Introduction
Multidrug resistance (MDR) in cancer refers to tumor
cells that not only exhibit resistance to a single drug to which
they have been exposed, but they also develop cross-resistance to
multiple drugs with different structures, cellular targets and
mechanisms of action (1,2). MDR thus reduces the sensitivity of tumor
cells to cytotoxic and targeted chemotherapeutic agents, and is one
reason why individuals with cancer may not respond to other
effective chemotherapeutic regimens. Traditional Chinese medicine
(TCM) is recognized by an increasing number of individuals for its
potential applications in tumor therapy due to the advantages of
its relatively low toxicity, its reported efficacy and its ability
to target multiple cellular pathways. Following a comprehensive
number of studies, it has been demonstrated that various components
of TCM may have the potential to reverse MDR in tumors. TCM may be
particularly effective in tumors in which resistance is mediated
through the elevated expression of the drug transporter membrane
protein, P-glycoprotein (P-gp). The present study provides a brief
review of the field of TCM and P-gp.
Mechanisms of MDR
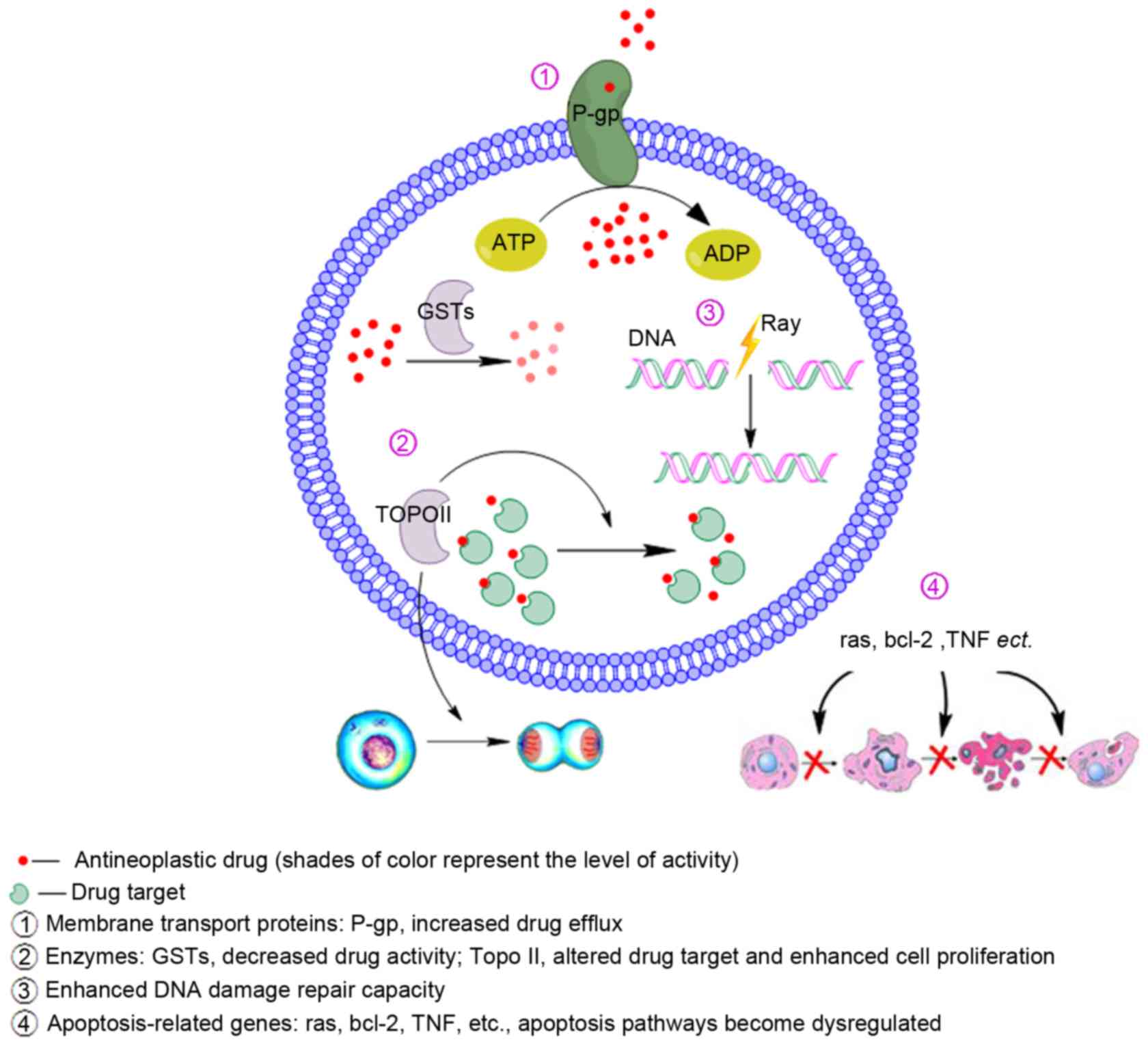
The mechanisms of MDR are multifaceted and complex,
and include several factors that are summarized in Fig. 1. Among these factors are: An adenosine
5′-triphosphate (ATP) dependent decrease in cellular drug
accumulation in tumor cell lines associated with elevated levels of
the 170 kDa drug transporter P-gp, the 190 kDa multidrug resistance
protein 1 (MRP1), or other ATP-binding cassette (ABC) drug efflux
pump (3–5); changes in enzymes involved in
detoxification and metabolism (e.g. increased levels of glutathione
S-transferases and decreased topoisomerase II levels) (6); changes in DNA damage repair capacity of
the tumor cells; and the dysregulation of apoptosis-related genes
(7) (e.g. increased B-cell lymphoma
2, mutation of tumor protein p53 and activation of RAS). Other
factors involved include changes in the tumor microenvironment
in vivo, decreases in cytokine secretion and changes in
hormone levels (8–10). Of all these factors, drug efflux
mediated by P-gp is perhaps the most studied.
