Introduction
Hepatocellular carcinoma (HCC) is the third most
common cause of cancer-associated mortality globally, and it is
more prevalent in men compared with women (1). According to one estimation, 745,500
mortalities occurred among the 782,500 new liver tumor cases
worldwide during 2012 (2). Currently,
surgical resection is the optimal treatment strategy to offer
long-term survival outcomes for patients with HCC. However, the
clinical behavior of HCC may vary. In certain patients, the disease
runs an aggressive course with a survival of several months,
whereas other patients may have a relatively slow clinical
evolution and survive for >5–10 years following diagnosis. An
improved understanding of the pathogenesis and identification of
novel biomarkers for HCC may provide physicians treating this
disease with additional therapeutic options. Although it has been
proposed that several molecular markers, including α-fetoprotein
(AFP), may be associated with the prognosis of HCC, their clinical
applications are limited (3–5). Therefore, previous studies have focused
on seeking new biomarkers for the prognosis and therapeutic
targeting of HCC (6–8).
The proliferation status of tumor cells is an
important parameter that reflects the biological characteristics of
the tumor, and affects the prognosis and efficiency of treatment of
the tumor directly. DNA topoisomerases are enzymes essential for
DNA replication, transcription and recombination, as well as for
chromosome compaction and segregation (9). Several families and subfamilies of the
two types (I and II) of DNA topoisomerases have been described
(9). There are two isoforms of
topoisomerase II in humans, α and β, coded on chromosomes 17q21-22
and 3p24, respectively (10,11). TopoIIα is essential for the survival
of actively growing cells. Enzyme concentrations are upregulated
markedly during periods of cell proliferation, and TopoIIα levels
increase over the cell cycle and have an increased peak in the G2/M
phase (9). Considering the previously
mentioned role of TopoIIα in cell division and its increased
expression in the G2/M phase of the cell cycle, immunohistochemical
staining for TopoIIα may be useful in the determination of cell
proliferation, and eventually malignant transformation (9–11).
Previous studies have considered TopoIIα as a prognostic factor in
patients with esophageal squamous cell carcinomas, breast cancer,
clear cell renal cell carcinoma and nasopharyngeal carcinoma
(12–15).
One of the established proliferation markers is Ki67
protein, which may be detected within the cell nucleus. As Ki67
protein is present during all active phases of the cell cycle (G1,
S, G2 and mitosis) but is absent in the resting cell (G0), it is an
effective marker for determining the growth fraction of a given
cell population (16). In a
multi-ethnic sample of patients with breast cancer, Ki67 was
revealed to be a significant independent prognostic marker
(17).
Various studies have been performed with TopoIIα or
Ki67 individually to investigate their role as prognostic markers
for the survival of patients with HCC. However, thus far, whether
TopoIIα or Ki67 affect the long-term survival of patients with HCC
is controversial. The present study investigated TopoIIα and Ki67
expression and evaluated their utility in predicting the outcomes
of patients with HCC.
Materials and methods
Patients and tumor samples
HCC tumor samples (353 in total) were obtained from
patients who underwent hepatic surgical resection without
preoperative chemotherapy at the Fuzhou General Hospital (Fujian,
China) between February 2003 and October 2012. Using the
International Union Against Cancer tumor-node-metastasis (TNM)
classification system (18) to
classify resected specimens, 251 cases at stage I/II and 102 cases
at stage IIIa were identified. Histological grades were classified
as well/moderately-differentiated (n=329) and poorly-differentiated
(n=24). All the patients enrolled in the present study conformed to
the following criteria: No tumor thrombus in the portal vein; only
one tumor lesion; 0–1 Eastern Cooperative Oncology Group score
prior to surgery (liver function grade Child-Pugh class A; no
distant metastasis, ascites or hepatic encephalopathy); no cancer
treatment prior to surgery, and pathology confirmed as primary HCC
following surgery; complete clinical records and follow-up
information available; no history of other tumors; radical
resection. Radical resection was defined as follows: All lesions
were resected and confirmed by intraoperative ultrasonography;
pathology proved to be negative; the tumor thrombus following two
branches were able to be resected. Exclusion criteria were:
Insufficient liver tissue on the biopsy specimen for extra analysis
or insufficient clinical data regarding patient outcome; and
without history of hepatitis B virus infection.
The clinical data analysis staff members were
blinded to the TopoIIα and Ki67 expression levels in the liver
cancer samples, and the laboratory and analysis staff members did
not view the clinical data. The present study protocol was approved
by the Ethical Committees of Fuzhou General Hospital (Fuzhou,
China) and informed consent was obtained from all patients.
Follow-up subsequent surgery
Patients who underwent hepatectomy between February
2003 and October 2012 were subjected to close clinical observation,
including abdominal computed tomography imaging, AFP level and
liver function tests at 2–4-month intervals. The follow-up end date
was October 1st 2014, and the median follow-up time was 34 (range,
2–122) months. The follow-up was conducted via telephone or
questionnaire as supplementary methods.
