Introduction
Patients with locally advanced or metastatic
urothelial cancer of the bladder exhibit poor prognoses, with an
overall survival of 12–14 months subsequent to first-line
chemotherapy with the combination of gemcitabine and cisplatin as
standard of care. Treatment failure is commonly caused by
resistance acquisition to chemotherapy subsequent to the primary
response (1,2).
In Europe, vinflunine is the only approved
second-line chemotherapy, with only moderate response rates
(3). There is currently no USA Food
and Drug Administration (FDA)-approved treatment subsequent to
first-line chemotherapy in the USA. However, taxanes are commonly
used for palliative chemotherapy based on modest response rates in
several small, nonrandomized phase II trials (4).
Nanoparticle albumin-bound (nab)-paclitaxel, also
termed Abraxane®, is already widely used in the clinical
treatment of breast cancer with FDA approval in 2005, non-small
cell lung cancer (NSCLC) with FDA approval in 2010 and pancreatic
cancer with FDA approval in 2013 (5,6).
Nab-paclitaxel achieved a marked overall response rate of 27.7% in
patients with metastatic urothelial cancer of the bladder who were
pretreated with cisplatin, and may be more effective than
conventional paclitaxel (7). At
present, a randomized phase II trial [National Clinical Trials
(NCT) no., 02033993] comparing nab-paclitaxel and paclitaxel is
ongoing.
It has been postulated that the increased efficacy
of nab-paclitaxel compared with that of paclitaxel is based on an
increased transendothelial glycoprotein (gp)60-mediated transport
and enhanced intratumoral accumulation as a result of the secreted
protein acidic and rich in cysteine (SPARC)-albumin interaction
(5,8,9). Dong
et al (10) demonstrated that
paclitaxel-loaded lipid-based nanoparticles containing the Brij 78
surfactant may overcome adenosine 5′-triphosphate-binding cassette
transporter subfamily B, member 1 (ABCB1)-mediated drug resistance.
By contrast, other studies hypothesized that resistance to
nanoparticle-bound paclitaxel may also be ABCB1 mediated (11,12).
However, it remains unclear whether albumin-bound paclitaxel
nanoparticles may overcome drug resistance caused by ABCB1
(5).
The present study evaluated whether ABCB1
transporters affect the antitumoral activity of nab-paclitaxel in a
panel of urothelial cancer cell lines. The results demonstrate that
ABCB1 overexpression mediates resistance to nab-paclitaxel.
Resistance to nab-paclitaxel may be overcome by inhibitors of ABCB1
transporters, including cabozantinib and crizotinib, two
FDA-approved small molecule inhibitors being tested at present as
second-line therapy for urothelial carcinoma (NCT nos., 02612194,
01688999 and 02496208).
Materials and methods
Drugs
Cisplatin was purchased from Gry-Pharma GmbH
(Dresden, Germany), gemcitabine from Lilly Germany GmbH (Bad
Homburg, Germany), paclitaxel from Bristol-Myers Squibb (New York,
NY, USA), vinblastine from Teva Pharmaceutical Industries Ltd.,
(Petah Tikva, Israel), and cabozantinib and crizotinib from Selleck
Chemicals (Houston, TX, USA). Nab-paclitaxel was purchased from
Celgene International (Boudry, Switzerland) and was stored at 4°C
subsequent to preparation.
Cell lines and lentiviral
transduction
The urothelial bladder cancer T24 and TCC-SUP cell
lines were obtained from the American Type Culture Collection
(Manassas, VA, USA). Drug-resistant sublines were established by
continuous exposure to increasing drug concentrations as described
previously (13) and are part of the
Resistant Cancer Cell Line collection (Institute of Medical
Virology, University Hospital Frankfurt, Frankfurt, Germany):
T24rGEMCI20 (gemcitabine-resistant, 20 ng
gemcitabine/ml), T24rVBL20
(vinblastine-resistant, 20 ng vinblastine/ml),
TCC-SUPrGEMCI20 and
TCC-SUPrVBL20 (vinblastine-resistant, 20 ng
vinblastine/ml).
The cell lines TCC-SUPABCB1 and
T24ABCB1 with ectopic overexpression of ABCB1
(University Medical Center Hamburg-Eppendorf, Hamburg, Germany),
and the corresponding control cell lines with empty vector
TCC-SUPCER2 and T24CER2 (University Medical
Center Hamburg-Eppendorf), were established by lentiviral
transduction using the Lentiviral Gene Ontology Vector technology
as described previously (14,15).
All cell lines were grown in Iscove's modified
Dulbecco's medium supplemented with 10% fetal calf serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cell line
authentication was performed by short tandem repeat profiling.
Cell viability assay
Cell viability was determined by the MTT dye
reduction assay after 120 h of incubation, as described previously
(13). Drug resistance was determined
according to resistance factors defined as half maximal inhibitory
concentration (IC50) drug in resistant
cells/IC50 drug in parental cells. The cell lines were
considered to be resistant to a drug if the resistance factor was
>2 (16). Sensitization to a drug
was determined according to sensitization factors defined as
IC50 drug in the tested cell line without tyrosine
kinase inhibitor (TKI)/IC50 drug in the tested cell line
plus TKI. To evaluate the in vitro stability of
nab-paclitaxel, efficacy factors were defined as IC50 of
7 or 28 days-old nab-paclitaxel/IC50 of freshly prepared
nab-paclitaxel.