As mentioned, P-gp is a member of the ABC
superfamily of membrane proteins, and was first identified in the
plasma membrane of mammalian cells that had been selected for
resistance to drugs (1,11). It is present in a number of normal
tissues (12), and uses the energy
from ATP binding and hydrolysis to effect the conformational
changes in the protein necessary to pump xenobiotics (including
anticancer drugs) across the cell membrane (13,14). Human
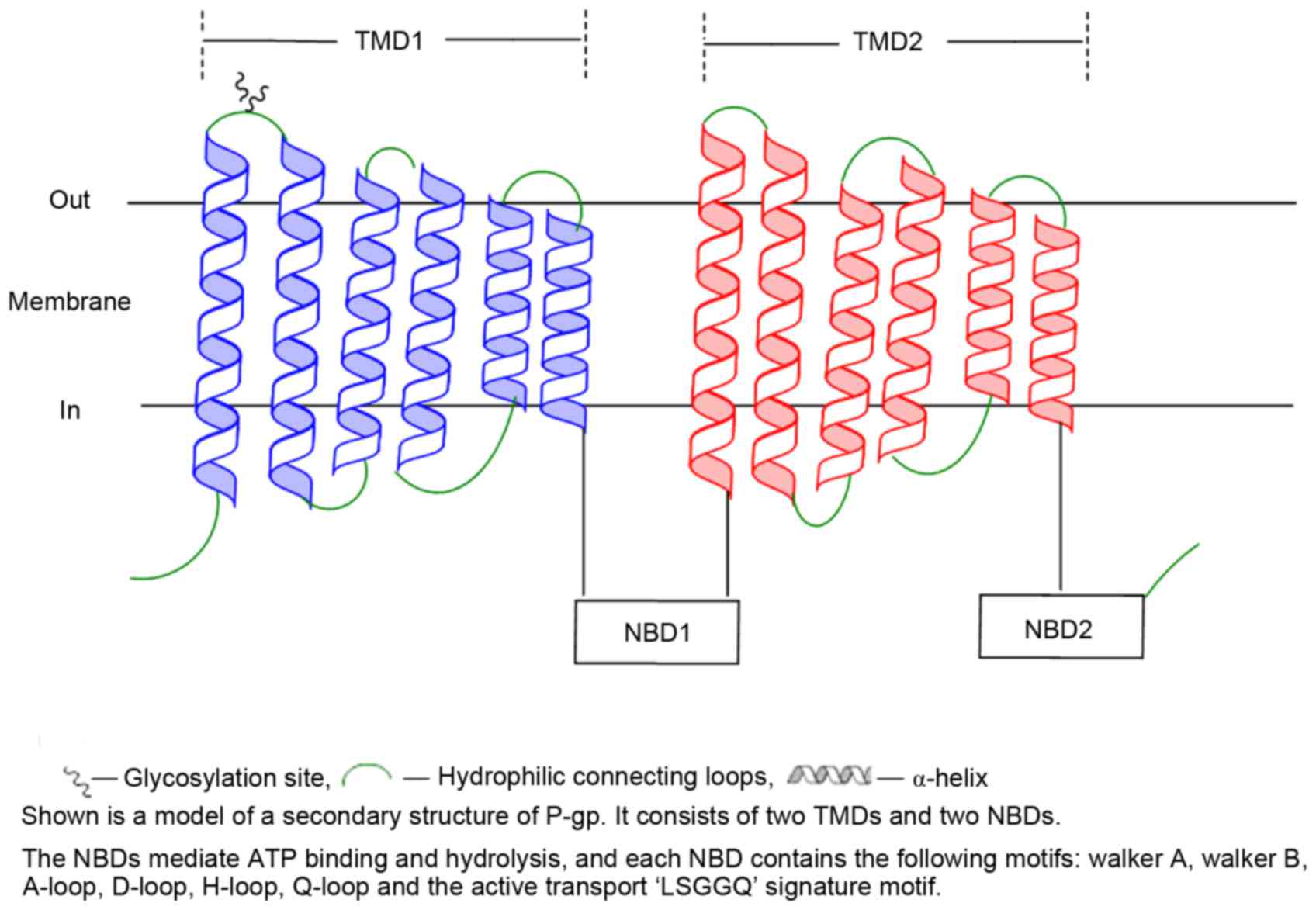
P-gp is encoded by the ATP-binding cassette sub family B member 1
(ABCB1) gene (formerly termed MDR1), and has 1,280 amino acids that
are organized in 2 homologous halves, each containing a hydrophobic
transmembrane domain (TMD) with 6 transmembrane segments (TM), and
a cytoplasmic nucleotide binding domain (NBD) (1,15).
Substrate binding and translocation occurs largely through the
TMDs, while ATP binding and hydrolysis requires the cooperation of
the two NBDs (Fig. 2) (1,13,16).
P-gp as a target for reversing MDR in
cancer
P-gp mediated drug efflux in tumor cells is an
important resistance mechanism, and studies into MDR reversal
agents almost always include this transporter protein as a target.
Numerous small molecule inhibitors of P-gp have been developed
during the last 4 decades (17).
Verapamil was the first small molecule reported to reverse MDR
mediated by P-gp in 1981 (18). The
multiple P-gp MDR reversal agents described since then have been
assigned to one of three generations, according to the timing of
their discovery and development, and their individual features,
selectivity and effectiveness. Although all MDR reversal agents
possess a certain degree of effectiveness, each individual agent
has shortcomings (see Table I for
details).
 | Table I.Selected examples of P-gp multi drug
resistance reversal agents. |
Table I.