Immunohistochemical (IHC)
analysis
IHC methods previously described by
Schmilovitz-Weiss et al (19),
for Ki67 assay were used to analyze the expression of Ki67 using
anti-Ki67 antibody (Maixin Biotechnology Co., Ltd., Fujian, China;
clone no., MID-1; dilution, 1:130) to replace the antibody. The
same method was used to analyze the expression of DNA TopoIIα,
using anti-DNA TopoIIα antibody (Maixin Biotechnology Co., Ltd;
clone no., 3F6; dilution, 1:150) to replace the antibody.
Evaluation of IHC staining
The 353 stained tissue sections (4-µm thick) were
evaluated on separate occasions by two pathologists with no
previous knowledge of any patient information. Semi-quantitative
IHC detection was used to determine TopoIIα protein level with a
4-point scale, as follows: No positive cells, 0; <25% positive
cells, 1; 25–50% positive cells, 2; ≥50% positive cells, 3. HCC
tissue samples graded 0 or 1 were judged as low TopoIIα expression,
whereas those graded 2 or 3 were regarded as high TopoIIα
expression.
As Ki67 expression was mostly homogenous, it was
scored as a percentage of positively-stained cells, based on the
following standards: (−), cancer cells unstained or stained <10%
(cancer cells stained ≥10% were identified as positive); (+),
cancer cells stained 10–25%; (++), cancer cells stained 26–50%;
(+++), cancer cells stained 51–75%; (++++) cancer cells stained
>75%. HCC tissue samples with (−) or (+) Ki67 expression levels
were judged as low Ki67 expression; samples with (++), (+++) or
(++++) Ki67 expression were regarded as having high Ki67
expression.
Statistical analysis
Analyses were conducted using SPSS statistical
software (version 20.0; IBM SPSS, Armonk, NY, USA). The data are
presented as the median and range. Categorical data were analyzed
using a χ2 test or Fisher's exact test. The correlation
between TopoIIα and Ki67 expression was analyzed using Spearman's
rank correlation test. Overall survival (OS) and recurrence-free
survival (RFS) rates were evaluated by the Kaplan-Meier method, and
the differences were examined with the log-rank test. Univariate
risk ratios with 95% confidence intervals (CIs) were calculated
using Cox proportional hazards regression models with
enter-stepwise selection. To evaluate the prognostic value of
TopoIIα and Ki67 expression, a Cox multivariate proportional
hazards regression analysis was performed with all the variables
adopted for their prognostic significance by univariate analysis
with enter-stepwise selection. P<0.05 was considered to indicate
a statistically significant difference.
Results
Demographic features and
clinicopathological data
The present study cohort included 309 men and 44
women with a median age of 53 years (range, 13–81 years) and a
median tumor size of 4 cm (range, 1–26 cm). Serum AFP level was
≤400 µg/l in 66.0% of the patients and >400 µg/l in 34.0%
(normal ranges, <20.0 ng/ml) The tumors were