Western blotting
Cells were lysed on ice in Triton X-100 sample
buffer, centrifuged at 4°C at 14,000 × g for 5 min, and
supernatant was stored at −20°C. Protein concentration was
determined using BioRad DC protein assay (catalog no. 5000112,
Bio-Rad Laboratories, Inc., Hercules, CA, USA), and the proteins
(concentration 1 mg/ml; 15 µl per lane) were separated by 12%
SDS-PAGE. Proteins were transferred onto nitrocellulose membrane
(catalog no. 88,018; Thermo Fisher Scientific, Inc.) and blocked
with 3% bovine serum albumin (Carl Roth, Karlsruhe, Germany) in
Tris buffered saline containing 0.05% (v/v) Tween®-20
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 40 min at room
temperature. Membranes were incubated overnight at 4°C with
specific primary antibodies against β-actin (1:1,000; catalog no.
A2228; Sigma-Aldrich; Merck KGaA), protein kinase B (Akt; 1:1,000;
catalog no. 9272; Cell Signaling Technology, Inc., Danvers, MA,
USA) and phosphorylated (p)-Akt (T308; 1:1,000; catalog no. 2965;
Cell Signaling Technology). Membranes were washed in Tris buffered
saline containing 0.05% (v/v) Tween®20 (Sigma-Aldrich;
Merck KGaA) for 40 min at room temperature, blocked and incubated
for 1 h with fluorescence-labeled secondary antibodies IRDye 800CW
goat anti-rabbit immunoglobulin G (IgG; heavy and light chains;
1:20,000; catalog no. 926-32211; LI-COR Biosciences, Lincoln, NE,
USA) and IRDye 800CW goat anti-mouse IgG (heavy and light chains;
1:20,000; catalog no. 926-32210; LI-COR Biosciences) at room
temperature. Fluorescence of the secondary antibody was measured
with the Odyssey CLx Imaging system and the Image Studio software
(version 3.1; LI-COR Biosciences).
Statistical analysis
For statistical analyses, unpaired Student's t-test,
analysis of variance and Student-Newman-Keuls test were performed
for comparison of IC50 values following treatment of
tumor cell lines with various anti-cancer compounds. Statistical
analyses were performed with GraphPad Prism® (Version
5.0c; GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
In vitro stability studies of
nab-paclitaxel
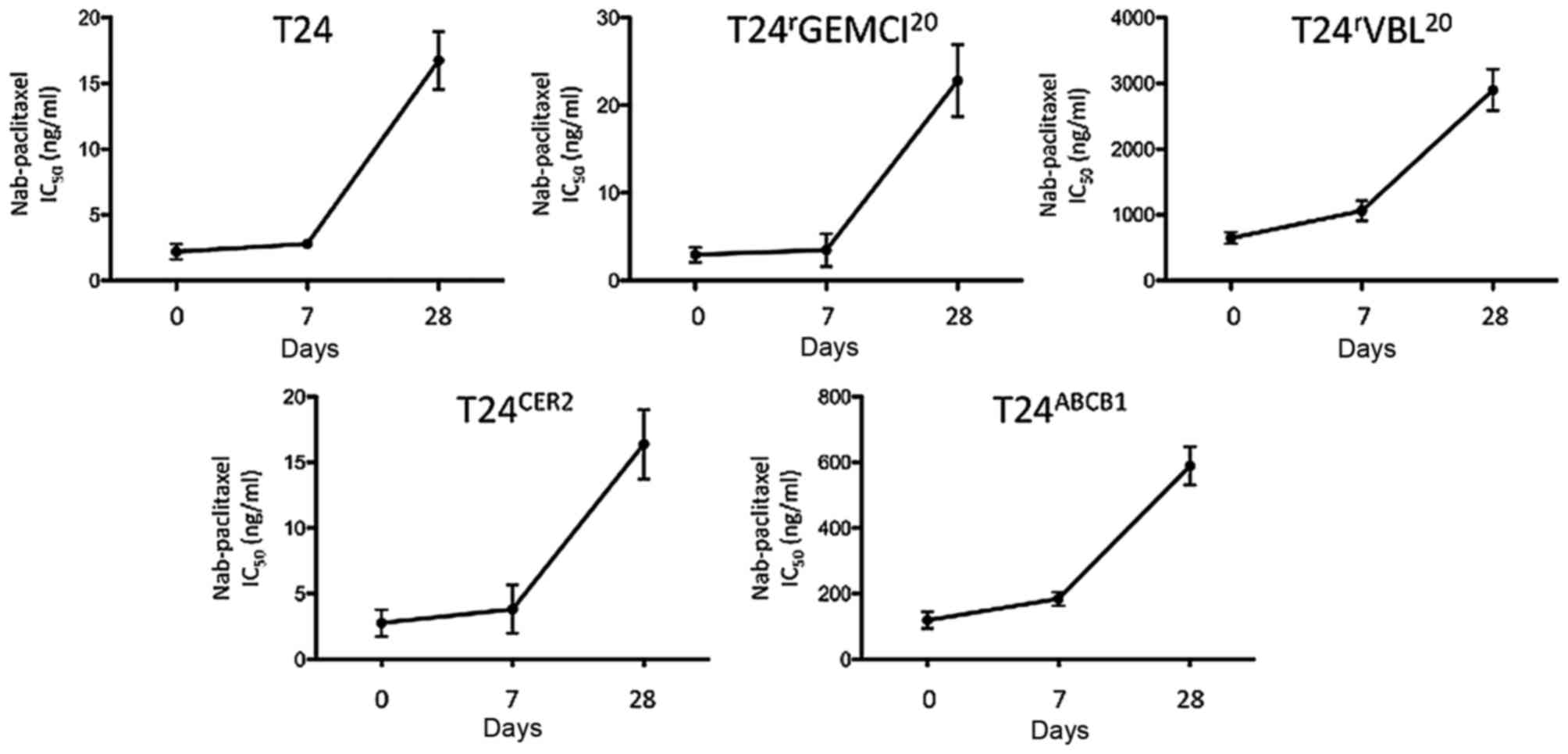
As the instability of nab-paclitaxel has been
examined previously in vivo (17),
The present study sought to evaluate for how long freshly prepared
nab-paclitaxel may be used in vitro. Therefore, functional
MTT assays were conducted with nab-paclitaxel on days 0, 7, and 28
subsequent to drug preparation. A slight loss of the antitumoral
efficacy of nab-paclitaxel after 7 days was observed, with efficacy
factors of 0.95–1.64 (efficacy factor of day 7=IC50 at
day 7/IC50 at day 0) compared with that revealed by MTT
assays at day 0. On day 28, a marked loss of antitumoral efficacy
with efficacy factors of 4.91–19.90 (efficacy factor of day
28=IC50 day at 28/IC50 at day 0) was observed
(Fig. 1). A significant
time-dependent loss of efficacy of nab-paclitaxel was also
observed. Therefore, nab-paclitaxel was used only on the day of
preparation for additional experiments.