Selected examples of P-gp multi drug
resistance reversal agents.
| Generation | Representative
agent |
Characteristics | Shortcomings |
|---|
| 1st | Verapamil,
cyclosporin A, auinidine | Competitive
inhibitors of P-gp | Weakly competitive,
no target specificity, adverse side effects |
| 2nd | R-verapamil,
cyclosporin A derivatives, quinidine analogues | Often structural
analogs of first generation of reversal agents | Alters
metabolism/pharmacokinetics of chemotherapeutic agent |
| 3rd | XR9576, LY335979,
R101933, Tariquidar | Designed according
to structure or activity predictions; more specific,
non-competitive P-gp inhibitors | May inhibit certain
P-gp pump functions in normal cells; may alter
pharmacokinetics |
The majority of small molecule reversal agents
interact with P-gp at its drug binding sites through a competitive
or non-competitive mechanism to inhibit the transport of anticancer
drugs. Despite promising pre-clinical results in experimental
systems, none have yet been approved for clinical use as reversal
agents (19). Studies of certain
reversal agents were terminated due to unacceptable patient
toxicities in clinical trials or apparent lack of efficacy
(20–22). Consequently, the search for effective
and less toxic tumor MDR reversal agents continues to be a target
pursued by numerous studies. An increasing number of non-toxic
natural plant medicines are now being studied for their potential
as MDR reversal agents. A variety of TCM components have been shown
to have good activity with respect to reversing tumor MDR in
experimental model systems. Studies on TCM components as P-gp
reversal agents have mainly been performed in vitro using
cultured cells and in rat models bearing MDR tumors.
In vitro experimental studies on TCM
components as P-gp reversal agents
MDR tumor cell lines with elevated P-gp levels have
been used in vitro to study the effect and mechanism of TCM
components on the reversal of MDR. Frequently, a colorimetric MTT
chemosensitivity assay is used to determine the effect of the P-gp
reversal agent on the IC50 (concentration which inhibits
cell viability by 50%) of the cytotoxic drug as well as the degree
of drug resistance (fold change in resistance). Changes of P-gp
content and the levels of related genes are typically measured in
the cells by reverse transcription-polymerase chain reaction
(RT-PCR), immunoblotting, and/or flow cytometry.
Rh2 ginsenosides
Rh2 ginsenosides are mainly derived from the dry
roots and leaves of Panax ginseng C.A. Meyer (Araliaceae),
and are given to patients with cancer to promote immunity against
cancer through enhancing immune cell activity. Different
concentrations of Rh2 ginsenosides were added to cultured MDR
breast cancer MCF7/ADM cells and then resistance to doxorubicin
(DOX; also termed adriamycin) and 5-fluorouracil (5FU), two agents
commonly used to treat breast cancer clinically, were examined
(23). The Rh2 ginsenosides were also
tested for their ability to influence the fluorescence intensity of
MDR cells incubated in rhodamine 123 as a measure of their effect
on P-gp efflux activity. The Rh2 ginsenosides increased the
sensitivity of MCF7/ADM cells to DOX and 5FU. In addition, the Rh2
ginsenosides significantly inhibited the cellular efflux of
rhodamine 123 from the MDR cells (23). This indicates that Rh2 ginsenosides
can effectively reduce P-gp activity to reverse tumor cell MDR.
Rh2 ginsenosides perform an additional important
role in leukemia and breast cancer cells. In addition to reducing
P-gp activity, they have been demonstrated to decrease the levels
of phospho-protein kinase B (p-AKT) and matrix metalloproteinase-2,
and reduce the invasion and metastasis of MCF7/ADM cells through
the suppression of the phosphoinositide 3-kinase/AKT signaling
pathway (24). Rh2 ginsenosides could
be excellent anti-leukemic agents due to their ability to inhibit
growth, induce apoptosis and reverse the MDR of human leukemia
K562/VCR cell lines (25).
Matrine
Matrine is a tetracycline quinolizidine alkaloid
found mainly in members of the legume genus Sophora flavescens.
Matrine has anti-inflammatory (26,27),
antiviral (28), and antitumor
effects (29–32), and can also reverse MDR (33–35). Thus,
the effect of celecoxib, alone and combined with matrine, on the
resistance of MDR erythroleukemia K562/AO2 cell lines was examined.
Gui et al (33) revealed that
in the presence of matrine, the DOX IC50 in
erythroleukemia K562/AO2 cells was reduced almost 4-fold (from
33.31 to 9.44 µg/ml). The extent of apoptosis also increased from
4.81 to 15.31%. RT-PCR analyses demonstrated that ABCB1 and
cyclooxygenase-2 (COX-2) mRNA levels were downregulated, as were
the levels of the corresponding P-gp and COX-2 proteins. These data
indicate that matrine likely reverses MDR (and enhances
chemosensitivity) by reducing P-gp levels through the
downregulation of ABCB1. In similarly designed studies using MDR
breast cancer MCF-7/ADR (34) and
hepatoma CRBH-7919/MDR1 cell lines (35), similar conclusions were reached that
matrine reverses P-gp mediated MDR.
Quercetin
Quercetin is a natural flavonoid compound that
exists widely in TCM, and in certain vegetables, fruits and grains.
Quercetin appears to act as an MDR reversal agent through a variety
of different mechanisms. For example, Wang et al (36) established a drug resistant glioma cell
line (U87/TR) by exposing drug sensitive cells to temozolomide
(TMZ). The authors determined that, compared with TMZ alone, the
cell survival rate with a combination of quercetin with TMZ was
significantly decreased (P<0.01), and cell toxicity was enhanced
in a dose-dependent manner. Wang et al (37) also revealed that quercetin inhibited
proliferation and enhanced apoptosis of a tamoxifen resistant
breast cancer MCF-7Ca/TAM-R cell line, in a dose-dependent manner.
In addition, Wang et al (38)
reported that quercetin could significantly diminish P-gp activity
in drug resistant lung adenocarcinoma A549/DDP cell lines and
enhance the accumulation of chemotherapeutic agents. Wei et
al (39) and He et al
(40) also reported that quercetin
resulted in a decrease in ABCB1 gene expression.