well/moderately-differentiated in 93.2% of the patients and
poorly-differentiated in 6.8%; 26.1% of the tumors were on the left
side and 73.9% were on the right side; and tumor TNM stage was I/II
in 71.1% of the patients and IIIa in 28.9%.
Ki67 expression and clinicopathological parameters
of HCC are presented in Table I and
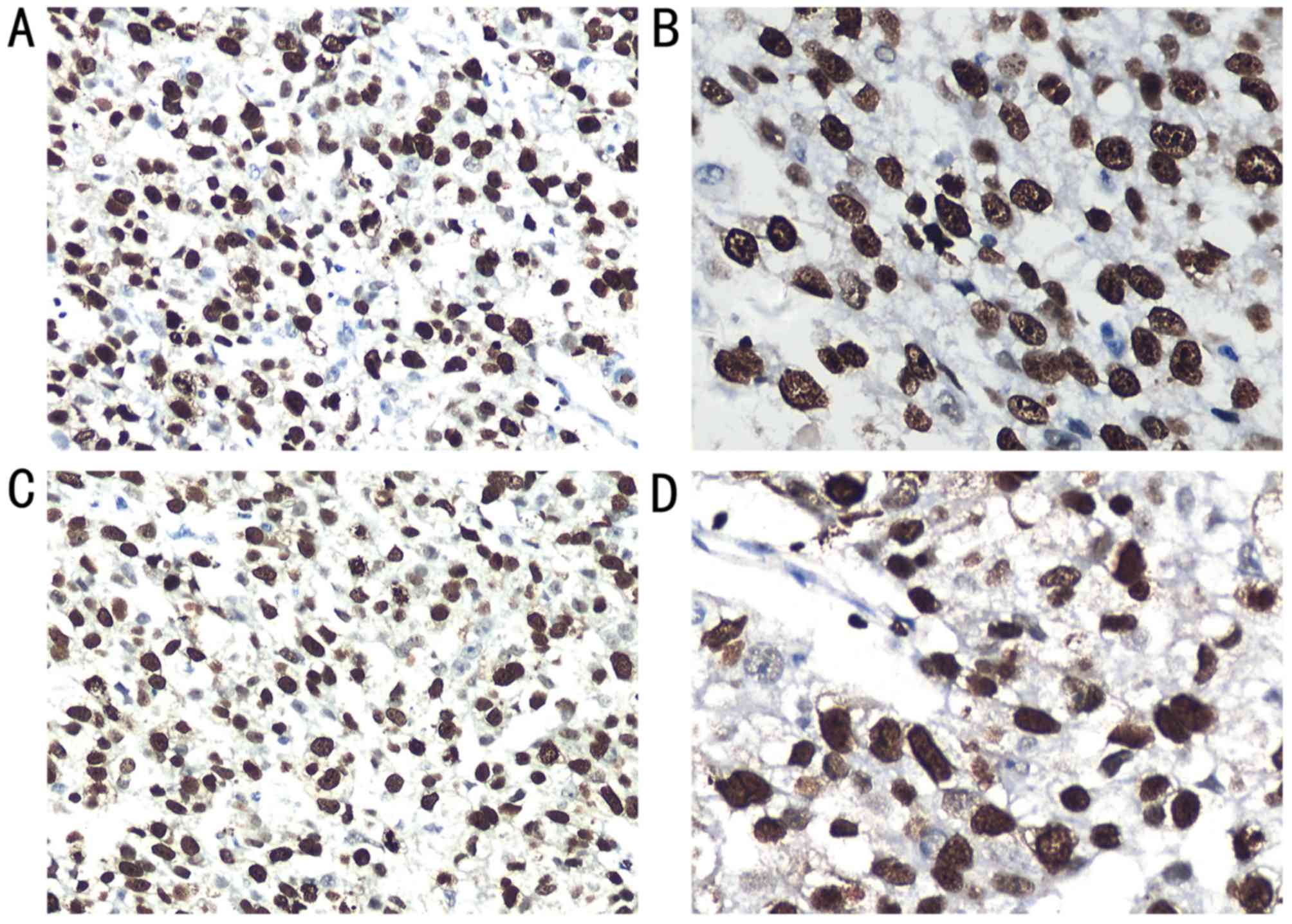
Fig. 1. Ki67 expression was detected
in the nuclei of the tumor cells in the HCC tissues (Fig. 1A and B). Among the 353 HCC tissues,
131 (37.1%) exhibited high expression and 222 (62.9%) had low
expression levels. The results revealed that Ki67 expression was
associated with TNM stage (P=0.014), tumor size (P=0.014) and high
AFP level (P=0.004). However, no association was observed between
Ki67 and age, sex, tumor location or histological grade.
 | Table I.Association between TopoIIα/Ki67
expression and characteristics of patients (n=353). |
Table I.
Association between TopoIIα/Ki67
expression and characteristics of patients (n=353).
|
| Ki67 expression |
| TopoIIα
expression |
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-valuea |
|---|
| Age |
|
| 0.427 |
|
| 0.218 |
| ≤55
years | 126 (56.8) | 80 (61.1) |
| 127 (55.9) | 79 (62.7) |
|
| >55
years | 96 (43.2) | 51 (38.9) |
| 100 (44.1) | 47 (37.3) |
|
| Gender |
|
| 0.373 |
|
| 0.921 |
| Male | 197 (88.7) | 112 (85.5) |
| 199 (87.7) | 110 (87.3) |
|
|
Female | 25 (11.3) | 19 (14.5) |
| 28 (12.3) | 16 (12.7) |
|
| Histological
grade |
|
| 0.176 |
|
| 0.489 |
|
Well-/moderately-differentiated | 210 (94.6) | 119 (90.8) |
| 210 (92.5) | 119 (94.4) |
|
|
Poorly-differentiated | 12 (5.4) | 12 (9.2) |
| 17 (7.5) | 7 (5.6) |
|
| Serum AFP level
(µg/l) |
|
| 0.004 |
|
| 0.017 |
|
≤400 | 159 (71.6) | 74 (56.5) |
| 160 (70.5) | 73 (57.9) |
|
|
>400 | 63 (28.4) | 57 (43.5) |
| 67 (29.5) | 53 (42.1) |
|
| Tumor location |
|
| 0.641 |
|
| 0.641 |
|
Left | 56 (25.2) | 36 (27.5) |
| 56 (25.2) | 36 (27.5) |
|
|
Right | 166 (74.8) | 95 (72.5) |
| 166 (74.8) | 95 (72.5) |
|
| TNM stage |
|
| 0.014 |
|
| 0.009 |
|
I/II | 168 (75.7) | 83 (63.4) |
| 172 (75.8) | 79 (62.7) |
|
|
IIIa | 54 (24.3) | 48 (36.6) |
| 55 (24.2) | 47 (37.3) |
|
| Survival |
|
| <0.001 |
|
| <0.001 |
|
Deceased | 72 (32.4) | 81 (61.8) |
| 71 (31.3) | 82 (65.1) |
|
|
Alive | 150 (67.6) | 50 (38.2) |
| 156 (68.7) | 44 (34.9) |
|
| Tumor size |
|
| 0.014 |
|
| 0.001 |
| ≤5
cm | 143 (64.4) | 67 (51.1) |
| 150 (66.1) | 60 (47.6) |
|
| >5
cm | 79 (35.6) | 64 (48.9) |
| 77 (33.9) | 66 (52.4) |
|
TopoIIα expression was also detected in the nuclei
of the tumor cells in the HCC tissues (Table I; Fig. 1C
and D). Of the 353 HCC tissues, 126 tissues (35.7%) exhibited
high expression and 227 (64.3%) showed low expression. Similar to
the expression pattern of Ki67, TopoIIα was associated with TNM
stage (P=0.009), tumor size (P=0.001) and AFP level (P=0.017),
whereas no association was observed between TopoIIα and age, sex,
tumor location or histological grade.
