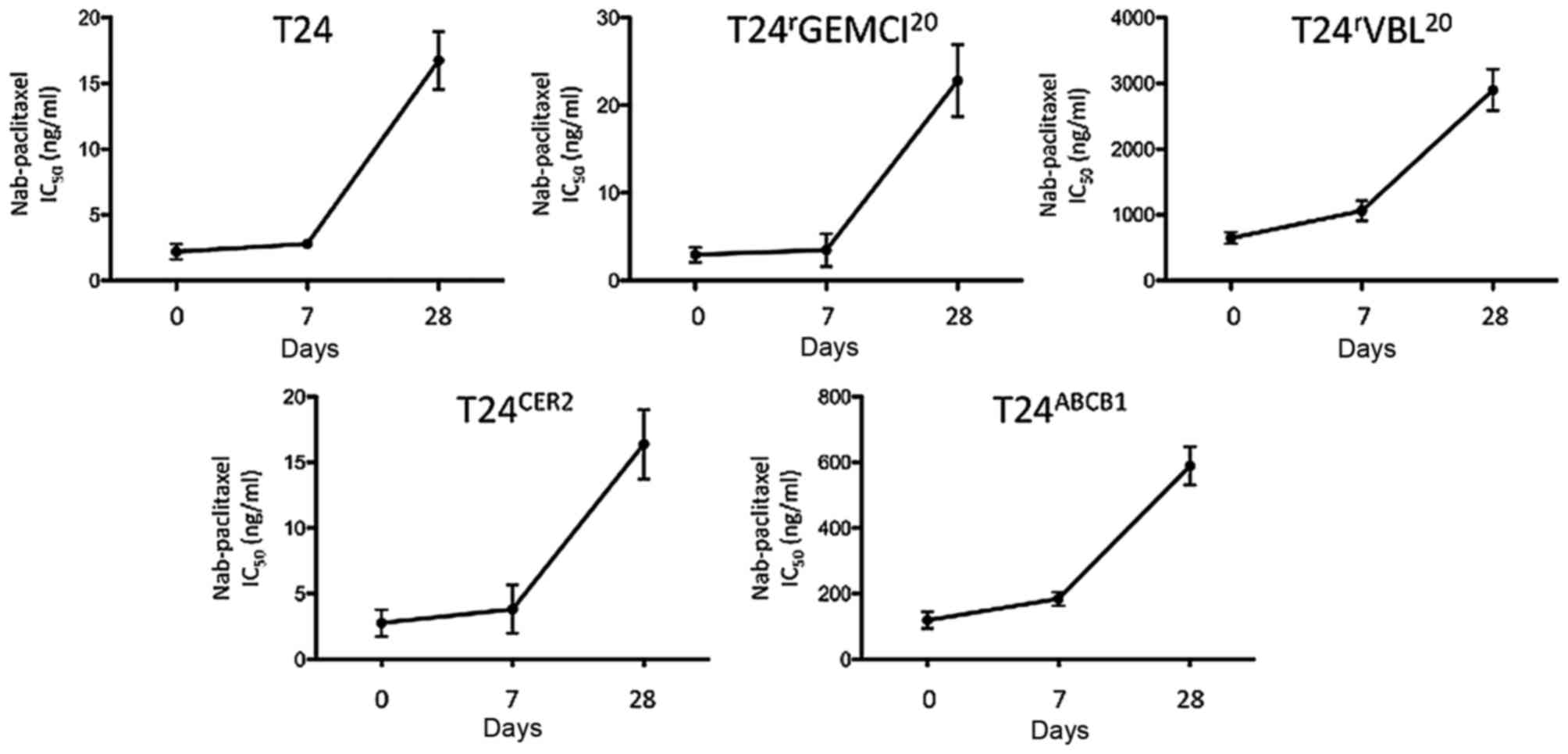
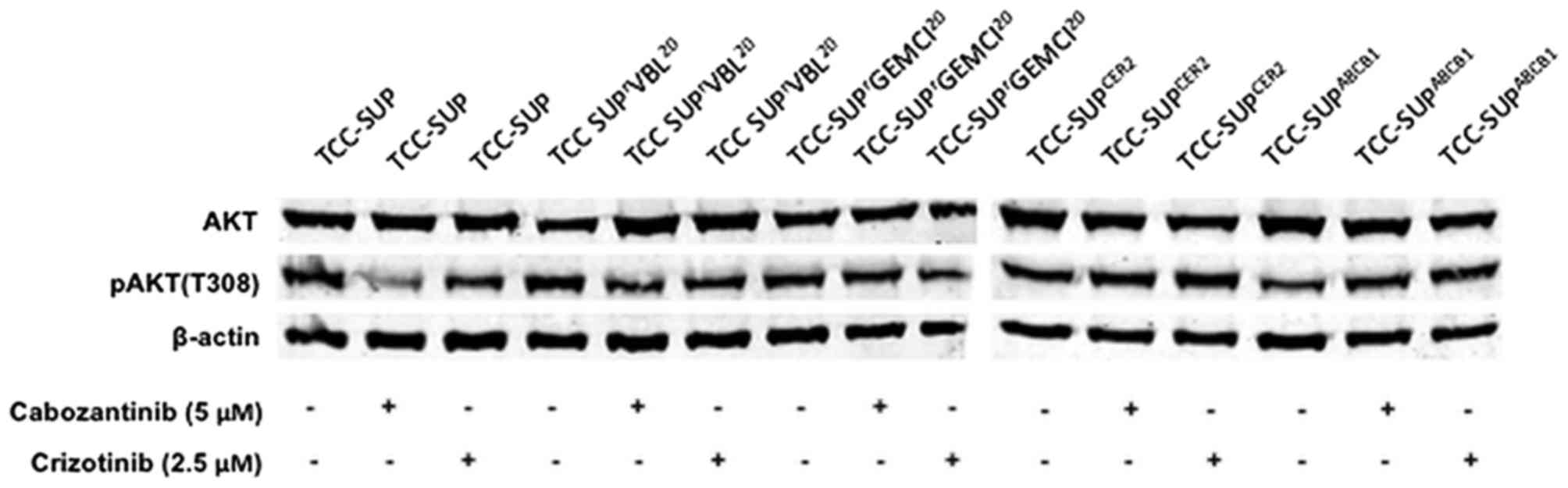
|
1
|
von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pectasides D, Pectasides M and
Economopoulos T: Systemic chemotherapy in locally advanced and/or
metastatic bladder cancer. Cancer Treat Rev. 32:456–470. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bellmunt J, Théodore C, Demkov T, Komyakov
B, Sengelov L, Daugaard G, Caty A, Carles J, Jagiello-Gruszfeld A,
Karyakin O, et al: Phase III trial of vinflunine plus best
supportive care compared with best supportive care alone after a
platinum-containing regimen in patients with advanced transitional
cell carcinoma of the urothelial tract. J Clin Oncol. 27:4454–4461.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oing C, Rink M, Oechsle K, Seidel C, von
Amsberg G and Bokemeyer C: Second line chemotherapy for advanced
and metastatic urothelial carcinoma: Vinflunine and beyond-A
comprehensive review of the current literature. J Urol.
195:254–263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma P and Mumper RJ: Paclitaxel
nano-delivery systems: A comprehensive review. J Nanomed
Nanotechnol. 4:10001642013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sparreboom A, Scripture CD, Trieu V,
Williams PJ, De T, Yang A, Beals B, Figg WD, Hawkins M and Desai N:
Comparative preclinical and clinical pharmacokinetics of a
cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and
paclitaxel formulated in Cremophor (Taxol). Clin Cancer Res.
