Introduction
Esophageal cancer is the eighth most common
malignant cancer in incidence and the sixth in cancer mortality
worldwide (1). In China, esophageal
cancer is the fourth most common cause of cancer-associated
mortalities, and the majority of cases are squamous cell carcinoma
(SCC) (2). Generally, patients with
esophageal cancer are diagnosed at a relatively late stage and have
a very poor prognosis. The 5-year survival rate of esophageal
cancer is <10% (3,4). The most important prognostic factors in
esophageal carcinoma include the extent of the primary tumor and
the metastasis of lymph nodes (5). An
increasing number of metastatic lymph nodes are associated with a
progressively poorer prognosis (6).
The identification of more efficient biomarkers associated with
lymph node metastasis is important for predicting the clinical
outcomes in esophageal cancer.
CD63, also known as lysosome-associated membrane
glycoprotein 3, melanoma-associated antigen ME491 or
melanoma-associated antigen MLA1, is a member of the
transmembrane-4 superfamily (TM4SF; tetraspan proteins), and is a
cell surface glycoprotein (7–9). TM4SF molecules mediate signal
transduction events that have a role in the regulation of cellular
processes, including growth, adhesion motility and differentiation,
and has been associated with tumor progression in human non small
cell lung cancer, prostate cancer, breast cancer, astrocytomas,
pancreatic cancer and melanoma (10–13). The
tetraspanin CD63 has been implicated in metastatic signaling
pathways in several cancer types. Kondoh et al (14) suggested that CD63 may act as an
anti-metastatic gene in human malignant melanoma. The precancerous
lesions of melanoma exhibit intense CD63 expression, whereas the
tissues from the melanomas with the dermis invasion or with distant
metastasis are weakly stained (14).
Radford et al (15) confirmed
the suppressive role of CD63 in melanoma invasion and progression,
due to its association with β1 integrins and their interaction with
specific extracellular matrix (ECM) substrates (fibronectin,
laminin, and collagen). Sordat et al (16) has reported that substrate-immobilized
anti-CD63 antibodies are able to enhance colon carcinoma cell
migration and invasion. Seubert et al (17) revealed that CD63 overexpression
enhanced the tumor cell-intrinsic metastatic potential by
initiating β-catenin-dependent epithelial-mesenchymal transition
(EMT), which contrasted the results of other studies. Kwon et
al (18) suggested that CD63
negativity is able to predict poor prognosis for early-stage lung
adenocarcinoma (15). Jang et
al (19) reported that reduced
CD63 expression contributes to the invasive and metastatic ability
of human melanoma cells due to the increased cell motility, matrix
proteolytic capability and ability to detach from the surrounding
matrix. The results reported by Toricelli et al (20) revealed that a supramolecular complex
containing CD63, tissue inhibitor of metalloproteinase-1 (TIMP-1)
and β1-integrins increased anoikis resistance in melanoma cells by
activating Phosphatidylinositol-4,5-bisphosphate 3-kinase signaling
pathway, independently of protein kinase B phosphorylation.
Previous studies have suggested that the tetraspanin
family may be involved in the metastasis of esophageal cancers. For
example, TM4SF3, a member of the tetraspanin family, has been
reported to be a pro-metastatic factor in esophageal carcinoma via
upregulating ADAM12 m expression (21). Ectopic expression of the tetraspanin
cell surface receptor uroplakin 1A inhibited cell proliferation,
clonogenicity, cell motility and tumor formation by inhibiting
nuclear translocation of β-catenin in esophageal carcinoma cells
(21). Inactivation of its downstream
targets, including Cyclin-D1, c-Jun, c-Myc, and matrix
metalloproteinase 7 (MMP-7) (22).
However, the role of the TM4SF member CD63 in esophageal cancer
remains unclear.
The current study examined the expression of CD63 in
primary esophageal cancer to examine its association with tumor
stage and lymph node metastasis. The invasiveness of esophageal
cancer cell lines following downregulation of CD63 expression was
also investigated. In addition, the underlying mechanisms by which
CD63 affects the invasiveness of esophageal carcinoma cells were
examined.
Materials and methods
Tissue samples
A total of 106 esophageal carcinoma (EC) samples and
49 matched adjacent esophagus tissues were collected by endoscopic
biopsies or surgical resection from 106 patients (Table I), and 17 normal esophagus mucosa
tissues were obtained from surgical resections of trauma patients.
The age of the EC patients ranged between 32 and 78, and the mean
age was 59.8. The age of the trauma patients ranged from 32 to 72,
and the mean age was 57.2. No patient had received any other
treatment prior to surgery. These tissues were obtained from the
Gastrointestinal Center at the Jiangyin People's Hospital, Medical
School of University of Southeast of China (Jiangyin, China) from
February 2010 to December 2012. This study was performed under the
approval the Ethics Committees of Jiangyin People's Hospital and
the patients gave written informed consent to participate. All
diagnoses were based on pathological and/or cytological evidence.
According to the classification criteria from the World Health
Organization (23), the histological
features of the specimens were evaluated by two senior
pathologists. For immunohistochemistry, tissues were frozen in
liquid nitrogen and maintained at −80°C immediately following
endoscopic biopsies or surgical resection.
 | Table I.Clinicopathological data from 106
patients with EC. |
Table I.