Emodin
Emodin (EM) is a type of anthraquinone extracted
from rhubarb that possesses a variety of physiological activities,
including inhibiting tumor cell proliferation, promoting apoptosis,
and reversing MDR. EM can augment cisplatin cytotoxicity in
platinum-resistant ovarian cancer COC1/DDP cells via reactive
oxygen species-dependent downregulation of MRP1 (41). Li et al (42) reported that EM (10 µM) could markedly
promote apoptosis in MDR human ovarian A2780/taxol tumor cells. It
could also enhance the sensitivity of these cells to paclitaxel,
and downregulate the intracellular levels of P-gp and inhibitor of
apoptosis protein. Consequently, the authors concluded that the
mechanism of EM-mediated reversal of MDR is associated with
P-gp.
Tetramethylpyrazine
Tetramethylpyrazine (TMP; also termed ligustrazine)
is a type of pyrazine alkaloid that occurs in the chuanqiong TCM.
TMP has calcium channel blocking activity, and thus may be
considered as an MDR reversal agent in tumors. The effect of TMP on
P-gp activity was examined by Yu et al (43) using hepatoma BEL-7402/ADM cells. In
one study, the authors examined 4 groups: BEL-7402 (drug sensitive)
cells; BEL-7402/ADM (drug resistant) cells; BEL-7402/ADM cells
exposed to verapamil (positive control group); and BEL-7402/ADM
exposed to TMP (experimental group). Levels of P-gp were then
measured by flow cytometry and immunohistochemistry. The results
indicated that P-gp expression in BEL-7402/ADM cells was
significantly increased compared with BEL-7402 cells, as expected.
However, the authors also revealed that P-gp expression in
BEL-7402/ADM cells was significantly lower following the treatment
of cells with TMP (P<0.01).
Zhang et al (44) examined the effects of TMP on MDR
breast cancer cells and demonstrated that TMP increased the
intracellular concentration of DOX and inhibited P-gp mediated
efflux of DOX in a dose-dependent manner. Additionally, TMP
inhibited the ATPase activity of P-gp and suppressed the expression
of P-gp in MCF-7/DOX cells.
Baicalin
Baicalin is a flavonoid compound isolated from the
Scutellaria baicalensis root and is reported to demonstrate
antibacterial, anti-inflammatory, anti-allergy and anticancer
activity. Yang et al (45)
observed that baicalin could reverse the resistance of leukemia
K562/ADR cells. Thus the DOX resistance of these cells was reversed
5.2 and 19.3 fold with baicalin at 10 and 20 mg/l, respectively.
Intracellular DOX accumulation was also increased significantly.
The authors concluded that resistance reversal by baicalin may be
associated with its ability to inhibit expression of the ABCB1
gene.
Schizandrin B
It has been reported that 5 schizandrins isolated
from the Chinese herb Fructus schizandrae (FS) could reverse P-gp
mediated MDR (46). Schizandrin B is
the biphenyl cyclooctene lignans present at the highest levels in
FS. It demonstrated effective reversal of drug resistance in
bladder tumor (47), human
osteosarcoma (48) and human colon
cancer cells (49). Pan et al
(50) has reported that Schizandrin B
demonstrated MDR reversal activity in 4 MDR human tumor cell lines,
which express elevated P-gp levels. These cell lines are K562/Adr,
MCF-7/Adr, KBv200 and Bcap37/Adr. Through direct interaction with
P-gp, Schizandrin B reduces drug efflux activity and thus
completely restores the ability of the tumor cells to accumulate
drugs.
5. Studies of TCM components as P-gp reversal
agents in tumor-bearing rat models
TCM components can also reverse P-gp mediated MDR
in vivo as demonstrated in studies conducted on tumor
bearing rats (45,51). The chemosensitizing abilities of TCM
components have been analyzed by studying changes in cell protein
levels (51–54), tumor growth (55) or survival rate of tumor-bearing rats
(56). A TCM component with MDR
reversing activity should show an ability to decrease resistance
related protein expression levels, to inhibit tumor growth or
prolong the survival rate of tumor-bearing rats.
Matrine
In addition to having MDR reversal activity in
vitro as described above, matrine also has activity in intact
mice. Li et al (52) emulated
the clinical chemotherapy of Cisplatin/5-FU/Cytoxan (PFC) to induce
resistance in S180 tumors in mice. Following 10 days of
continuous lavage with matrine solution, the study obtained
S180 cells from mouse ascites. The S180 cells
were then analyzed by flow cytometry, and it was determined that
P-gp levels and the drug target topoisomerase II were both reduced.
The authors concluded that the MDR reversing activity of matrine
was associated with its ability to regulate a variety of drug
resistance-related macromolecules.
Cepharanthine
Cepharanthine is a type of bisbenzylisoquinoline
alkaloid extracted from the radix, stem or leaves of Menispermaceae
stephania. It has been reported that cepharanthine hydrochloride
can downregulate the ABCB1 gene, and may activate c-Jun/c-Jun
N-terminal kinases in K562/ADR cells (53). Due to the difficulty of directly
analyzing intratumoral drug concentrations or P-gp changes in
vivo, Han (54) established an
alternative surrogate method that took advantage of the presence of
P-gp in peripheral CD8+ lymphocytes. Thus, P-gp activity
was measured in CD8+ peripheral lymphocytes rather than
in whole tumor bearing rats. The mean fluorescence intensity (MFI)
of CD8+ cells incubated with the fluorescent P-gp
substrate Rhodamine123 was used as a measure of P-gp activity, and
used to study the MDR reversing effect of cepharanthine
hydrochloride in vivo. Different doses of cepharanthine
(2.5, 5.0 and 10.0 mg/kg), verapamil (5.0 mg/kg; positive control)
and saline (vehicle control) were injected in hepatoma
tumor-bearing mice (Hca/Fap), which had received tail-vein
injections of Rhodamine 123. The MFI was 8.6±0.4 in the absence of
cepharanthine or verapamil. Following cepharanthine treatment, the
MFI in CD8+ peripheral lymphocytes increased in a
dose-dependent manner to 18.9±0.8, 13.1±0.8 and 11.9±0.4,
respectively; following treatment with verapamil, the MFI was
10.2±0.2. The differences among the groups were statistically
significant (P<0.05), indicating that cepharanthine can reverse
MDR by inhibiting the P-gp activity.