Cell viability assays
There was no significant difference between the
antitumoral activity of nab-paclitaxel and paclitaxel in
chemosensitive urothelial cancer cells or their chemoresistant
sublines (Tables I–IV). The ABCB1-overexpressing cell lines
were similarly resistant to these two compounds. Cabozantinib and
crizotinib sensitized the ABCB1-overexpressing tumor cell lines
T24rVBL20, T24ABCB1,
TCC-SUPrGEMCI20,
TCC-SUPrVBL20 and TCC-SUPABCB1
(18) to nab-paclitaxel and
paclitaxel in a dose-dependent manner (Tables I–IV).
In addition, cabozantinib sensitized these ABCB1-overexpressing
tumor cell lines to vinblastine therapy in a dose-dependent manner
(Tables I and III). The IC50 values of
cabozantinib and crizotinib monotherapy were in the low-micromolar
range. Resistance against gemcitabine, vinblastine or stable
transduction with a lentiviral vector encoding for ABCB1 did not
appear to significantly change the sensitivity profiles to
cabozantinib or crizotinib (Table
V).
 | Table I.IC50 values of paclitaxel,
nab-paclitaxel, gemcitabine and vinblastine in TCC-SUP,
TCC-SUPrGEMCI20,
TCC-SUPrVBL20, TCC-SUPCER2 and
TCC-SUPABCB1 cells in the presence of cabozantinib. |
Table I.
IC50 values of paclitaxel,
nab-paclitaxel, gemcitabine and vinblastine in TCC-SUP,
TCC-SUPrGEMCI20,
TCC-SUPrVBL20, TCC-SUPCER2 and
TCC-SUPABCB1 cells in the presence of cabozantinib.
|
|
| IC50
(sensitization factor)a,
ng/ml |
|---|
|
|
|
|
|---|
| Cell line | Cabozantinib,
µM | Paclitaxel | Nab-paclitaxel | Gemcitabine | Vinblastine |
|---|
| TCC-SUP | 0 | 1.14±0.12 | 0.78±0.34 | 1.20±0.39 | 0.36±0.14 |
| TCC-SUP |
0.5 | 1.07±0.07
(1.07) | 0.93±0.31
(0.84) | 0.92±0.01
(1.3) | 0.47±0.02
(0.77) |
| TCC-SUP |
1.25 | 1.18±0.14
(0.97) | 0.79±0.14
(0.99) | 2.27±0.30
(0.53) | 0.31±0.16
(1.16) |
|
TCC-SUPrGEMCI20 | 0 | 4.98±1.50 | 0.69±0.45 |
42.56±0.90 | 0.69±0.23 |
|
TCC-SUPrGEMCI20 |
0.5 | 2.01±0.35
(2.48) | 0.56±0.15
(1.23) |
37.72±0.60 (1.13) | 0.60±0.07
(1.15) |
|
TCC-SUPrGEMCI20 |
1.25 | 1.23±0.36
(4.05) | 0.31±0.17
(2.22) |
43.40±13.60 (0.98) | 0.35±0.07
(1.97) |
|
TCC-SUPrVBL20 | 0 | 159.60±24.50 | 395.40±65.80 | 1.24±0.30 |
35.29±6.50 |
|
TCC-SUPrVBL20 |
0.5 |
66.33±13.50 (2.41) |
79.52±6.90 (4.97) | 1.19±0.40
(1.04) | 8.19±1.16
(4.31) |
|
TCC-SUPrVBL20 |
1.25 |
20.86±3.90 (7.65) |
22.64±1.90 (17.46) | 1.92±0.21
(0.65) | 3.44±0.69
(10.26) |
|
TCC-SUPCER2 | 0 | 1.96±0.44 | 2.43±0.25 | 1.84±0.18 | 0.43±0.19 |
|
TCC-SUPCER2 |
0.5 | 1.88±0.58
(1.04) | 2.11±0.23
(1.15) | 1.71±0.11
(1.08) | 0.58±0.09
(0.74) |
|
TCC-SUPCER2 |
1.25 | 1.42±0.42
(1.38) | 1.77±0.26
(1.37) | 1.57±0.02
(1.17) | 0.31±0.14
(1.39) |
|
TCC-SUPABCB1 | 0 |
93.14±18.40 | 140.0±91.30 | 1.46±0.22 |
11.33±3.70 |
|
TCC-SUPABCB1 |
0.5 |
19.12±7.10 (4.87) |
25.96±17.30 (5.39) | 1.53±0.01
(0.95) | 2.00±0.83
(5.67) |
|
TCC-SUPABCB1 |
1.25 | 7.81±1.60
(11.93) | 5.03±2.50
(27.83) | 1.61±0.23
(0.91) | 0.87±0.35
(13.06) |
 | Table IV.IC50 values of paclitaxel,
nab-paclitaxel, gemcitabine and vinblastine in T24,
T24rGEMCI20, T24rVBL20,
T24CER2 and T24ABCB1 cells in the presence of
crizotinib. |
Table IV.