TopoIIα is associated with Ki67
expression in HCC
The IHC results revealed that TopoIIα expression
rate was positively correlated with Ki67 (Spearman's correlation
coefficient, r=0.444; P<0.001).
RFS
The results of the survival analyses are presented
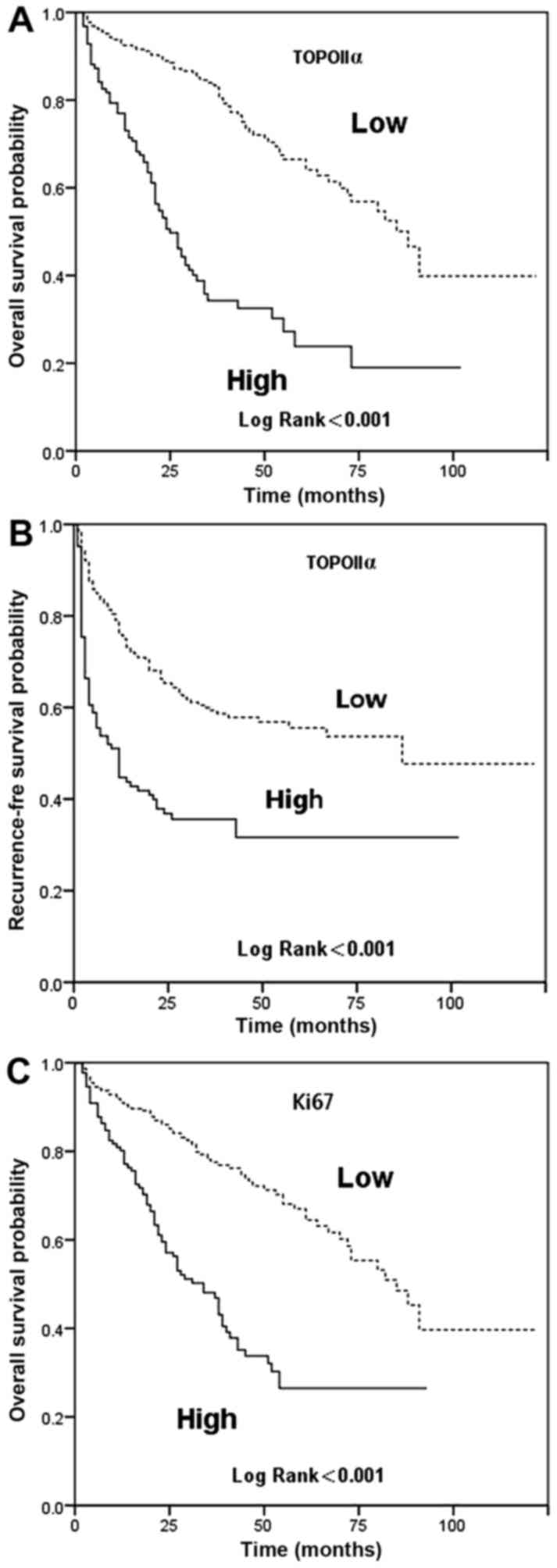
in Tables II and III and Fig.
2. In the present study, the 5-year RFS rate of the patients in
the study cohort was 47.2%. During the observation period, 171
(48.4%) patients developed tumor recurrence. As predicted, a highly
significant association was detected between tumor size (HR, 1.933;
95% CI, 1.429–2.613; P<0.001), TNM stage (HR, 2.843; 95% CI,
2.088–3.871; P<0.001), TopoIIα (HR, 2.320; 95% CI, 1.708–3.151;
P<0.001), Ki67 (HR, 1.760; 95% CI, 1.297–2.389; P<0.001) and
the development of tumor recurrence by the univariate analysis
method. By multivariate analysis of all the factors that were
significant in the univariate analysis, the independent prognostic
indicators were TNM stage (HR, 3.757; 95% CI, 2.033–6.945;
P<0.001) and TopoIIα (HR, 2.002; 95% CI, 1.429–2.806;
P<0.001). The TopoIIα-low group had a significantly increased
RFS rate compared with the TopoIIα-high group (55.6 vs. 31.7%;
P<0.001).
 | Table II.Univariate and multivariate analysis
of factors associated with overall survival. |
Table II.