Curcumin
In a study by Lu et al (55), human colon tumor HCT-8/VCR cells were
implanted in nude mice to establish MDR tumor-bearing mice. The
mice were then divided into 4 groups, and administered saline
(vehicle control), vincristine (VCR) alone, curcumin alone, and VCR
+ curcumin together, respectively. The tumors were excised and
weighed following 2 weeks of treatment, and RT-PCR and
immunoblotting were used to detect levels of ABC1 and survivin
mRNAs as well as P-gp and survivin proteins in tumor tissue,
respectively. The tumor mass and levels of ABCB1 mRNA, survivin
mRNA and P-gp and survivin proteins in the VCR/curcumin combination
group and the curcumin alone group were significantly lower than
the control (saline) group and VCR alone group, indicating that
curcumin is more effective than VCR in MDR. Other studies have also
shown that curcumin could inhibit the migration and invasion of
Hca-F cells (57), and induce
apoptosis in gallbladder carcinoma GBC-SD cells (58).
Other TCM components
Diallyltrisulfide could overcome P-gp-mediated MDR
in K562/A02 cells by the downregulation of nuclear factor-κB
(NF-κB)/p65 (59). In addition, grape
seed procyanidin (GSP) belongs to a class of polyphenol flavonoid
compounds that significantly increase the efficacy of paclitaxel
and adriamycin in A2780/T cells by blocking the function of P-gp
and inhibiting the transcription of ABCB1. Additionally, GSP
suppressed the NF-kB activity and mitogen activated protein kinase
(MAPK)/extracellular signal-related kinases (ERK) pathway mediated
YB-1 nuclear translocation, which may be associated with the
downregulation of P-gp (60).
Annonaceous acetogenins can also reverse MDR by
reducing P-gp pump function and increasing the intracellular
concentration of chemotherapeutic drugs. Thus, 15 annonaceous
acetogenins demonstrated significant inhibitory activities against
MCF-7/ADR cells; among them, annotemoyin-1 was 190X more active
than verapamil (61).
Tetrandrine also significantly reduced P-gp
expression in a concentration-dependent manner, and thus can
reverse MDR by increasing the intracellular concentration of
anticancer drugs (62,63). In addition, the study demonstrated
that H1 (a novel derivative of tetrandrine) inhibited P-gp
expression in a dose-dependent manner by promoting P-gp degradation
apparently through decreasing its half-life, which may be
associated with a downregulated MAPK-ERK signaling pathway. H1 also
inhibited the ATPase activity of P-gp in a dose-dependent manner
(64). Psoralen (65), neferine (66,67),
peimine (68,69), guggulsterone (69,70) and
artemisinin (71) are also reported
to reverse MDR by reducing P-gp expression or promoting the ATPase
activity of P-gp in drug-resistant tumor cells (for details of TCM
components, see Table II).
 | Table II.Physicochemical characteristics of
selected TCM components. |
Conclusions
Reversing MDR in cancer is a challenging task, and
over the past several decades (72,73), a
number of studies have proposed numerous strategies to combat it
and 4 generations of small molecule MDR P-gp inhibitors have been
reported (17). Among these are a
variety of TCM components that have been shown to reverse P-gp
mediated MDR by several mechanisms including inhibiting P-gp drug
efflux activity, hindering P-gp ATPase activity and reducing P-gp
levels by downregulating the expression levels of the ABCB1 gene.
Despite these interesting observations, there are still a number of
challenges that need to be addressed. Firstly, MDR is the result of
numerous biochemical and cellular factors. Therefore, if studies
remain narrowly focused on the classical resistance mechanism
mediated by P-gp (i.e. a single target), the contributions of other
resistance mechanisms will remain unexplored. Secondly, studies to
date have mostly used cultured MDR tumor cell model systems. By
contrast, studies utilizing tumor-bearing animals are relatively
rare, and clinical studies with human patients even rarer. In
addition, the majority of studies use TCM components in their
purified forms, and studies of TCM components in relevant and
appropriate formulations should also be examined.
To date, no single compound (or combination of
compounds) has been used as a MDR reversal agent successfully in
the clinic. However, as TCM components with higher efficacy and
lower toxicity, and with confirmed MDR reversing mechanisms are
identified, it remains a promising prospect. Additional studies are
also required to explore the influence of TCM components on
pharmacokinetic processes in vitro and in vivo in
order to choose the optimal formulation and dosage for clinical
cancer treatment.
Acknowledgements
The present work was supported in part by The
National Natural Science Foundation of China (grant nos. 81273707
and 81173215), The Ministry of Education in the New Century
Excellent Talents (grant no. NECT-12-0677), the Natural Science
Foundation of Guangdong (grant no. S2013010012880), the Science and
Technology Program of Guangzhou (grant no. 2014J4500005), the
Science Program of Department of Education of Guangdong (grant nos.
2013KJCX0021 and 2015KGJHZ012) and the Science and Technology
Program of Guangdong (grant no. 2015A050502027).