IC50 values of paclitaxel,
nab-paclitaxel, gemcitabine and vinblastine in T24,
T24rGEMCI20, T24rVBL20,
T24CER2 and T24ABCB1 cells in the presence of
crizotinib.
|
|
| IC50
(sensitization factor)a,
ng/ml |
|---|
|
|
|
|
|---|
| Cell line | Crizotinib, µM | Paclitaxel | Nab-paclitaxel | Gemcitabine | Vinblastine |
|---|
| T24 | 0 | 4.92±1.07 | 4.31±0.48 | 3.82±1.10 | 0.61±0.28 |
| T24 |
0.5 | 3.31±0.47
(1.49) | 2.54±0.33
(1.70) | 3.03±0.05
(1.26) | 0.86±0.30
(0.71) |
| T24 |
1.25 | 2.29±0.10
(2.15) |
19.92±4.30 (0.22) | 4.46±0.74
(0.86) | 4.65±2.32
(0.13) |
|
T24rGEMCI20 | 0 | 7.09±1.24 | 9.33±2.54 |
51.54±3.20 | 0.62±0.13 |
|
T24rGEMCI20 |
0.5 | 2.54±0.55
(2.79) | 2.73±0.37
(3.42) |
52.60±0.03 (0.98) | 0.45±0.07
(1.38) |
|
T24rGEMCI20 |
1.25 | 8.60±2.86
(0.82) | 7.50±0.01
(1.24) |
47.42±6.60 (1.09) |
11.20±4.39 (0.06) |
|
T24rVBL20 | 0 | 575.30±46.30 |
1,006.60±106.00 | 1.97±0.64 | 118.82±15.20 |
|
T24rVBL20 |
0.5 | 305.80±82.70
(1.88) | 565.62±33.20
(1.78) | 1.58±0.03
(1.25) |
87.76±8.16 (1.35) |
|
T24rVBL20 |
1.25 | 118.50±80.10
(4.85) | 168.70±41.80
(5.96) | 1.94±0.51
(1.02) |
58.13±8.85 (2.04) |
|
T24CER2 | 0 | 4.51±1.56 | 7.02±1.97 | 5.40±2.16 | 0.66±0.11 |
|
T24CER2 |
0.5 | 2.56±0.66
(1.76) | 3.83±1.55
(1.83) | 3.44±0.10
(1.57) | 0.83±0.20
(0.80) |
|
T24CER2 |
1.25 | 1.52±0.35
(2.97) | 5.32±0.75
(1.32) |
10.31±1.30 (0.52) | 0.96±0.27
(0.69) |
|
T24ABCB1 | 0 | 100.80±23.40 | 129.99±15.60 | 4.43±1.80 |
13.24±2.48 |
|
T24ABCB1 |
0.5 |
64.80±17.50 (1.56) |
77.07±16.20 (1.69) | 2.81±0.04
(1.58) |
13.92±3.30 (0.95) |
|
T24ABCB1 |
1.25 |
36.20±3.97 (2.78) |
68.22±21.70 (1.91) | 5.99±0.90
(0.74) |
10.32±4.25 (1.28) |
 | Table III.IC50 values of paclitaxel,
nab-paclitaxel, gemcitabine and vinblastine in T24,
T24rGEMCI20, T24rVBL20,
T24CER2 and T24ABCB1 cell viability in the
presence of cabozantinib. |
Table III.
IC50 values of paclitaxel,
nab-paclitaxel, gemcitabine and vinblastine in T24,
T24rGEMCI20, T24rVBL20,
T24CER2 and T24ABCB1 cell viability in the
presence of cabozantinib.
|
|
| IC50
(sensitization factor)a,
ng/ml |
|---|
|
|
|
|
|---|
| Cell line | Cabozantinib,
µM | Paclitaxel | Nab-paclitaxel | Gemcitabine | Vinblastine |
|---|
| T24 | 0 | 5.48±1.15 | 4.65±0.75 | 3.60±0.80 | 0.62±0.16 |
| T24 |
0.5 | 4.89±0.52
(1.12) | 4.78±0.85
(0.97) | 3.10±0.04
(1.16) | 0.73±0.16
(0.85) |
| T24 |
1.25 | 5.05±0.88
(1.09) | 3.87±0.32
(1.20) | 6.40±0.24
(0.56) | 0.60±0.11
(1.03) |
|
T24rGEMCI20 | 0 | 7.05±0.90 | 8.36±0.50 |
54.28±1.60 | 0.56±0.22 |
|
T24rGEMCI20 |
0.5 | 4.49±0.52
(1.57) | 4.76±0.60
(1.76) |
56.37±0.90 (0.96) | 0.67±0.05
(0.84) |
|
T24rGEMCI20 |
1.25 | 4.22±0.35
(1.67) | 4.09±0.20
(2.04) |
78.48±0.40 (0.69) | 0.47±0.11
(1.19) |
|
T24rVBL20 | 0 | 576.00±81.50 |
1,174.00±278.00 | 1.31±0.50 | 117.28±13.00 |
|
T24rVBL20 |
0.5 | 155.20±49.90
(3.70) | 257.50±48.20
(4.56) | 1.20±0.05
(1.09) |
32.86±2.19 (3.57) |
|
T24rVBL20 |
1.25 |
61.99±3.90 (9.29) |
72.76±7.10 (16.14) |
2.30±0.15(0.60) | 9.44±1.09
(12.42) |
|
T24CER2 | 0 | 4.68±1.60 | 7.28±1.97 | 4.90±1.60 | 0.67±0.29 |
|
T24CER2 |
0.5 | 4.54±1.50
(1.36) | 6.52±1.76
(1.12) | 3.40±0.06
(1.44) | 0.93±0.35
(0.72) |
|
T24CER2 |
1.25 | 3.34±0.50
(1.40) | 5.04±1.17
(1.44) | 6.40±0.09
(0.77) | 0.52±0.12
(1.29) |
|
T24ABCB1 | 0 | 105.80±28.70 | 139.30±27.10 | 3.30±2.50 |
11.62±1.37 |
|
T24ABCB1 |
0.5 |
26.15±4.80 (4.05) |
32.30±3.95 (4.31) | 3.10±0.07
(1.06) | 6.99±1.20
(1.66) |
|
T24ABCB1 |
1.25 | 8.97±2.01
(11.97) |
12.80±2.58 (10.88) | 3.80±0.16
(0.87) | 5.54±0.89
(2.10) |
 | Table V.IC50 values of
cabozantinib and crizotinib in TCC-SUP,
TCC-SUPrGEMCI20,
TCC-SUPrVBL20, TCC-SUPCER2,
TCC-SUPABCB1, T24, T24rGEMCI20,
T24rVBL20, T24CER2 and
T24ABCB1 cells. |
Table V.