Univariate and multivariate analysis
of factors associated with overall survival.
| Characteristic | Univariate
analysis, HR (95% CI) | P-value | Multivariate
analysis, HR (95% CI) | P-value |
|---|
| Age |
| 0.322 |
|
|
| ≤55
years | 1 |
|
|
|
| >55
years | 0.848
(0.612–1.175) |
|
|
|
| Gender |
| 0.183 |
|
|
|
Male | 1 |
|
|
|
|
Female | 0.688
(0.397–1.193) |
|
|
|
| Histological
grade |
| 0.157 |
|
|
|
Well-/moderately-differentiated | 1 |
|
|
|
|
Poorly-differentiated | 1.730
(0.810–3.693) |
|
|
|
| Tumor location |
| 0.822 |
|
|
|
Left | 1 |
|
|
|
|
Right | 0.959
(0.664–1.384) |
|
|
|
| Serum AFP level
(µg/l) |
| 0.284 |
|
|
|
≤400 | 1 |
|
|
|
|
>400 | 1.198
(0.861–1.558) |
|
|
|
| TNM stage |
| <0.001 |
| 0.599 |
|
I/II | 1 |
| 1 |
|
|
IIIa | 2.265
(1.638–3.131) |
| 1.137
(0.704–1.836) |
|
| Tumor size |
| <0.001 |
| 0.014 |
| ≤5
cm | 1 |
| 1 |
|
| >5
cm | 2.472
(1.794–3.406) |
| 1.821
(1.129–2.937) |
|
| Ki67
expression |
| <0.001 |
|
|
|
Low | 1 |
|
|
|
|
High | 2.892
(2.093–3.995) |
|
|
|
| TopoIIα
expression |
| <0.001 |
|
|
|
Low | 1 |
|
|
|
|
High | 3.804
(2.741–5.278) |
|
|
|
|
TopoIIα-Ki67expression |
| <0.001 |
| <0.001 |
| TopoIIα
(low) or Ki67 (low) | 1 |
| 1 |
|
| TopoIIα
(high) and Ki67 (high) | 4.264
(3.030–6.000) |
| 3.569
(2.518–5.059) |
|
 | Table III.5-year overall survival percentage
according to clinicopathological variables. |
Table III.
5-year overall survival percentage
according to clinicopathological variables.
| Characteristic | Cases, n
(n=353) | 5-year OS, % | P-value |
|---|
| Age |
|
| 0.319 |
| ≤55
years | 206 | 51.0 |
|
| >55
years | 147 | 54.1 |
|
| Sex |
|
| 0.178 |
|
Male | 309 | 51.4 |
|
|
Female | 44 | 59.1 |
|
| TNM stage |
|
| <0.001 |
|
I/II | 251 | 61.7 |
|
|
IIIa | 102 | 29.3 |
|
| Tumor location |
|
| 0.821 |
|
Left | 92 | 59.0 |
|
|
Right | 261 | 50.9 |
|
| Histological
grade |
|
| 0.150 |
|
Well/moderately
differentiated | 329 | 51.5 |
|
| Poorly
differentiated | 24 | 63.1 |
|
| Serum AFP level
(µg/l) |
|
| 0.281 |
|
≤400 | 233 | 53.7 |
|
|
>400 | 120 | 49.8 |
|
| Tumor size |
|
| <0.001 |
| ≤5
cm | 210 | 65.7 |
|
| >5
cm | 143 | 33.5 |
|
| TopoIIα
expression |
|
| <0.001 |
|
Low | 227 | 66.5 |
|
|
High | 126 | 23.8 |
|
| Ki67expression |
|
| <0.001 |
|
Low | 222 | 67.0 |
|
|
High | 131 | 26.5 |
|
| TopoIIα-Ki67
expression |
|
| <0.001 |
| TopoIIα
(low) or Ki67 (low) | 270 | 61.9 |
|
| TopoIIα
(high) and Ki67 (high) | 83 | 18.2 |
|
OS
In the present study, the 5-year OS rate of the
patients in the study cohort was 52.3%. Univariate analysis
revealed that tumor size (HR, 2.472; 95% CI, 1.794–3.406), TNM
stage (HR, 2.625; 95% CI, 1.638–3.131), TopoIIα (HR, 3.804; 95% CI,
2.741–5.278) and Ki67 (HR, 2.829; 95% CI, 2.093–3.995) were poor
predictors for OS, while age, sex, histological grade, tumor
location and serum AFP level had no prognostic significance for OS.
Multivariate analyses of factors demonstrated that the TNM stage
(HR, 3.757; 95% CI, 2.033–6.945), TopoIIα (HR, 2.749; 95% CI,
1.919–3.939), tumor size (HR, 1.938; 95% CI, 1.203–3.124) and Ki67
(HR, 1.816; 95% CI, 1.273–2.589) were the independent prognostic
indicators. The TopoIIα-low group had a significantly increased OS
rate compared with the TopoIIα-high group (66.5 vs. 23.8%;
P<0.001). The OS rate was also significantly increased in the
Ki67-low group compared with in the Ki67-high group (67.0 vs.