Clinicopathological data from 106
patients with EC.
| Clinicopathological
factor | No. of patients
(%) |
|---|
| Sex |
|
|
Male | 62
(58.48) |
|
Female | 44
(41.52) |
| Histologic
type |
|
|
SqC | 58
(54.72) |
|
AdC | 48
(45.28) |
| Stage |
|
| Early
(I/II) | 68
(64.15) |
|
Advanced (III/IV) | 38
(35.85) |
| Tumor
statusa |
|
| T1 | 18
(16.98) |
| T2 | 38
(35.85) |
| T3 | 22
(20.75) |
| T4 | 28
(26.42) |
| Lymph node
metastasisb |
|
| N0 | 64
(60.38) |
| N1 | 27
(25.47) |
| N2 |
7 (6.60) |
| N3 |
8 (7.55) |
| Distant
metastasis |
|
|
Negative | 104 (98.1) |
|
Positive | 2
(1.9) |
Immunohistochemistry
The expression patterns of CD63 in human esophageal
cancer samples, the adjacent normal esophagus samples and the
normal esophagus mucosa tissues were analyzed using
immunohistochemistry. Sections of formalin-fixed and
paraffin-embedded tissue with a thickness of 6 µm were
deparaffinized and heated in citrate buffer (pH 6.0; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at 95°C for 30 min as an antigen
retrieval protocol. Endogenous peroxidase activity was blocked with
0.3% hydrogen peroxide, and non-specific-binding sites were reduced
by incubating the sections at room temperature with 4% skim milk
powder for 30 min. The sections were then incubated overnight at
4°C with a rabbit monoclonal antibody to CD63 protein (cat. no.
ab134045; dilution, 1:1,000; Abcam, Cambridge, MA USA).
Biotinylated secondary antibody mouse anti-rabbit immunoglobulin
G-B (cat. no., sc-2491; dilution, 1:1,000, Santa Cruz
Biotechnology, Santa Cruz, CA, USA) was then added to the sections
followed by incubation for 30 min at room temperature. An
avidin-biotin-peroxidase complex (Beyotime Institute of
Biotechnology, Nantong, China) was added for an additional 30 min.
Following treatment with a 3,3′-diaminobenzidine
substrate-chromogen system (Dako; Agilent Technologies Inc., Santa
Clara, CA, USA) for 8 min, counterstaining was performed with
hematoxylin. All histological assessments were performed by the
same pathologist. For statistical analysis, the sections with
<10% of the whole tissue mass stained were classified as
negative; the sections with 10–25% of the whole tissue mass stained
were classified as weakly positive (+1); the sections with 25–75%
of the whole tissue mass stained were defined as moderately
positive (+2); the sections with >75% of the tissue stained
positive were regarded as strongly positive (+3).
Cell culture and transfection
The human esophageal cancer cell line TE-1 was
cultured in Dulbecco's modified Eagle's medium (Thermo Fisher
Scientific Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
antibiotics (100 U/ml penicillin and 100 µg/streptomycin,
Sigma-Aldrich; Merck KGaA). Cells were grown at 37°C in a 5%
CO2 atmosphere and were transfected according to the
manufacturer's protocol using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) with CD63 siRNA (Santa Cruz
Biotechnology Inc., Dallas, TX, USA).
Western blot analysis
Esophageal carcinoma TE-1 cells were divided into
three groups: Control (with no transfection protocol), NC (cells
transfected with empty plasmid), and siCD63 (cells transfected with
small interfering RNA targeting CD63). Following treatment, cells
were lysed with radioimmunoprecipitation assay lysis buffer (50 mM
Tris-HCl, pH 7.4; 150 mM NaCl; 1% Triton X-100; 1% sodium
deoxycholate; 0.1% SDS) and protease inhibitor cocktail (P8340;
Sigma-Aldrich; Merck KGaA) for 30 min at 4°C. BCA protein assay
(Bio-Rad Laboratories, Hercules, CA, USA) was used to determine the
protein concentration. Equal quantities of protein lysates (40 µg
total protein) were electrophoretically separated by 10% SDS-PAGE
and transferred to polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Following blocking with 5% nonfat
milk in TBST (20 mM Tris, pH 7.5; 500 mM NaCl; 0.05% Tween-20) for
1 h at room temperature, the membranes were then incubated
overnight at 4°C with the primary antibody to CD63 (cat. no.,
ab134045; dilution, 1:1,000, Abcam), E-cadherin (cat. no.,
sc-59778; dilution, 1:200, Santa Cruz Biotechnology), vimentin
(cat. no. sc-6260; dilution, 1:200, Santa Cruz Biotechnology),
MMP-2 (cat. no. sc-13594; dilution, 1:200, Santa Cruz
Biotechnology) and MMP-9 (cat. no. sc-21733; dilution, 1:200, Santa
Cruz Biotechnology). β-Actin (cat. no. sc-8432; dilution, 1:200,
Santa Cruz Biotechnology) was used as a loading control. Following
washing four times with TBST, the membranes were incubated at room
temperature with a horseradish peroxidase-conjugated secondary
antibody (cat. nos. sc-2005, sc-2357; dilution, 1:1,000, Santa Cruz
Biotechnology) for 2 h, and visualized by chemiluminescence using
an enhanced chemiluminescence system (GE Healthcare Life Sciences,
Chalfont, UK).
In vitro migration assay
Esophageal carcinoma TE-1 cells were seeded in
6-well plates and allowed to reach a confluent state, then were
divided into three groups: Control (with no transfection protocol),
NC (cells transfected with empty plasmid), and siCD63 (cells
transfected with small interfering RNA targeting CD63). Following
treatment, the monolayer was scratched with the tip of a 200 µl
pipette. The floating and detached cells were removed by washing
twice with PBS. Subsequently, fresh serum-free medium was added. At
0 and 24 h, images were captured to assess the extent of cell
migration using an Olympus BX 40 Light Microscope (Olympus
Corporation, Tokyo, Japan).