IC50 values of
cabozantinib and crizotinib in TCC-SUP,
TCC-SUPrGEMCI20,
TCC-SUPrVBL20, TCC-SUPCER2,
TCC-SUPABCB1, T24, T24rGEMCI20,
T24rVBL20, T24CER2 and
T24ABCB1 cells.
|
| IC50
(resistance factor)a,
µM |
|---|
|
|
|
|---|
| Cell line | Cabozantinib | Crizotinib |
|---|
| TCC-SUP | 5.08±0.17 | 0.90±0.02 |
|
TCC-SUPrGEMCI20 | 8.45±1.25
(1.66) | 2.87±0.33
(3.12) |
|
TCC-SUPrVBL20 | 6.67±0.21
(1.31) | 1.55±0.15
(1.72) |
|
TCC-SUPCER2 | 5.80±0.10
(1.14) | 0.74±0.01
(0.82) |
|
TCC-SUPABCB1 | 7.70±1.43
(1.52) | 1.80±0.09
(2.00) |
| T24 |
11.02±0.27 | 6.16±0.74 |
|
T24rGEMCI20 |
11.64±1.68 (1.06) | 3.33±0.34
(0.54) |
|
T24rVBL20 | 7.16±0.09
(0.65) | 1.50±0.13
(0.24) |
|
T24CER2 |
10.21±0.04 (0.93) | 3.54±0.31
(0.57) |
|
T24ABCB1 | 8.91±0.63
(0.81) | 5.32±0.21
(0.86) |
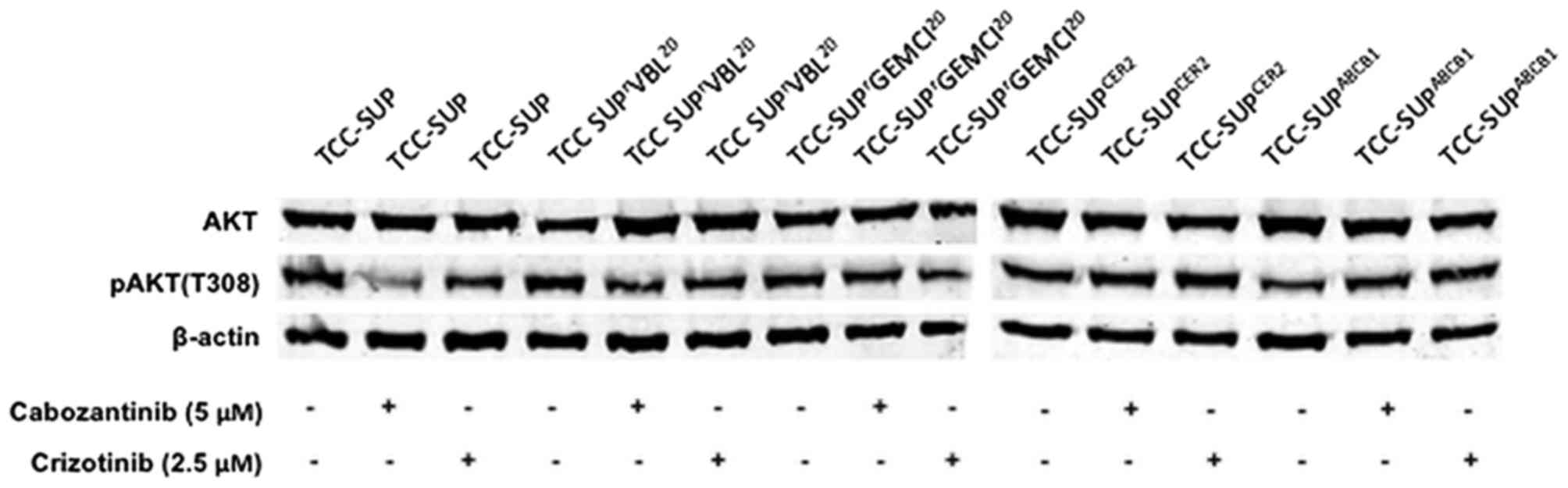
Western blot analysis
Akt is a well-known downstream marker of the
tyrosine-protein kinase Met (c-MET) and vascular endothelial growth
factor receptor 2 inhibitor cabozantinib, and of the anaplastic
lymphoma kinase, hepatocyte growth factor receptor (MET) and
proto-oncogene tyrosine-protein kinase ROS inhibitor crizotinib
(19,20). Therefore, differences in Akt/pAkt
expression subsequent to TKI treatment were evaluated. There were
no significant differences in the basal expression of Akt or pAkt
(T308) observed in TCC-SUP, TCC-SUPrGEMCI20,
TCC-SUPrVBL20, TCC-SUPCER2 or
TCC-SUPABCB1 cells. In addition, treatment with 5 µM
cabozantinib or 2.5 µM crizotinib did not appear to affect Akt or
pAkt (T308) expression (Fig. 2).