11:4136–4143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ko YJ, Canil CM, Mukherjee SD, Winquist E,
Elser C, Eisen A, Reaume MN, Zhang L and Sridhar SS: Nanoparticle
albumin-bound paclitaxel for second-line treatment of metastatic
urothelial carcinoma: A single group, multicentre, phase II study.
Lancet Oncol. 14:769–776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yardley DA: Nab-Paclitaxel mechanisms of
action and delivery. J Control Release. 170:365–372. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Desai N, Trieu V, Damascelli B and
Soon-Shiong P: SPARC expression correlates with tumor response to
albumin-bound paclitaxel in head and neck cancer patients. Transl
Oncol. 2:59–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong X, Mattingly CA, Tseng MT, Cho MJ,
Liu Y, Adams VR and Mumper RJ: Doxorubicin and paclitaxel-loaded
lipid-based nanoparticles overcome multidrug resistance by
inhibiting P-glycoprotein and depleting ATP. Cancer Res.
69:3918–3926. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chavanpatil MD, Patil Y and Panyam J:
Susceptibility of nanoparticle-encapsulated paclitaxel to
P-glycoprotein-mediated drug efflux. Int J Pharm. 320:150–156.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao M, Lei C, Yang Y, Bu X, Ma H, Gong H,
Liu J, Fang X, Hu Z and Fang Q: Abraxane, the nanoparticle
formulation of paclitaxel can induce drug resistance by
up-regulation of P-gp. PLoS One. 10:e01314292015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Michaelis M, Rothweiler F, Barth S, Cinatl
J, van Rikxoort M, Löschmann N, Voges Y, Breitling R, von Deimling
A, Rödel F, et al: Adaptation of cancer cells from different
entities to the MDM2 inhibitor nutlin-3 results in the emergence of
p53-mutated multi-drug resistant cancer cells. Cell Death Dis.
2:e2432011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rothweiler F, Michaelis M, Brauer P, Otte
J, Weber K, Fehse B, Doerr HW, Wiese M, Kreuter J, Al-Abed Y, et
al: Anticancer effects of the nitric oxide-modified saquinavir
derivative saquinavir-NO against multidrug-resistant cancer cells.
Neoplasia. 12:1023–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weber K, Thomaschewski M, Benten D and
Fehse B: RGB marking with lentiviral vectors for multicolor clonal
cell tracking. Nat Protoc. 7:839–849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rohde D, Brehmer B, Kapp T, Valdor M and
Jakse G: Induction of drug-resistant bladder carcinoma cells in
vitro: Impact on polychemotherapy with cisplatin, methotrexate
and vinblastine (CMV). Urol Res. 26:249–257. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Li Y, Gao Y, Wei N, Zhao X, Wang C,
Li Y, Xiu X and Cui J: Direct comparison of two albumin-based
paclitaxel-loaded nanoparticle formulations: Is the crosslinked
version more advantageous? Int J Pharm. 468:15–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vallo S, Michaelis M, Rothweiler F,
Bartsch G, Gust KM, Limbart DM, Rödel F, Wezel F, Haferkamp A and
Cinatl J Jr: Drug-resistant urothelial cancer cell lines display
diverse sensitivity profiles to potential second-line therapeutics.
Transl Oncol. 8:210–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zillhardt M, Christensen JG and Lengyel E:
An orally available small-molecule inhibitor of c-Met, PF-2341066,
reduces tumor burden and metastasis in a preclinical model of
ovarian cancer metastasis. Neoplasia. 12:1–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiang QF, Zhang DM, Wang JN, Zhang HW,
Zheng ZY, Yu DC, Li YJ, Xu J, Chen YJ and Shang CZ: Cabozantinib
reverses multidrug resistance of human hepatoma HepG2/adr cells by
modulating the function of P-glycoprotein. Liver Int. 35:1010–1023.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fojo T and Menefee M: Mechanisms of
multidrug resistance: The potential role of microtubule-stabilizing
agents. Ann Oncol. 18 Suppl 5:v3–v8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Childs S and Ling V: The MDR superfamily
of genes and its biological implications. Important Adv Oncol.