In vitro invasion assay
The invasive potential of TE-1 cells was assessed
using 24-well Matrigel invasion chambers (pore size 8 µm, Corning
Costar Corporation, Corning, NY, USA). Inserts were pre-coated with
40 µl Matrigel (1:4 dilution; BD Biosciences, San Jose, CA, USA).
Then, 5×104 cells/ml pre-transfected with CD63 siRNA in
serum-free medium were added to the upper chambers. The lower
chambers contained 500 µl medium supplemented 10% FBS. Following
incubation for 24 h at 37°C, the cells remaining in the upper
chambers were removed with PBS-moistened cotton swabs, and the
invading cells on the underside of the insert filter were fixed
with 3.7% paraformaldehyde for 30 min at room temperature and
stained with Giemsa for 1 h at room temperature. Images of 5 random
microscopic fields gathered by an Olympus BX 40 Light Microscope
(Olympus Corporation) were captured to calculate the average number
of invaded cells.
Statistical analysis
Mean ± standard error of the mean of several
independent experiments were calculated. One-way analysis of
variance was applied to analyze the data, and then the significance
of differences among different groups was evaluated by
Student-Newman-Keuls post hoc test. Statistical tests were
performed using Graph-Pad PRISM version 6.0 (Graph-Pad Software,
San Diego, CA). P<0.05 was considered to indicate a
statistically significant difference.
Results
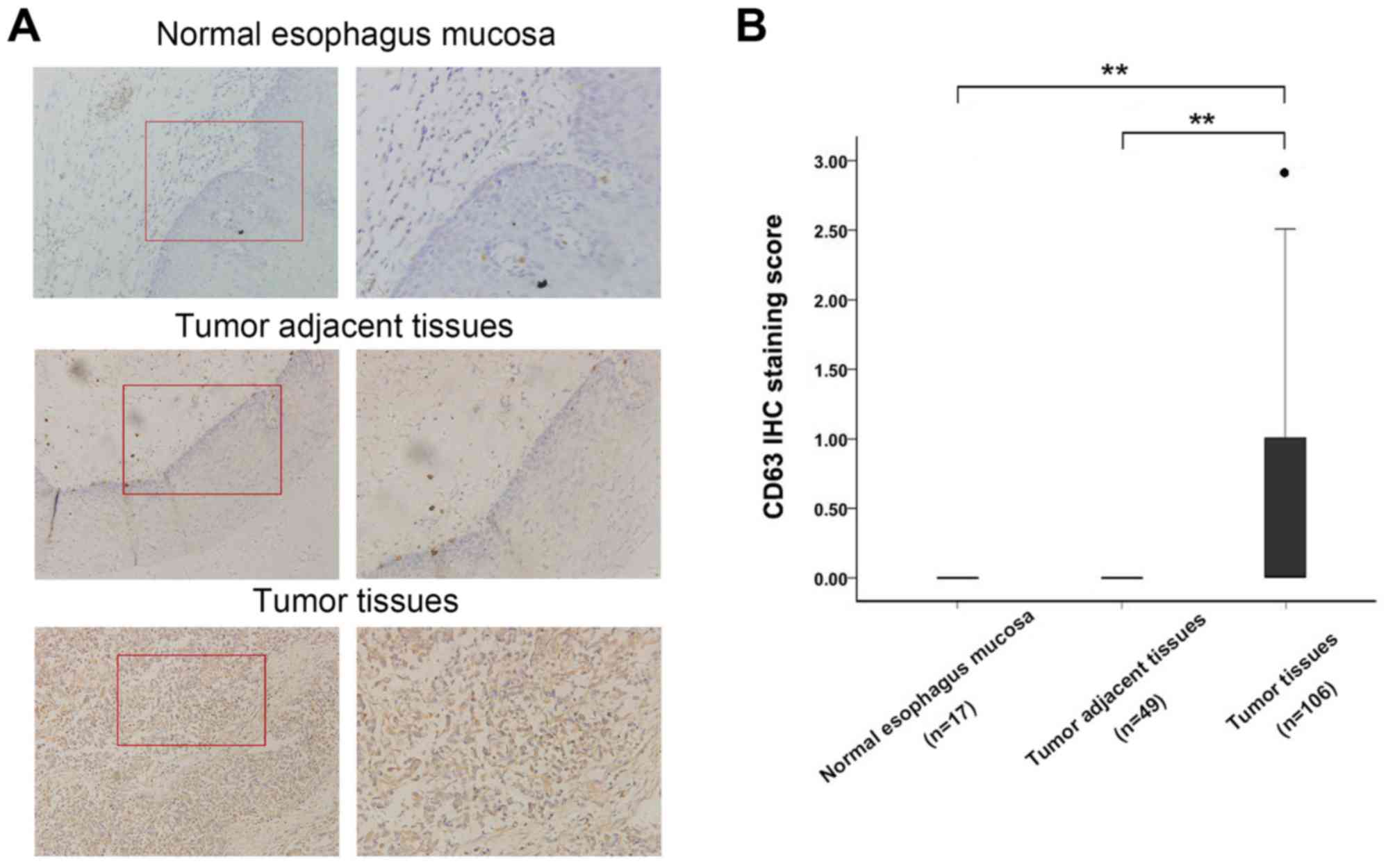
Expression of CD63 is upregulated in
esophageal cancer tissues
To investigate the variation in expression levels
between the normal and cancerous esophageal tissues, CD63 protein
expression levels were evaluated by immunohistochemistry staining
in 106 paraffin-embedded human esophageal cancer tissues, 49
adjacent esophagus tissues and the 17 normal esophagus mucosa
tissues. Overall positive staining for CD63 was frequently observed
in the cytoplasm of EC tissues, whereas no staining of CD63 was
observed in the adjacent esophagus tissues and the normal esophagus
mucosa tissues (Fig. 1A). The
sections were subsequently scored by a pathologist; there were
significant differences in CD63 expression between esophageal
cancer tissues, adjacent esophagus tissues and the 17 normal
esophagus mucosa tissues (Fig. 1B,
P<0.01). This demonstrated that CD63 expression was increased in
esophageal carcinoma and may be a regulator in the development of
EC.