Discussion
Acquired taxane resistance is considered to be
mediated by multiple mechanisms, including overexpression of drug
pumps such as ABCB1, variations in tubulin structure, altered
signal transduction and apoptotic pathways (21). One of the most important factors for
taxane chemoresistance appears to be ABCB1 overexpression, which
has been frequently detected subsequent to the administration of
taxanes (22). Conventional
paclitaxel is usually solved in a Cremophor® EL/ethanol
vehicle, due to the very low aqueous solubility of the compound,
which may cause severe anaphylactic reactions (23–25).
Nab-paclitaxel is a colloidal suspension of 130-nm
particles homogenized in albumin and bound to paclitaxel (26). The superior antitumor activity of
nab-paclitaxel compared with that of paclitaxel was demonstrated to
be caused by increased transendothelial gp60-mediated transport and
increased intratumoral accumulation as a result of the
SPARC-albumin interaction (5,8,9). Zhang
et al (23) revealed that
paclitaxel-relapsed tumors are responsive to nab-paclitaxel
treatment, and Dong et al (10) demonstrated that paclitaxel-loaded
lipid-based nanoparticles containing the Brij 78 surfactant may
overcome ABCB1-mediated drug resistance. By contrast, there are
studies postulating that ABCB1 overexpression is a possible reason
for resistance to nanoparticle-bound paclitaxel (11,12).
Therefore, the role of ABCB1 as a resistance mechanism to
nab-paclitaxel remains unclear (5).
In the present study, a well-established panel of
urothelial cancer cell lines with acquired resistance to
gemcitabine was used as a part of the standard chemotherapy of
patients with metastasized urothelial bladder cancer (18). In addition, cell lines with acquired
resistance to vinblastine, a well-known substrate of ABCB1 that
forms part of the alternative bladder cancer chemotherapy regimen
methotrexate, vinblastine, doxorubicin and cisplatin (MVAC)
(27), were used. Furthermore, cell
lines with stable ectopic expression of ABCB1 were used to
elucidate the resistance mechanisms against nab-paclitaxel
treatment. ABCB1 overexpression and functional drug transport were
previously demonstrated in the T24rVBL20,
TCC-SUPrGEMCI20 and
TCC-SUPrVBL20 cell lines with acquired
chemoresistance, and in the T24ABCB1 and
TCC-SUPABCB1 cell lines with stable ectopic expression
of ABCB1 (18).
Nano-sized drug carrier systems have been
demonstrated to circumvent ABC transporter-mediated drug efflux
(28,29), and the increased efficacy of
nanoparticle-bound paclitaxel was explained by a decreased efflux
rate through the inhibition of ABCB1 (10). By contrast, in the cell line model of
the present study, a similar cross-resistance profile in
ABCB1-overexpressing cell lines to paclitaxel and nab-paclitaxel
was demonstrated (Tables I–IV). Zhao et al (12) identified that an NSCLC cell line with
acquired resistance to nab-paclitaxel was also resistant to ABCB1
substrates. The authors suggested that the overexpression of ABCB1
serves an important role in resistance to nab-paclitaxel in NSCLC,
similarly to the common resistance mechanism for paclitaxel
(12). They hypothesized that
paclitaxel, being the active component in nab-paclitaxel, is
responsible for the development of drug resistance (12). Additionally, they concluded that
paclitaxel is likely to be dissociated from albumin inside the cell
and pumped out by ABCB1 as a free molecule, as the albumin is too
large to be transported by ABC drug pumps (12). Therefore, the reason for
ABCB1-mediated resistance to nab-paclitaxel may be the
aforementioned dissociation of paclitaxel from albumin subsequent
to endocytosis, and nab-paclitaxel may cause the same cytotoxicity
to tumor cells as unbound paclitaxel (12,30). This
would suggest that tumor cells in vitro are sensitive or
resistant to nab-paclitaxel and paclitaxel at the same level.
Numerous studies have been conducted to develop
inhibitors for ABC transporters to circumvent ABCB1-associated
resistance. At present, several clinical trials are ongoing to
evaluate the clinical role of ABCB1 inhibitors to prevent drug
resistance (31–34). However, none of the tested compounds
had been approved for clinical use until 2010 (34). In the present study, the TKI
crizotinib reversed ABCB1-mediated drug resistance of paclitaxel
and nab-paclitaxel without changing Akt/pAkt expression. The
phosphorylation of Akt and extracellular signal-related kinase
(ERK) 1/2 are known downstream markers of crizotinib (19). These molecules may be used to test the
targeted activity of crizotinib (19). The results of the present study are in
accordance with the study of Zhou et al (33). In that study, the authors demonstrated
that crizotinib reversed multidrug resistance in different cancer
cell lines by inhibiting the function of ABCB1 without incurring
significant changes to the expression of Akt, ERK or c-MET
(33). Additionally in the present
study, the second tested TKI, cabozantinib, re-sensitized
chemoresistant cancer cell lines to ABCB1 substrates without
affecting the expression of downstream molecules. These results are
consistent with the study of Xiang et al (20), who revealed that cabozantinib
treatment in hepatocellular cancer cells reversed ABCB1-mediated
chemoresistance with no significant change to the levels of the
downstream molecules Akt, ERK1/2 or MET. As promising overall
response rates have been demonstrated for nab-paclitaxel treatment
in patients with metastatic platinum-refractory urothelial cancer
(7), a combination of nab-paclitaxel
and cabozantinib or crizotinib should be clinically evaluated to
avoid the development of resistance against nab-paclitaxel and to
extend the antitumoral effect of the drug.