26.5%; P<0.001).
Discussion
HCC is a common malignant tumor with a high
mortality rate in humans (20,21). Due
to the vast heterogeneity of patients with HCC, prognosis following
surgery may differ, and current clinicopathological factors,
including AFP, TNM stage and tumor size, are not able to accurately
predict the outcomes of all patients with HCC (22). Thus, there is an urgent requirement to
find new biomarkers for prognosis and therapeutic HCC targets.
The proliferation status of tumor cells is an
important parameter that reflects the biological characteristics of
the tumor, affects prognosis and the efficiency of treating the
tumor directly (16). TopoIIα and
Ki67 are markers of cell proliferation in normal and neoplastic
tissues; their expression levels may be used as a predictive
parameter (9,16).
It is established that Ki67 protein is a traditional
proliferation marker, and various studies have explored the
predictive value of the Ki67-labeling index (17,23–27). The
associations between Ki-67 and clinicopathological features, as
well as its prognostic significance in numerous cancer types,
including breast cancer, pituitary adenomas, laryngeal carcinoma,
adrenocortical carcinoma, ovarian carcinoma and lymphoma, have been
investigated, and the results were similar (17,23–27). These
previous studies demonstrated that the expression of the Ki67
protein was associated with a more advanced tumor stage,
underlining the importance of Ki-67 as a prognostic parameter.
A meta-analysis of 54 studies with a total of 4,996
patients examined the effect of high Ki67 on HCC
clinicopathological features and patient DFS, RFS and OS (28). The results revealed that a high Ki67
level was significantly associated with advanced HCC stage, which
included poor differentiation, large tumors and an increased number
of tumor nodes, with metastasis, cirrhosis and vein invasion In
accordance with previous studies, the present study demonstrated
that Ki67 expression is associated with TNM stage, tumor size and
AFP level. Using multivariate analysis, Ki-67 was an independent
factor for OS, but was not associated with RFS. In addition, the OS
rate was increased in the Ki67-low group compared with the
Ki67-high group, which may indicate that a high expression level of
Ki67 is associated with poor prognosis in patients with HCC.
Similar to Ki67, TopoIIα is another proliferation
marker that is considered to function in growth-dependent
processes, including DNA replication and chromosome segregation,
due to its ability to break the DNA double helix. TopoIIα is
involved in the DNA replication process and chromosome segregation,
which are crucial for cell growth and development (11,9) and may
facilitate tumor cell growth (29,30).
Enzyme concentrations are upregulated dramatically during periods
of cell proliferation, and they are associated with the
proliferation of the cells (31,32). The
prognostic value of TopoIIα has been pointed out by different
researchers in various types of cancer (33).
While studying patients with HCC, Wong et al
(34) identified that TopoIIα
expression was associated with advanced histological grading,
microvascular invasion and early age onset of the malignancy, and
that high TopoIIα protein score (Grade 3) was associated with
non-responsiveness to chemotherapy and early disease-associated
mortalities. Similar to their study, the present study revealed
that TopoIIα may be detected in the nuclei of tumor cells in HCC
tissues. Of the 353 HCC tissues in the present study, 126 (35.7%)
displayed high expression. In addition, TopoIIα was associated with
TNM stage, tumor size and high AFP level. By multivariate analysis,
TopoIIα remained an independent factor for OS and RFS. In addition,
the low-TopoIIα group had increased OS and RFS rates compared with
the high-TopoIIα group.
In 2001, Watanuki et al (35) reported an association between the
TopoIIα index and the Ki-67 index in 70 resected HCC samples, and
patients with lower TopoIIα had prolonged DFS and OS times. Most
recently, the prognostic significance of TopoIIα in HCC was further
elucidated by Panvichian et al (36), who studied gene aberrations of TopoIIα
and protein expression levels of TopoIIα and Ki-67 in tumor and
corresponding non-tumor tissues. The overexpression of TopoIIα was
only identified in tumor tissues; TopoIIα gene amplification was
not detected in tumor or non-tumor tissues, and overexpression of
TopoIIα was associated with Ki-67. Based on previous studies, the
present study investigated whether TopoIIα expression in HCC is
associated with the expression of other, more established markers
of proliferation (such as Ki67). Notably, the present study
revealed that TopoIIα expression rate is positively associated with
Ki67, and the presence of TopoIIα predicted reduced RFS, which is
in accordance with earlier work by Watanuki et al (35).
The present study was limited by its retrospective
nature, and all the patients in the present cohort were confined by
the inclusion criteria. As the number of poorly differentiated
tumors in the present study was small, additional research with a
larger sample of HCC cases from different centers may be of great
value.