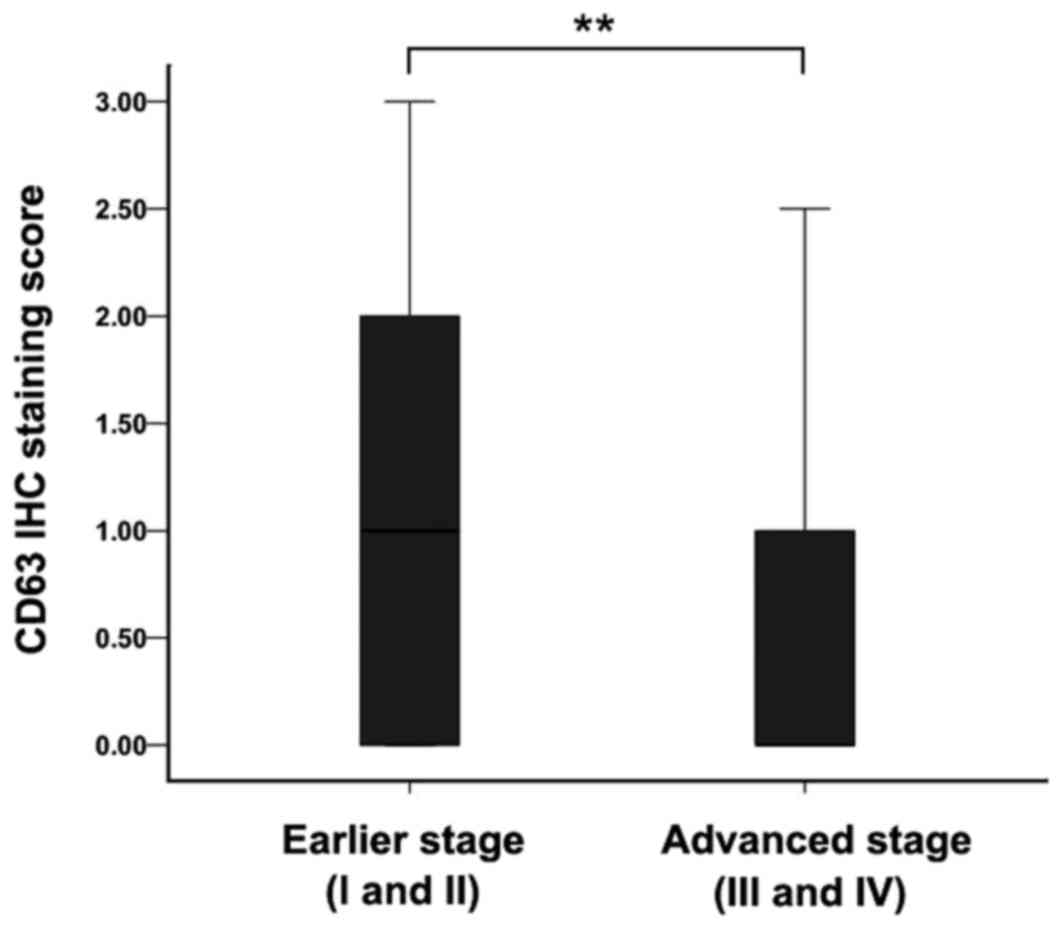
Association between CD63 expression
and clinicopathological factors
The CD63 expression differences in the EC tissues in
different clinical stages were subsequently analyzed. The average
CD63 expression level in earlier stage (I and II) esophageal
carcinoma was higher than the level in advanced stage (III and IV)
(Fig. 2; P<0.01). CD63 expression
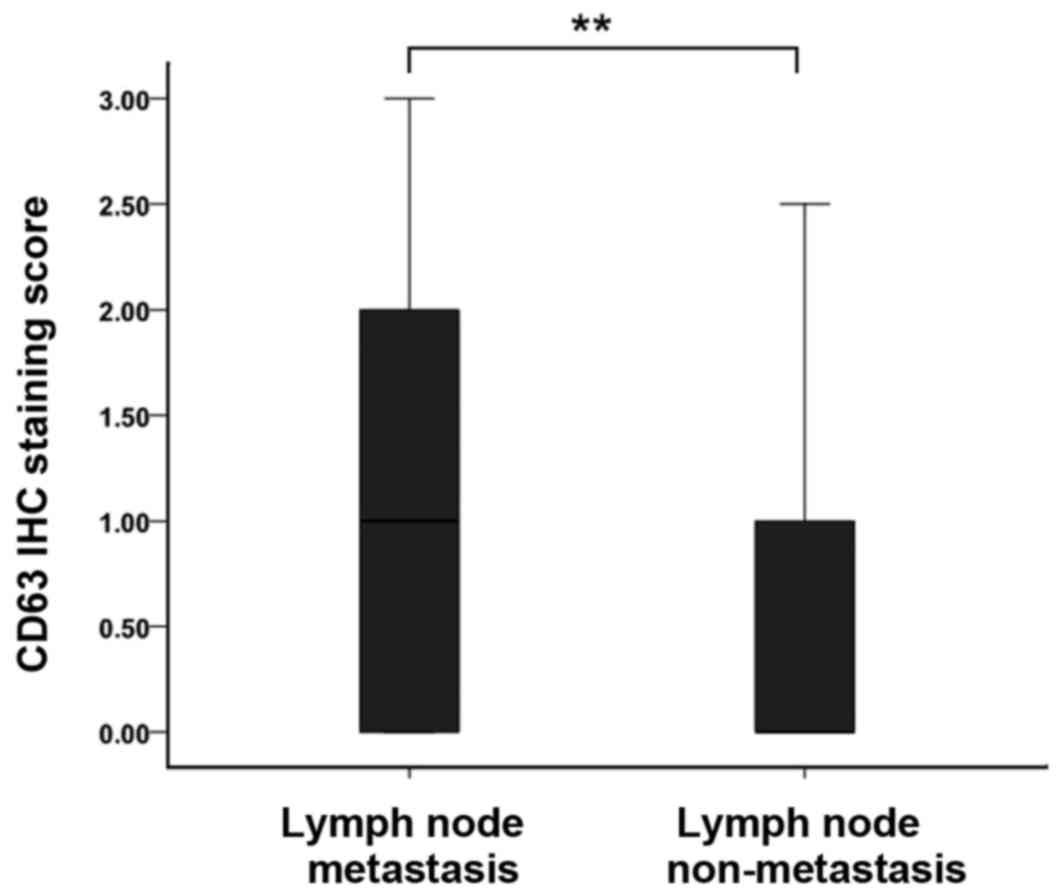
levels were also found to be associated with lymph node metastasis
in EC cases. Weak positive staining of EC tissues was detected in
patients with lymph node metastasis. However, in the patients
without metastatic lymph nodes, the CD63 staining was strongly
positive (Fig. 3). This suggests that
CD63 may have an important role in the lymph node metastasis of
EC.