Glossary
Abbreviations
Abbreviations:
|
MDR
|
multidrug resistance
|
|
TCM
|
traditional Chinese medicine
|
|
P-gp
|
P-glycoprotein
|
|
MRP1
|
multidrug resistance protein 1
|
|
TMD
|
transmembrane domain
|
|
TM
|
transmembrane segment
|
|
NBD
|
nucleotide binding domain
|
References
|
1
|
Gottesman MM and Ling V: The molecular
basis of multidrug resistance in cancer: The early years of
P-glycoprotein research. FEBS Lett. 580:998–1009. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi CH: ABC transporters as multidrug
resistance mechanisms and the development of chemosensitizers for
their reversal. Cancer Cell Int. 5:302005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leslie EM, Deeley RG and Cole SP:
Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2,
and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol.
204:216–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cole SP: Targeting multidrug resistance
protein 1 (MRP1, ABCC1): Past, present, and future. Annu Rev
Pharmacol Toxicol. 54:95–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharom FJ: ABC multidrug transporters:
Structure, function and role in chemoresistance. Pharmacogenomics.
9:105–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chikamori K, Grozav AG, Kozuki T,
Grabowski D, Ganapathi R and Ganapathi MK: DNA topoisomerase II
enzymes as molecular targets for cancer chemotherapy. Curr Cancer
Drug Targets. 10:758–771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kartal-Yandim M, Adan-Gokbulut A and Baran
Y: Molecular mechanisms of drug resistance and its reversal in
cancer. Crit Rev Biotechnol. 36:716–726. 2016.PubMed/NCBI
|
|
8
|
Ren SX: The potential of Chinese herb on
multidrug resistance. Pharmacy Clinics Chinese Materia Med.
3:57–60. 2012.
|
|
9
|
Liao SL and Wang P: Research progress on
multidrug resistance mechanism of cancer cells and its reversal
agents. Guowai Yiyao Kangshengsu Fence. 1:7–11. 2008.(In
Chinese).
|
|
10
|
Pan GD and Yan L: Advances in gene therapy
of multidrug resistance reversal induced by Mdr1. Huaxi Yixue.
22:205–207. 2007.(In Chinese).
|
|
11
|
Sharom FJ: The P-glycoprotein multidrug
transporter. Essays Biochem. 50:161–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cascorbi I: P-glycoprotein: Tissue
distribution, substrates, and functional consequences of genetic
variations. Handb Exp Pharmacol. 261–283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones PM and George AM: Mechanism of the
ABC transporter ATPase domains: Catalytic models and the
biochemical and biophysical record. Crit Rev Biochem Mol Biol.
48:39–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ward AB, Szewczyk P, Grimard V, Lee CW,
Martinez L, Doshi R, Caya A, Villaluz M, Pardon E, Cregger C, et
al: Structures of P-glycoprotein reveal its conformational
flexibility and an epitope on the nucleotide-binding domain. Proc
Natl Acad Sci USA. 110:13386–13391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ueda K, Cornwell MM, Gottesman MM, Pastan
I, Roninson IB, Ling V and Riordan JR: The mdr1 gene, responsible
for multidrug-resistance, codes for P-glycoprotein. Biochem Biophys
Res Commun. 141:956–962. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loo TW and Clarke DM: Recent progress in
understanding the mechanism of P-glycoprotein-mediated drug efflux.
J Membr Biol. 206:173–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crowley E, McDevitt CA and Callaghan R:
Generating inhibitors of P-glycoprotein: Where to, now? Methods Mol
Biol. 596:405–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsuruo T, Iida H, Tsukagoshi S and Sakurai
Y: Overcoming of vincristine resistance in P388 leukemia in vivo
and in vitro through enhanced toxicity of vincristine and
vinblastine by verapamil. Cancer Res. 41:1967–1972. 1981.PubMed/NCBI
|
|
19
|
Tamaki A, Ierano C, Szakacs G, Robey RW
and Bates SE: The controversial role of ABC transporters in
clinical oncology. Essays Biochem. 50:209–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang SC, Xu J and Cai SH: Research
progress of targeted reverse MDR mediated by P-glycoprotein. Huaxi
Yaoxue Zazhi. 24:551–554. 2009.(In Chinese).
|
|
21
|
Shen XL, Hu YJ, Yu ZL and Fong WF:
Research progress on reversal of multidrug resistance of
P-glycoprotein mediated by Chinese Herbs. Chinese J Natural Med.
7:465–475. 2009. View Article : Google Scholar
|
|
22
|
Pusztai L, Wagner P, Ibrahim N, Rivera E,
Theriault R, Booser D, Symmans FW, Wong F, Blumenschein G, Fleming
DR, et al: Phase II study of tariquidar, a selective P-glycoprotein
inhibitor, in patients with chemotherapy resistant, advanced breast
carcinoma. Cancer. 104:682–691. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li P and Chen S: Study of the ginsenoside
Rh2 reversal MCF-7/ADM multidrug resistance. Guide Chin Med.
11:8–10. 2013.(In Chinese).
|
|
24
|
Piao L, Cai Y, Zhang M, Jiang J, Jin Z and
Xu Z: Effects of gensenoside Rh2 combined with PI3K/AKT pathway
inhibitor LY294002 on invasion and migration of breast cancer
cells. China Pharmacy. 24:4050–4052. 2013.
|
|
25
|
Xu X, Shi S, Tang Y, Shen H and Qian B:
Therapeutic effects of ginsenoside Rh2 on multi-drug resistant
leukemia cell line K562/VCR. Chin Trad Herbal Drugs. 41:1131–1135.
2010.(In Chinese).
|
|
26
|
Zhang B, Liu ZY, Li YY, Luo Y, Liu ML,
Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG and Li ZC:
Antiinflammatory effects of matrine in LPS-induced acute lung
injury in mice. Eur J Pharm Sci. 44:573–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhaowu Z, Xiaoli W, Yangde Z and Nianfeng
L: Preparation of matrine ethosome, its percutaneous permeation in
vitro and anti-inflammatory activity in vivo in rats. J Liposome
Res. 19:155–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Xiu J, Zhang X, Zhang L, Yan K,
Qin C and Liu J: Antiviral effect of matrine against human
enterovirus 71. Molecules. 17:10370–10376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li LQ, Li XL, Wang L, Du WJ, Guo R, Liang
HH, Liu X, Liang DS, Lu YJ, Shan HL and Jiang HC: Matrine inhibits
breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells.
