, Li S, Mou Y, et al: Chinese Study Group for
Pancreatic Cancer (CSPAC): The clinical utility of CA125/MUC16 in
pancreatic cancer: a consensus of diagnostic, prognostic and
predictive updates by the Chinese Study Group for Pancreatic Cancer
(CSPAC). Int J Oncol. 48:900–907. 2016.PubMed/NCBI
|
|
16
|
Zhou F, Liu H, Zhang X, Shen Y, Zheng D,
Zhang A, Lai Y and Li H: Proline-rich protein 11 regulates
epithelial-to-mesenchymal transition to promote breast cancer cell
invasion. Int J Clin Exp Pathol. 7:8692–8699. 2014.PubMed/NCBI
|
|
17
|
Ho HY, Rohatgi R, Ma L and Kirschner MW:
CR16 forms a complex with N-WASP in brain and is a novel member of
a conserved proline-rich actin-binding protein family. Proc Natl
Acad Sci USA. 98:11306–11311. 2001. View Article : Google Scholar : PubMed/NCBI
|