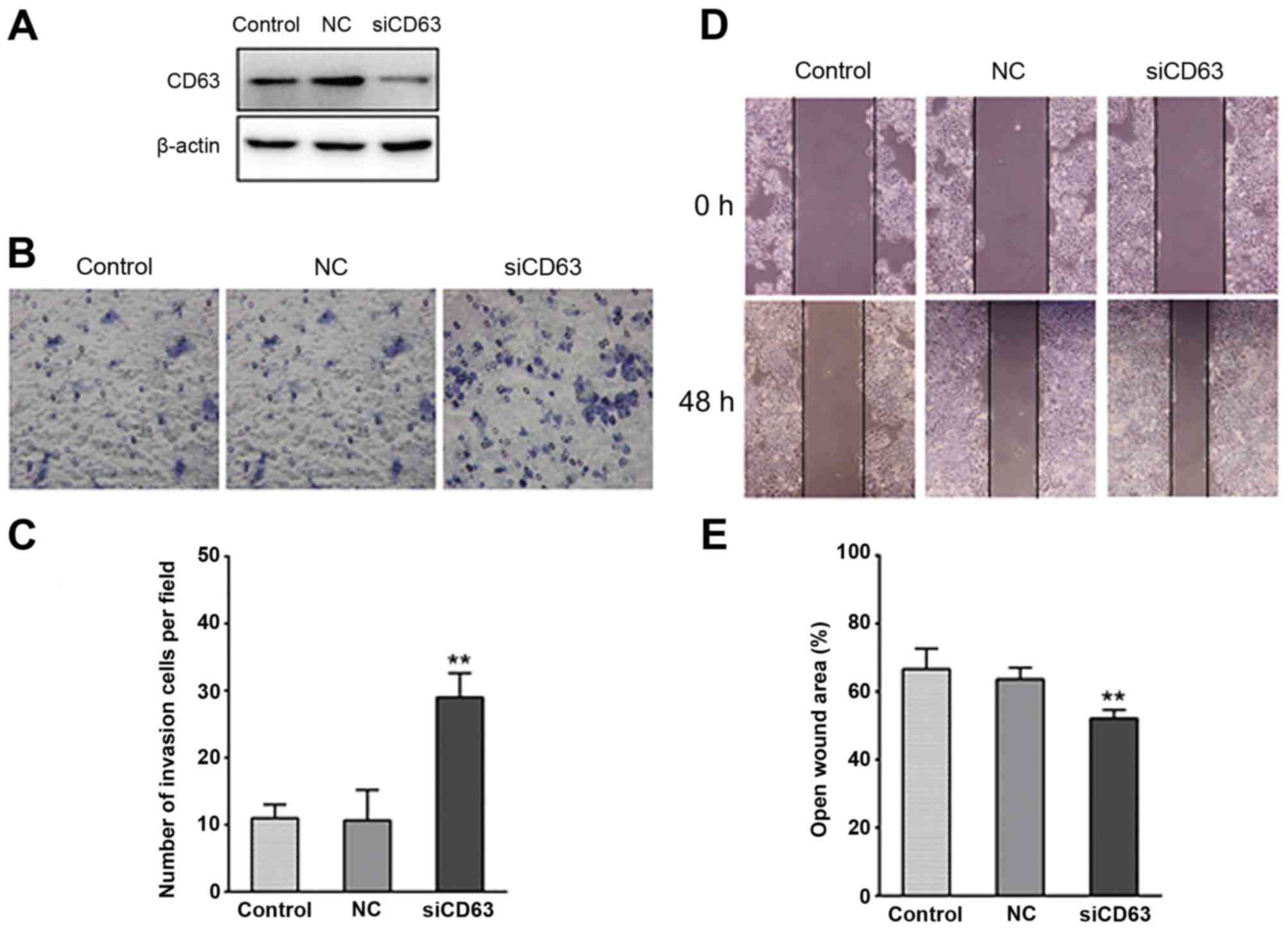
Downregulation of CD63 promotes
migration of EC cells
To investigate the role of reduced CD63 expression
in the migration of EC, the esophageal cancer cells TE-1 were
transfected with CD63-silencing RNA. Western blot analysis revealed
that CD63 protein expression was markedly reduced by the siRNA
transfection (Fig. 4A). Matrigel
invasion assays were performed to explore the effect of CD63 on the
invasiveness of TE-1 cells. As presented in Fig. 4B and C, the ability of TE-1 cells to
invade through the Matrigel and membrane was increased by CD63
knockdown when compared with the control cells or the empty plasmid
transfected cells. The CD63 siRNA transfected TE-1 cells exhibited
a ~2-fold higher invasiveness than the control cells. In
vitro wound healing assays were performed to detect the
motility of TE-1 cells, which is another important characteristic
of metastatic cells. As presented in Fig.