In conclusion, TopoIIα and Ki67 may serve as an
unfavorable prognostic factor for patients with HCC undergoing
curative tumor resection. Examination of TopoIIα and Ki67 may help
to stratify subgroups of patients and establish targeted therapies.
Thus, undertaking adjuvant treatment early in Ki67 (high) or
TopoIIα (high) patients following surgery may prolong survival.
However, the underlying mechanism requires additional study.
Acknowledgements
The authors would like to thank Professor Yu Yinghao
for his technical support in experiments. The present study was
jointly supported by grants from the Key project of Natural Science
Foundation of Fujian Province (grant no. 2014Y0034) and the
Innovation Team Foundation of Fuzhou General Hospital (grant no.
2015Y0026).
References
|
1
|
Kew MC: Hepatocellular carcinoma:
Epidemiology and risk factors. J Hepatocell Carcinoma. 1:115–125.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanaka S and Arii S: Molecular targeted
therapies in hepatocellular carcinoma. Semin Oncol. 39:486–492.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olsen SK, Brown RS and Siegel AB:
Hepatocellular carcinoma: Review of current treatment with a focus
on targeted molecular therapies. Therap Adv Gastroenterol. 3:55–66.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Furihata T, Sawada T, Kita J, Iso Y, Kato
M, Rokkaku K, Shimoda M and Kubota K: Serum alpha-fetoprotein level
per tumor volume reflects prognosis in patients with hepatocellular
carcinoma after curative hepatectomy. Hepatogastroenterology.
55:1705–1709. 2008.PubMed/NCBI
|
|
6
|
Bhattacharya S, Steele R, Shrivastava S,
Chakraborty S, Di Bisceglie AM and Ray RB: Serum miR-30e and
miR-223 as novel noninvasive biomarkers for hepatocellular
carcinoma. Am J Pathol. 186:242–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cubeddu A, Astara G, Madeddu C, Ruggiero
V, Cabula C, Serra G, Ganga R, Faloppi L, Cascinu S and Scartozzi
M: L47Osteopontin (OPN) and interleukin-6 (IL-6) as predictive
biomarkers in HCC receiving loco-regional treatment: Preliminary
results. Ann Oncol. 26:(Suppl 6). vi.124–vi. 2015. View Article : Google Scholar
|
|
8
|
Xu B, Cai Z, Zeng Y, Chen L, Du X, Huang
A, Liu X and Liu J: α-Methylacyl-CoA racemase (AMACR) serves as a
prognostic biomarker for the early recurrence/metastasis of HCC. J
Clin Pathol. 67:974–979. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watt PM and Hickson ID: Structure and
function of type II DNA topoisomerases. Biochem J. 303:681–695.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Forterre P, Gribaldo S, Gadelle D and
Serre MC: Origin and evolution of DNA topoisomerases. Biochimie.
89:427–446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McClendon AK and Osheroff N: DNA
topoisomerase II, genotoxicity, and cancer. Mutat Res. 623:83–97.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu XL, Zheng WH, Fu ZX, Li ZP, Xie HX, Li
XX, Jiang LH, Wang Y, Zhu SM and Mao WM: Topo2A as a prognostic
biomarker for patients with resectable esophageal squamous cell
carcinomas. Med Oncol. 32:3962015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Parker AS, Eckel-Passow JE, Serie D,
Hilton T, Parasramka M, Joseph RW, Wu KJ, Cheville JC and Leibovich
BC: Increased expression of topoisomerase II alpha is an
independent marker of increased risk of cancer-specific death in
patients with clear cell renal cell carcinoma. Eur Urol.
66:929–935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mrklić I, Pogorelić Z, Ćapkun V and Tomić
S: Expression of topoisomerase II-α in triple negative breast
cancer. Appl Immunohistochem Mol Morphol. 22:182–187. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lan J, Huang HY, Lee SW, Chen TJ, Tai HC,
Hsu HP, Chang KY and Li CF: TOP2A overexpression as a poor
prognostic factor in patients with nasopharyngeal carcinoma. Tumour
Biol. 35:179–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mahmoud AM, Macias V, Al-alem U, Kresovich
JK, Khramtsova G, Kajdacsy-Balla A, Wiley EL and Rauscher GH: Ki67
is an independent prognostic marker in breast cancer even after
accounting for molecular subtype. Cancer Res. 74:55692014.
View Article : Google Scholar
|
|
18
|
Kee KM, Wang JH, Lee CM, Chen CL,
Changchien CS, Hu TH, Cheng YF, Hsu HC, Wang CC, Chen TY, et al:
Validation of clinical AJCC/UICC TNM staging system for
hepatocellular carcinoma: Analysis of 5,613 cases from a medical
center in southern Taiwan. Int J Cancer. 120:2650–2655. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmilovitz-Weiss H, Tobar A, Halpern M,
Levy I, Shabtai E and Ben-Ari Z: Tissue expression of squamous
cellular carcinoma antigen and Ki67 in hepatocellular
carcinoma-correlation with prognosis: A historical prospective
study. Diagn Pathol. 6:1212011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li WQ, Park Y, McGlynn KA, Hollenbeck AR,
Taylor PR, Goldstein AM and Freedman ND: Index-based dietary
patterns and risk of incident hepatocellular carcinoma and
mortality from chronic liver disease in a prospective study.