In the present study, the cytotoxic effects of
vinblastine were demonstrated to be increased when vinblastine was
administered in combination with cabozantinib. The Vinca
alkaloid vinblastine binds to tubulin and inhibits the assembly of
microtubules, similarly to the mechanisms of paclitaxel and
nab-paclitaxel (35). Furthermore,
ABCB1 serves a similar role in the development of vinblastine
resistance (36). Vinblastine, as a
part of the alternative bladder cancer chemotherapy regimen MVAC,
is administered as first-line therapy, or subsequent to the failure
of gemcitabine/cisplatin treatment (27,37).
Therefore, a combination of vinblastine with cabozantinib may be a
reasonable option for the treatment of patients with metastatic
urothelial bladder cancer. However, additional in vivo
studies are required to evaluate the efficacy of a combination
therapy of TKI with vinblastine.
In conclusion, resistance to nab-paclitaxel in ABC
transporter-expressing urothelial cancer cells appears to be
mediated by ABCB1. The data of the present study suggest that the
previously identified beneficial clinical effects of nab-paclitaxel
compared with those of paclitaxel are possibly due to improved
pharmacokinetics and decreased systemic toxicity. In addition,
ABCB1 inhibition by the small molecule inhibitors cabozantinib or
crizotinib may improve clinical response to chemotherapy.
Acknowledgements
The present study was supported by the charity of
‘Hilfe für krebskranke Kinder Frankfurt e.V.’ (Children's Cancer
Aid Frankfurt) and its trust, the ‘Patenschaftsmodell’ program
(in-house scholarship of the University Hospital Frankfurt) and the
Kent Cancer Trust. The authors thank Kristoffer Riecken and Boris
Fehse of the University Medical Center Hamburg, Germany) for
provision of and support with LeGO vectors.
References
|
1
|
von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pectasides D, Pectasides M and
Economopoulos T: Systemic chemotherapy in locally advanced and/or
metastatic bladder cancer. Cancer Treat Rev. 32:456–470. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bellmunt J, Théodore C, Demkov T, Komyakov
B, Sengelov L, Daugaard G, Caty A, Carles J, Jagiello-Gruszfeld A,
Karyakin O, et al: Phase III trial of vinflunine plus best
supportive care compared with best supportive care alone after a
platinum-containing regimen in patients with advanced transitional
cell carcinoma of the urothelial tract. J Clin Oncol. 27:4454–4461.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oing C, Rink M, Oechsle K, Seidel C, von
Amsberg G and Bokemeyer C: Second line chemotherapy for advanced
and metastatic urothelial carcinoma: Vinflunine and beyond-A
comprehensive review of the current literature. J Urol.
195:254–263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma P and Mumper RJ: Paclitaxel
nano-delivery systems: A comprehensive review. J Nanomed
Nanotechnol. 4:10001642013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sparreboom A, Scripture CD, Trieu V,
Williams PJ, De T, Yang A, Beals B, Figg WD, Hawkins M and Desai N:
Comparative preclinical and clinical pharmacokinetics of a
cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and
paclitaxel formulated in Cremophor (Taxol). Clin Cancer Res.
11:4136–4143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ko YJ, Canil CM, Mukherjee SD, Winquist E,
Elser C, Eisen A, Reaume MN, Zhang L and Sridhar SS: Nanoparticle
albumin-bound paclitaxel for second-line treatment of metastatic
urothelial carcinoma: A single group, multicentre, phase II study.
Lancet Oncol. 14:769–776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yardley DA: Nab-Paclitaxel mechanisms of
action and delivery. J Control Release. 170:365–372. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Desai N, Trieu V, Damascelli B and
Soon-Shiong P: SPARC expression correlates with tumor response to
albumin-bound paclitaxel in head and neck cancer patients. Transl
Oncol. 2:59–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong X, Mattingly CA, Tseng MT, Cho MJ,
Liu Y, Adams VR and Mumper RJ: Doxorubicin and paclitaxel-loaded
lipid-based nanoparticles overcome multidrug resistance by
inhibiting P-glycoprotein and depleting ATP. Cancer Res.
69:3918–3926. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chavanpatil MD, Patil Y and Panyam J:
Susceptibility of nanoparticle-encapsulated paclitaxel to
P-glycoprotein-mediated drug efflux. Int J Pharm. 320:150–156.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao M, Lei C, Yang Y, Bu X, Ma H, Gong H,
Liu J, Fang X, Hu Z and Fang Q: Abraxane, the nanoparticle
formulation of paclitaxel can induce drug resistance by
up-regulation of P-gp. PLoS One. 10:e01314292015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Michaelis M, Rothweiler F, Barth S, Cinatl
J, van Rikxoort M, Löschmann N, Voges Y, Breitling R, von Deimling
A, Rödel F, et al: Adaptation of cancer cells from different
entities to the MDM2 inhibitor nutlin-3 results in the emergence of
p53-mutated multi-drug resistant cancer cells. Cell Death Dis.
2:e2432011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rothweiler F, Michaelis M, Brauer P, Otte
J, Weber K, Fehse B, Doerr HW, Wiese M, Kreuter J, Al-Abed Y, et
al: Anticancer effects of the nitric oxide-modified saquinavir
derivative saquinavir-NO against multidrug-resistant cancer cells.