4D and E, CD63 knockdown noticeably reduced the wound area,
suggesting that CD63 knockdown enhanced the migration capability of
esophageal cancer.
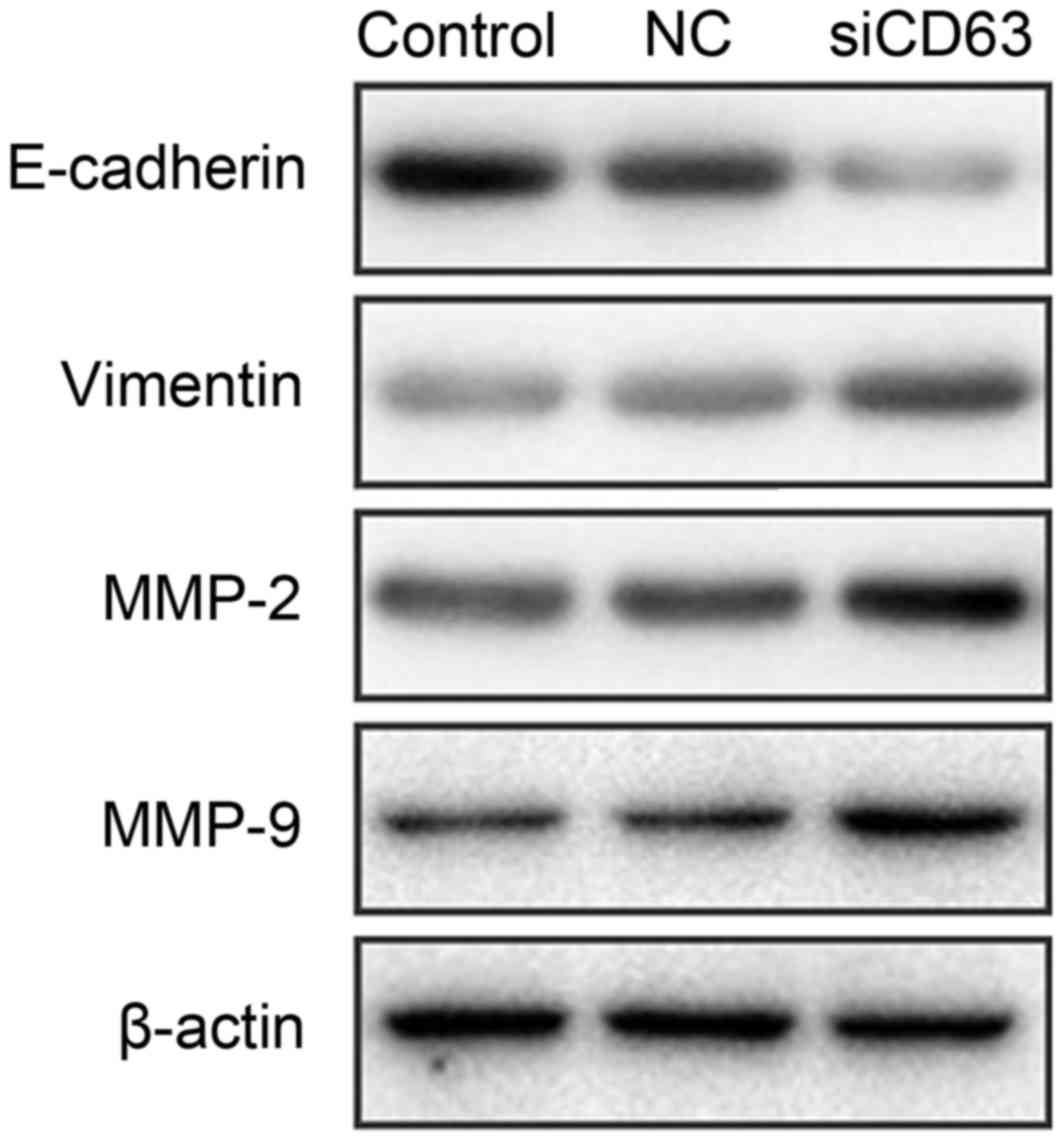
CD63 knockdown enhances invasiveness
of EC cells via promoting EMT
To explore the mechanisms underlying the effect of
CD63 on the invasiveness of EC, TE-1 cells were transfected with
CD63 siRNA or empty plasmid. After 48 h of incubation, western
blotting assays were used to detect the expression of
EMT-associated proteins and MMPs. E-cadherin was established as the
epithelial maker, and vimentin as the mesenchymal maker. As
presented in Fig. 5, TE-1 cells
transfected with CD63 siRNA exhibited downregulation of E-cadherin
expression and upregulation of vimentin when compared with the
control cells or the empty plasmid transfected cells. The matrix
metalloproteinase MMP-2 and MMP-9 expression was increased by CD63
knockdown. These results suggest that CD63 knockdown regulates the
invasiveness of esophageal cancer cells via promoting EMT.