Cell Physiol Biochem. 30:631–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin H, Sun Y, Wang S and Cheng X: Matrine
activates PTEN to induce growth inhibition and apoptosis in
V600EBRAF harboring melanoma cells. Int J Mol Sci. 14:16040–16057.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J,
Zhang S, Wu J, Yu K and Han Y: Matrine induces apoptosis in human
acute myeloid leukemia cells via the mitochondrial pathway and Akt
inactivation. PLoS One. 7:e468532012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan F, Liu Y and Wang W: Matrine inhibited
the growth of rat osteosarcoma UMR-108 cells by inducing apoptosis
in a mitochondrial-caspase-dependent pathway. Tumour Biol.
34:2135–2140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gui L, Li J, Chen B, Gao F, Wang J and Cai
X: Effect of COX-2 inhibitor celecoxib, matrine and combination on
multidrug resistance of K562/AO2 cell line. J Southeast Univ (Med
Sci Edi). 31:174–178. 2012.
|
|
34
|
Wei C, Shen Y, Zhang Z, Wang J and Zhou B:
Matrine reverses multidrug resistance in breast cancer
drug-resistant cell line MCF-7/ADR through inhibiting PI3K/AKT
signal pathway. J Third Mil Med Univ. 36:2254–2258. 2014.
|
|
35
|
Zhan T: Matrine induces apoptosis of
multidrug resistant CRBH-7919/mdr1 cells. Jilin Univ 1–84. 2012.(In
Chinese).
|
|
36
|
Wang J, Wang G and Liu M: Effects of
quercetin on reversion of temozolomide resistance in U87/TR cell
line. Yiyao Dao Bao. 32:710–714. 2013.(In Chinese).
|
|
37
|
Wang H, Zhang H, Tao L, et al:
Quercetin-induced strengthening efficiency to tamoxifen in
tamoxifen-resistant breast cancer cells. Chin J Modern Med.
24:39–44. 2014.(In Chinese).
|
|
38
|
Wang Y, Liu Z, Zhang Q, Gao X, Zhang L and
Liu H: Effect of quercetin on multidrug resistance reversal of lung
adenocarcinoma cell line A549/DDP. J Binzhou Med Univ. 26:86–89.
2013.
|
|
39
|
Wei Y, Zhang H and Liang G: The reverse
effect of quercetin on multidrug resistance of human hepatocellular
carcinoma. Tianjin Med J. 40:1022–1025. 2013.
|
|
40
|
He HL, Ji LJ, Li QZ, Zhang R, Huang JM and
Li G: Effect of quercetin on doxorubicin-induced expression of MDR1
gene in HL-60 cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 23:70–76.
2015.(In Chinese). PubMed/NCBI
|
|
41
|
Ma J, Yang J and Shen H: The chemotherapy
sensitivity analysis of the impact of reactive oxygen species on
ovarian cancer cells COC1 and COC1/DDP. Biol Med Res Intl.
30:535–539. 2014.(In Chinese).
|
|
42
|
Li J, Liu P, Mao H, Wanga A and Zhang X:
Emodin sensitizes paclitaxel-resistant human ovarian cancer cells
to paclitaxel-induced apoptosis in vitro. Oncol Rep. 21:1605–1610.
2009.PubMed/NCBI
|
|
43
|
Yu X, Wang S, Cao Y, Mo C, Li X and Li M:
Effects of tetramethylpyrazine on P-glycoprotein in human drug
resistant hepatocellular carcinoma cells BEL-7402/ADM. J Guangdong
Pharmaceut Coll. 26:635–639. 2010.(In Chinese).
|
|
44
|
Zhang Y, Liu X, Zuo T, Liu Y and Zhang JH:
Tetramethylpyrazine reverses multidrug resistance in breast cancer
cells through regulating the expression and function of
P-glycoprotein. Med Oncol. 29:534–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang L, Zhao T, Bai Q and Dong H: Reversal
effect and mechanism induced by baicalin on leukemia cell line.
Shaxi Yixue Zazhi. 7:775–779. 2012.(In Chinese).
|
|
46
|
Huang M, Jin J, Sun H and Liu GT: Reversal
of P-glycoprotein-mediated multidrug resistance of cancer cells by
five schizandrins isolated from the Chinese herb Fructus
Schizandrae. Cancer Chemother Pharmacol. 62:1015–1026. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang M, Liu H, Yan M and Liu Z: Reversal
effect of SchB on bladder tumor multidrug resistance. Natl Med
Front Chin. 5:28–29. 2010.(In Chinese).
|
|
48
|
Li QP and Gai YN: Schisandrin B reverses
multidrug resistance due to MDR1-mediatied human osteosarcoma cell
line U-2 OS/ADR. Anhui Med Pharmaceut J. 18:1642–1645. 2014.
|
|
49
|
Xu Q, Jin X, Guo R, Zhang X and Liu G:
Schisandrin B reverses multidrug resistance of human colon cancer
cells. Food Nutr Chin. 17:64–66. 2011.(In Chinese).
|
|
50
|
Qiangrong P, Wang T, Lu Q and Hu X:
Schisandrin B-a novel inhibitor of P-glycoprotein. Biochem Biophys
Res Commun. 335:406–411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
He JB, Liao HZ, Zhang YQ, et al: Reversal
effect of ginsenoside Rg3 on multidrug resistance of lung
adenocarcinoma in mice. J Practical Oncol. 4:373–377. 2006.(In
Chinese).