Neoplasia. 12:1023–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weber K, Thomaschewski M, Benten D and
Fehse B: RGB marking with lentiviral vectors for multicolor clonal
cell tracking. Nat Protoc. 7:839–849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rohde D, Brehmer B, Kapp T, Valdor M and
Jakse G: Induction of drug-resistant bladder carcinoma cells in
vitro: Impact on polychemotherapy with cisplatin, methotrexate
and vinblastine (CMV). Urol Res. 26:249–257. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Li Y, Gao Y, Wei N, Zhao X, Wang C,
Li Y, Xiu X and Cui J: Direct comparison of two albumin-based
paclitaxel-loaded nanoparticle formulations: Is the crosslinked
version more advantageous? Int J Pharm. 468:15–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vallo S, Michaelis M, Rothweiler F,
Bartsch G, Gust KM, Limbart DM, Rödel F, Wezel F, Haferkamp A and
Cinatl J Jr: Drug-resistant urothelial cancer cell lines display
diverse sensitivity profiles to potential second-line therapeutics.
Transl Oncol. 8:210–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zillhardt M, Christensen JG and Lengyel E:
An orally available small-molecule inhibitor of c-Met, PF-2341066,
reduces tumor burden and metastasis in a preclinical model of
ovarian cancer metastasis. Neoplasia. 12:1–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiang QF, Zhang DM, Wang JN, Zhang HW,
Zheng ZY, Yu DC, Li YJ, Xu J, Chen YJ and Shang CZ: Cabozantinib
reverses multidrug resistance of human hepatoma HepG2/adr cells by
modulating the function of P-glycoprotein. Liver Int. 35:1010–1023.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fojo T and Menefee M: Mechanisms of
multidrug resistance: The potential role of microtubule-stabilizing
agents. Ann Oncol. 18 Suppl 5:v3–v8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Childs S and Ling V: The MDR superfamily
of genes and its biological implications. Important Adv Oncol.
194:21–36. 1994.
|
|
23
|
Zhang L, Marrano P, Kumar S, Leadley M,
Elias E, Thorner P and Baruchel S: Nab-paclitaxel is an active drug
in preclinical model of pediatric solid tumors. Clin Cancer Res.
19:5972–5983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rowinsky EK and Calvo E: Novel agents that
target tublin and related elements. Semin Oncol. 33:421–435. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kloover JS, den Bakker MA, Gelderblom H
and van Meerbeeck JP: Fatal outcome of a hypersensitivity reaction
to paclitaxel: A critical review of premedication regimens. Br J
Cancer. 90:304–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al-Hajeili M, Azmi AS and Choi M:
Nab-paclitaxel: Potential for the treatment of advanced pancreatic
cancer. Onco Targets Ther. 7:187–192. 2014.PubMed/NCBI
|
|
27
|
Kitamura H, Tsukamoto T, Shibata T,
Masumori N, Fujimoto H, Hirao Y, Fujimoto K, Kitamura Y, Tomita Y,
Tobisu K, et al: Randomised phase III study of neoadjuvant
chemotherapy with methotrexate, doxorubicin, vinblastine and
cisplatin followed by radical cystectomy compared with radical
cystectomy alone for muscle-invasive bladder cancer: Japan Clinical
Oncology Group Study JCOG0209. Ann Oncol. 25:1192–1198. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kapse-Mistry S, Govender T, Srivastava R
and Yergeri M: Nanodrug delivery in reversing multidrug resistance
in cancer cells. Front Pharmacol. 5:1592014.PubMed/NCBI
|
|
29
|
Zhang Q and Li F: Combating
P-glycoprotein-mediated multidrug resistance using therapeutic
nanoparticles. Curr Pharm Des. 19:6655–6666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gardner ER, Dahut WL, Scripture CD, Jones
J, Aragon-Ching JB, Desai N, Hawkins MJ, Sparreboom A and Figg WD:
Randomized crossover pharmacokinetic study of solvent-based
paclitaxel and nab-paclitaxel. Clin Cancer Res. 14:4200–4205. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choi DH, Chung JH and Choi JS:
Pharmacokinetic interaction between oral lovastatin and verapamil
in healthy subjects: Role of P-glycoprotein inhibition by
lovastatin. Eur J Clin Pharmacol. 66:285–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Helgason HH, Koolen SL, Werkhoven Ev,
Malingre MM, Kruijtzer CM, Huitema AD, Schot ME, Smit WM, Beijnen
JH and Schellens JH: Phase II and pharmacological study of oral
docetaxel plus cyclosporin A in anthracycline pre-treated
metastatic breast cancer. Curr Clin Pharmacol. 9:139–147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou WJ, Zhang X, Cheng C, Wang F, Wang
XK, Liang YJ, To KK, Zhou W, Huang HB and Fu LW: Crizotinib
(PF-02341066) reverses multidrug resistance in cancer cells by
inhibiting the function of P-glycoprotein. Br J Pharmacol.
166:1669–1683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Coley HM: Overcoming multidrug resistance
in cancer: Clinical studies of p-glycoprotein inhibitors. Methods
Mol Biol. 596:341–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Q, Bian Y and Zeng S: Involvement of
AP-1 and NF-κB in the up-regulation of P-gp in vinblastine
resistant Caco-2 cells. Drug Metab Pharmacokinet. 29:223–226. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim KH, Hong SJ and Han KS: Predicting the
response of patients with advanced urothelial cancer to
methotrexate, vinblastine, Adriamycin, and cisplatin (MVAC) after
the failure of gemcitabine and platinum (GP). BMC Cancer.
15:8122015. View Article : Google Scholar : PubMed/NCBI
|