Discussion
The prognosis of esophageal carcinoma remains poor
despite advances in aggressive treatment for EC (3,4), due to
late diagnosis and the rapid metastasis of cancer cells (24,25). The
present study investigated CD63 as a potential marker for the
migration capability of esophageal carcinoma.
The TM4SF is a group of cell surface proteins with
four transmembrane domains. CD63 was the first tetraspanin to be
characterized and it was found to be located on human chromosome
12q13 (26). Various studies have
demonstrated that CD63 regulates intracellular transport and
localization by interacting with a number of proteins (27,28). CD63
is a member of the tetraspanin proteins, and is a cell surface
glycoprotein. CD63 has been reported to be upregulated in breast
cancer, astrocytoma and melanoma, and is involved in various
biological processes (18,26,29). In
the present study, the protein levels of CD63 in EC tissues were
revealed to be significantly increased compared with adjacent tumor
tissues or the normal esophagus mucosa tissues. Furthermore, the
tetraspanin CD63 has been suggested to be associated with the
biological behavior of solid tumors, and has particularly been
implicated in metastatic signaling pathways (30). The hematogenous metastasis of
CD63-negative human melanoma cells in nude mice may be reduced by
CD63 transfection (15). Kwon et
al (18) suggested that CD63
negativity has a potential as a biomarker for predicting poor
prognosis in earlier stage lung adenocarcinoma. Jung et al
(31) identified CD63 as a cell
surface-binding partner for TIMP-1, regulating the polarization and
survival of breast epithelial cells. However, the expression
pattern and clinicopathological roles of CD63 have not been
investigated in EC in previous studies. In the current study, CD63
negativity was significantly associated with advanced tumor stage
and a greater number of metastatic lymph nodes in EC. Intensely
immunostained samples were identified in earlier stage (I and II)
EC. In advanced stage (III and IV) EC, the samples were weakly
immunostained. A previous study has demonstrated that CD63
expression is weaker as the stage advances in melanoma (14), consistent with the findings of the
current study in esophageal cancer. Lymph node metastasis is one of
the most important prognostic factors in EC, and the number of
metastatic lymph nodes is consistently associated with the
long-term outcomes of patients with EC (6). Regarding a potential correlation with
lymph node metastasis, the current study revealed that CD63 protein
expression was weaker as the number of metastatic lymph nodes
increased. To further elucidate the role of CD63 in EC progression,
wound healing migration and matrigel invasion assays were performed
to detect the invasiveness of the TE-1 esophageal cancer cell line.
Following siRNA-induced CD63 knockdown, the invasiveness of TE-1
cells was enhanced. In the current study, CD63 suppressed the
metastasis of EC cells. In a previous study, CD63 was originally
described as being involved in cancer metastasis (30). Tominaga et al (32) suggested that RPN2-mediated CD63
glycosylation regulates breast cancer cell malignancy, including
drug resistance and invasion. Jang et al (19) reported that reduced CD63 expression
contributes to the invasive and metastatic ability of human
melanoma cells, due to increased cell motility, matrix proteolytic
capability and ability to detach from the surrounding matrix. CD63
has also been implicated in regulating the functions of the tumor
development-associated protein, membrane-associated type-1 MMP in
ECM turnover, thereby increasing cell invasiveness and metastasis
(33). The association between CD63
and integrins, including α4β1, α3β1, α6β1, LFA-1 and β2, was
reported to be involved in increased tumor cell motility and
metastasis, which mediates binding to the ECM (34–37). CD63
potentially interacts with numerous other proteins such as CD9,
CD81 and β1 integrins, contributing to the downstream cell
signaling pathway (38). The
association between CD63 and β1 integrins has been observed in
human melanoma cells, and integrins were described as having major
roles in the invasive capacity acquisition of cancer cells
(30). TIMP-1 was identified to
regulate the integrin signaling complex via its interaction with
CD63 on the cell surface (31). CD63
associated with CD9 has also been revealed to suppress the motility
and metastasis of mouse melanoma cells by downregulation of CD9
expression (39). It has been
reported that a complex in the surface membrane of melanoma cells
formed by CD63, CD9 and CD81 was involved in signal transduction
(38). Seubert et al (17) reported that CD63 increased the tumor
cell intrinsic metastatic potential by initiating
β-catenin-dependent EMT and then affecting cell plasticity in human
ovarian carcinoma, human gastric carcinoma and mouse melanoma
cells. The current study identified the pro-metastatic role of
downregulated CD63 expression in esophageal cancer, and examined
the underlying mechanism. In addition, the epithelial marker
E-cadherin was downregulated and the mesenchymal maker vimentin was
upregulated by CD63 knockdown, suggesting that CD63 may function as
a potent inhibitor of metastasis in EC via regulating the EMT. MMP
family proteins are involved in the breakdown of ECM and
participate in the process of cancer metastasis (40). A number of studies have reported that
EMT-inducing transcription factors are directly or indirectly
involved in cancer cell metastasis through various signaling
cascades, with the ultimate consequence of the upregulation of
metastatic proteins, including MMPs (41). The present study identified that MMP-2
and MMP-9 expression levels were increased by CD63 knockdown,
suggesting that CD63 knockdown-induced EC invasion may be due to
MMP-2 and MMP-9 overexpression through the activation of EMT signal
pathway. However, the underlying mechanism by which CD63 activates
EMT in EC remains to be investigated.
In conclusion, to the best of our knowledge the
present study revealed for the first time that CD63 expression was
upregulated in EC tissues, and was negatively correlated with tumor
stage and lymph node metastasis. CD63 knockdown enhanced EC cell
invasion, and was mediated by MMP-2 and MMP-9 overexpression
through the activation of EMT signaling pathway. This indicates
that CD63 may be a promising biomarker to predict the risk of EC
progression.