Hepatology. 60:588–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qin LX and Tang ZY: Recent progress in
predictive biomarkers for metastatic recurrence of human
hepatocellular carcinoma: A review of the literature. J Cancer Res
Clin Oncol. 130:497–513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiloiro S, Bianchi A, Doglietto F, de
Waure C, Giampietro A, Fusco A, Iacovazzo D, Tartaglione L, Di
Nardo F, Signorelli F, et al: Radically resected pituitary
adenomas: Prognostic role of Ki 67 labeling index in a monocentric
retrospective series and literature review. Pituitary. 17:267–276.
2014.PubMed/NCBI
|
|
24
|
Rademakers SE, Hoogsteen IJ, Rijken PF,
Terhaard CH, Doornaert PA, Langendijk JA, van den Ende P, van der
Kogel AJ, Bussink J and Kaanders JH: Prognostic value of the
proliferation marker Ki-67 in laryngeal carcinoma: Results of the
accelerated radiotherapy with carbogen breathing and nicotinamide
phase III randomized trial. Head Neck. 37:171–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beuschlein F, Weigel J, Saeger W, Kroiss
M, Wild V, Daffara F, Libé R, Ardito A, Al Ghuzlan A, Quinkler M,
et al: Major prognostic role of Ki67 in localized adrenocortical
carcinoma after complete resection. J Clin Endocrinol Metab.
100:841–849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Battista MJ, Mantai N, Sicking I, Cotarelo
C, Weyer V, Lebrecht A, Solbach C and Schmidt M: Ki-67 as an
independent prognostic factor in an unselected cohort of patients
with ovarian cancer: Results of an explorative, retrospective
study. Oncol Rep. 31:2213–2219. 2014.PubMed/NCBI
|
|
27
|
He X, Chen Z, Fu T, Jin X, Yu T, Liang Y,
Zhao X and Huang L: Ki-67 is a valuable prognostic predictor of
lymphoma but its utility varies in lymphoma subtypes: Evidence from
a systematic meta-analysis. BMC Cancer. 14:1532014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo Y, Ren F, Liu Y, Shi Z, Tan Z, Xiong
H, Dang Y and Chen G: Clinicopathological and prognostic
significance of high Ki-67 labeling index in hepatocellular
carcinoma patients: A meta-analysis. Int J Clin Exp Med.
8:10235–10247. 2015.PubMed/NCBI
|
|
29
|
Coss A, Tosetto M, Fox EJ, Sapetto-Rebow
B, Gorman S, Kennedy BN, Lloyd AT, Hyland JM, O'Donoghue DP,
Sheahan K, et al: Increased topoisomerase IIalpha expression in
colorectal cancer is associated with advanced disease and
chemotherapeutic resistance via inhibition of apoptosis. Cancer
Lett. 276:228–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bidgoli SA, Azizi E and Zavarhei MD:
Association between p53 expression and Bcl-2, P-glycoprotein,
topoisomerase II alpha, thymidylate synthase and thymidine
phosphorylase as potential therapeutic targets in colorectal cancer
patients. Pak J Biol Sci. 10:3350–3355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shvero J, Koren R, Shvili I, Yaniv E,
Sadov R and Hadar T: Expression of human DNA topoisomerase II-alpha
in squamous cell carcinoma of the larynx and its correlation with
clinicopathologic variables. Am J Clin Pathol. 130:934–849. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim EJ, Lee YS, Kim YJ, Kim MJ, Ha YS,
Jeong P, Lee OJ and Kim WJ: Clinical implications and prognostic
values of topoisomerase-II alpha expression in primary
non-muscle-invasive bladder cancer. Urology. 75:1516.e9–e13. 2010.
View Article : Google Scholar
|
|
33
|
Ali Y and Hamid Abd S: Human topoisomerase
II alpha as a prognostic biomarker in cancer chemotherapy. Tumour
Biol. 37:47–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wong N, Yeo W, Wong WL, Wong NL, Chan KY,
Mo FK, Koh J, Chan SL, Chan AT, Lai PB, et al: TOP2A overexpression
in hepatocellular carcinoma correlates with early age onset,
shorter patients survival and chemoresistance. Int J Cancer.
124:644–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Watanuki A, Ohwada S, Fukusato T, Makita
F, Yamada T, Kikuchi A and Morishita Y: Prognostic significance of
DNA topoisomerase IIalpha expression in human hepatocellular
carcinoma. Anticancer Res. 22:1113–1119. 2002.PubMed/NCBI
|
|
36
|
Panvichian R, Tantiwetrueangdet A,
Angkathunyakul N and Leelaudomlipi S: TOP2A amplification and
overexpression in hepatocellular carcinoma tissues. Biomed Res Int.
2015:3816022015. View Article : Google Scholar : PubMed/NCBI
|