194:21–36. 1994.
|
|
23
|
Zhang L, Marrano P, Kumar S, Leadley M,
Elias E, Thorner P and Baruchel S: Nab-paclitaxel is an active drug
in preclinical model of pediatric solid tumors. Clin Cancer Res.
19:5972–5983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rowinsky EK and Calvo E: Novel agents that
target tublin and related elements. Semin Oncol. 33:421–435. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kloover JS, den Bakker MA, Gelderblom H
and van Meerbeeck JP: Fatal outcome of a hypersensitivity reaction
to paclitaxel: A critical review of premedication regimens. Br J
Cancer. 90:304–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al-Hajeili M, Azmi AS and Choi M:
Nab-paclitaxel: Potential for the treatment of advanced pancreatic
cancer. Onco Targets Ther. 7:187–192. 2014.PubMed/NCBI
|
|
27
|
Kitamura H, Tsukamoto T, Shibata T,
Masumori N, Fujimoto H, Hirao Y, Fujimoto K, Kitamura Y, Tomita Y,
Tobisu K, et al: Randomised phase III study of neoadjuvant
chemotherapy with methotrexate, doxorubicin, vinblastine and
cisplatin followed by radical cystectomy compared with radical
cystectomy alone for muscle-invasive bladder cancer: Japan Clinical
Oncology Group Study JCOG0209. Ann Oncol. 25:1192–1198. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kapse-Mistry S, Govender T, Srivastava R
and Yergeri M: Nanodrug delivery in reversing multidrug resistance
in cancer cells. Front Pharmacol. 5:1592014.PubMed/NCBI
|
|
29
|
Zhang Q and Li F: Combating
P-glycoprotein-mediated multidrug resistance using therapeutic
nanoparticles. Curr Pharm Des. 19:6655–6666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gardner ER, Dahut WL, Scripture CD, Jones
J, Aragon-Ching JB, Desai N, Hawkins MJ, Sparreboom A and Figg WD:
Randomized crossover pharmacokinetic study of solvent-based
paclitaxel and nab-paclitaxel. Clin Cancer Res. 14:4200–4205. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choi DH, Chung JH and Choi JS:
Pharmacokinetic interaction between oral lovastatin and verapamil
in healthy subjects: Role of P-glycoprotein inhibition by
lovastatin. Eur J Clin Pharmacol. 66:285–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Helgason HH, Koolen SL, Werkhoven Ev,
Malingre MM, Kruijtzer CM, Huitema AD, Schot ME, Smit WM, Beijnen
JH and Schellens JH: Phase II and pharmacological study of oral
docetaxel plus cyclosporin A in anthracycline pre-treated
metastatic breast cancer. Curr Clin Pharmacol. 9:139–147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou WJ, Zhang X, Cheng C, Wang F, Wang
XK, Liang YJ, To KK, Zhou W, Huang HB and Fu LW: Crizotinib
(PF-02341066) reverses multidrug resistance in cancer cells by
inhibiting the function of P-glycoprotein. Br J Pharmacol.
166:1669–1683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Coley HM: Overcoming multidrug resistance
in cancer: Clinical studies of p-glycoprotein inhibitors. Methods
Mol Biol. 596:341–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Q, Bian Y and Zeng S: Involvement of
AP-1 and NF-κB in the up-regulation of P-gp in vinblastine
resistant Caco-2 cells. Drug Metab Pharmacokinet. 29:223–226. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim KH, Hong SJ and Han KS: Predicting the
response of patients with advanced urothelial cancer to
methotrexate, vinblastine, Adriamycin, and cisplatin (MVAC) after
the failure of gemcitabine and platinum (GP). BMC Cancer.
15:8122015. View Article : Google Scholar : PubMed/NCBI
|