|
|
52
|
Li G, Wang M, Sun F, Wang X, Li X and Yin
G: Reversal effect of multidrug resistance associated gene
overexpression mediated induced by matrine on S180-tumor-bearing
mice. J Chin Med Mater. 29:40–42. 2006.(In Chinese).
|
|
53
|
Han L, Wang Y, Guo X, Zhou Y, Zhang J,
Wang N, Jiang J, Ma F and Wang Q: Downregulation of MDR1 gene by
cepharanthine hydrochloride is related to the activation of
c-Jun/JNK in K562/ADR cells. Biomed Res Int. 2014:1643912014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Han L: The multidrug resistance reversal
effect of cepharanthine hydrochloride and its mechanism. Zhengzhou:
Zhengzhou Univ 1–119. 2013.(In Chinese).
|
|
55
|
Lu W, Fu Z, Qin Y, Li L and Yang C:
Curcumin reverses multidrug resistance in HCT-8/VCR nude mice
xenograft. Di San Jun Yi Da Xue Xue Bao Bian Ji Bu. 33:376–380.
2011.(In Chinese).
|
|
56
|
Xu DH: Overcome multidrug resistance by
doxorubicin nanocarriers. Zhejiang Univ 1–153. 2008.(In
Chinese).
|
|
57
|
Wang S, Yu S, Shi W, Ge L, Yu X, Fan J and
Zhang J: Curcumin inhibits the migration and invasion of mouse
hepatoma Hca-F cells through down-regulating caveolin-1 expression
and epidermal growth factor receptor signaling. IUBMB Life.
63:775–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu TY, Tan ZJ, Jiang L, Gu JF, Wu XS, Cao
Y, Li ML, Wu KJ and Liu YB: Curcumin induces apoptosis in
gallbladder carcinoma cell line GBC-SD cells. Cancer Cell Int.
13:642013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xia Q, Wang ZY, Li HQ, Diao YT, Li XL, Cui
J, Chen XL and Li H: Reversion of P-glycoprotein mediated multidrug
resistance in human leukemic cell line by diallyl trisulfide. Evid
Based Complement Alternat Med. 2012:7198052012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao B: Molecular mechanisms of multi-drug
resistance reversal via inhibition of P-glycoprotein function and
expression by Grape seed procyanidin. Guangdong: Southern Medical
Univ 1–210. 2014.(In Chinese).
|
|
61
|
Yuan F, Bai G, Miao YJ, Chen Y, Chen JW
and Li X: Effects of annonaceous acetogenins against multidrug
resistant human breast cancer cell line MCF-7/ADR in vitro. Chin
Trad Herbal Drugs. 45:2815–2819. 2014.
|
|
62
|
Sun YF and Wink M: Tetrandrine and
fangchinoline, bisbenzylisoquinoline alkaloids from Stephania
tetrandracan reverse multidrug resistance by inhibiting
P-glycoprotein activity in multidrug resistant human cancer cells.
Phytomedicine. 21:1110–1119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Susa M, Choy E, Yang C, Schwab J, Mankin
H, Hornicek F and Duan Z: Multidrug resistance reversal agent,
NSC77037, identified with a cell-based screening assay. J Biomol
Screen. 15:287–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wei N, Sun H, Wang F and Liu G: H1, a
novel derivative of tetrandrine reverse P-glycoprotein-mediated
multidrug resistance by inhibiting transport function and
expression of P-glycoprotein. Cancer Chemother Pharmacol.
67:1017–1025. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cai Y and Cai T: Reversal effect of
psoralen on leukemia cell K562/ADR multidrug resistance. Zhongguo
Yaolixue Tongbao. 1164–1166. 2003.(In Chinese).
|
|
66
|
Qing Q, Xiao X and Xie Z: Effect of
neferine combined with mdr-1shRNA on the expression of mdr-1/P-gp
in K562/A02 cell line. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
35:445–450. 2010.(In Chinese). PubMed/NCBI
|
|
67
|
Huang C, Cao P, Xie Z and Zhu H: Effect of
different heating methods combined with neferine on the expressions
of γH2AX and mdr-1/P-gp in MCF-7/Adr breast cancer cells. Zhong Nan
Da Xue Xue Bao Yi Xue Ban. 36:317–322. 2011.(In Chinese).
PubMed/NCBI
|
|
68
|
Li Z, An C, Hu K, Zhou K, Duan H and Tang
M: Multidrug resistance reversal activity of total alkaloid from
Fritillaria thunbergii on cisplatin-resistant human lung
adenocarcinoma A549/DDP cells. Chin J Pharmacol Toxicol.
27:315–320. 2013.
|
|
69
|
Xu HB, Li L and Liu GQ: Reversal of
P-glycoprotein-mediated multidrug resistance by guggulsterone in
doxorubicin-resistant human myelogenous leukemia (K562/DOX) cells.
Pharmazie. 64:660–665. 2009.PubMed/NCBI
|
|
70
|
Xu HB, Xu LZ, Li L, Fu J and Mao XP:
Reversion of P-glycoprotein-mediated multidrug resistance by
guggulsterone in multidrug-resistant human cancer cell lines. Eur J
Pharmacol. 694:39–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yu H, Cui L and Pan Y: Reversal of drug
resistance in multidrug-resistant MCF-7/ADR cells of breast cancer
by artemisinin. Acta Med Univ Sci Technol Huazhong. 40:91–94.
2011.(In Chinese).
|
|
72
|
Liao K, Niu F, Hao H and Wang G: Advances
on structure-activity relationship of NQO1-target antitumor
quinones. Chin J Nat Med. 10:170–176. 2012. View Article : Google Scholar
|
|
73
|
Liao SG, Wang Z, Li J, Liu Y, Li YT, Zhang
LJ, Long QD and Wang YL: Cytotoxic sesquiterpene lactones from
Vernonia bockiana. Chin J Nat Med. 10:230–233. 2012. View Article : Google Scholar
|