Acknowledgements
The present study was supported by grants from the
Zhejiang Medical Science and Technology Foundation (grant nos.
2014KYA007 and 2014KYA009).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dipetrillo T, Suntharalingam M, Ng T,
Fontaine J, Horiba N, Oldenburg N, Perez K, Birnbaum A, Battafarano
R, Burrows W and Safran H: Neoadjuvant paclitaxel poliglumex,
cisplatin, and radiation for esophageal cancer: A phase 2 trial. Am
J Clin Oncol. 35:64–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Hagen P, Hulshof MC, van Lanschot JJ,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al: Preoperative
chemoradiotherapy for esophageal or junctional cancer. N Engl J
Med. 366:2074–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eloubeidi MA, Desmond R, Arguedas MR, Reed
CE and Wilcox CM: Prognostic factors for the survival of patients
with esophageal carcinoma in the U.S.: The importance of tumor
length and lymph node status. Cancer. 95:1434–1443. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho JW, Choi SC, Jang JY, Shin SK, Choi
KD, Lee JH, Kim SG, Sung JK, Jeon SW, Choi IJ, et al: Lymph node
metastases in esophageal carcinoma: An Endoscopist's view. Clin
Endosc. 47:523–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maecker HT, Todd SC and Levy S: The
tetraspanin superfamily: Molecular facilitators. FASEB J.
11:428–442. 1997.PubMed/NCBI
|
|
8
|
Hunziker W and Geuze HJ: Intracellular
trafficking of lysosomal membrane proteins. Bioessays. 18:379–389.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Atkinson B, Ernst CS, Ghrist BF, Herlyn M,
Blaszczyk M, Ross AH, Herlyn D, Steplewski Z and Koprowski H:
Identification of melanoma-associated antigens using fixed tissue
screening of antibodies. Cancer Res. 44:2577–2581. 1984.PubMed/NCBI
|
|
10
|
Hemler ME: Tetraspanin proteins mediate
cellular penetration, invasion and fusion events and define a novel
type of membrane microdomain. Annu Rev Cell Dev Biol. 19:397–422.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hemler ME, Mannion BA and Berditchevski F:
Association of TM4SF proteins with integrins: Relevance to cancer.
Biochim Biophys Acta. 1287:67–71. 1996.PubMed/NCBI
|
|
12
|
Hölters S, Anacker J, Jansen L,
Beer-Grondke K, Dürst M and Rubio I: Tetraspanin 1 promotes
invasiveness of cervical cancer cells. Int J Oncol. 43:503–512.
2013.PubMed/NCBI
|
|
13
|
Kwon MS, Shin SH, Yim SH, Lee KY, Kang HM,
Kim TM and Chung YJ: CD63 as a biomarker for predicting the
clinical outcomes in adenocarcinoma of lung. Lung Cancer. 57:46–53.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kondoh M, Ueda M, Ichihashi M and Mishima
Y: Decreased expression of human melanoma-associated antigen ME491
along the progression of melanoma pre-canceroses to invasive and
metastatic melanomas. Melanoma Res. 3:241–245. 1993.PubMed/NCBI
|
|
15
|
Radford KJ, Mallesch J and Hersey P:
Suppression of human melanoma cell growth and metastasis by the
melanoma-associated antigen CD63 (ME491). Int J Cancer. 62:631–635.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sordat I, Decraene C, Silvestre T,
Petermann O, Auffray C, Piétu G and Sordat B: Complementary DNA
arrays identify CD63 tetraspanin and alpha3 integrin chain as
differentially expressed in low and high metastatic human colon
carcinoma cells. Lab Invest. 82:1715–1724. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seubert B, Cui H, Simonavicius N, Honert
K, Schäfer S, Reuning U, Heikenwalder M, Mari B and Kruger A:
Tetraspanin CD63 acts as a pro-metastatic factor via β-catenin
stabilization. Int J Cancer. 136:2304–2315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwon MS, Shin SH, Yim SH, Lee KY, Kang HM,
Kim TM and Chung YJ: CD63 as a biomarker for predicting the
clinical outcomes in adenocarcinoma of lung. Lung Cancer. 57:46–53.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jang HI and Lee H: A decrease in the
expression of CD63 tetraspanin protein elevates invasive potential
of human melanoma cells. Exp Mol Med. 35:317–323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Toricelli M, Melo FH, Peres GB, Silva DC
and Jasiulionis MG: Timp1 interacts with beta-1 integrin and CD63
along melanoma genesis and confers anoikis resistance by activating
PI3-K signaling pathway independently of Akt phosphorylation. Mol
Cancer. 12:222013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Z, Ran YL, Hu H, Pan J, Li ZF, Chen
LZ, Sun LC, Peng L, Zhao XL, Yu L, et al: TM4SF3 promotes
esophageal carcinoma metastasis via upregulating ADAM12m
expression. Clin Exp Metastasis. 25:537–548. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kong KL, Kwong DL, Fu L, Chan TH, Chen L,
Liu H, Li Y, Zhu YH, Bi J, Qin YR, et al: Characterization of a
candidate tumor suppressor gene uroplakin 1A in esophageal squamous
cell carcinoma. Cancer Res. 70:8832–8841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamasaki M, Miyata H, Miyazaki Y,
Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mori M and Doki
Y: Evaluation of the nodal status in the 7th edition of the
UICC-TNM classification for esophageal squamous cell carcinoma:
Proposed modifications for improved survival stratification: Impact
of lymph node metastases on overall survival after esophagectomy.
Ann Surg Oncol. 21:2850–2856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
D'Amico TA: Outcomes after surgery for
esophageal cancer. Gastrointest Cancer Res. 1:188–196.
2007.PubMed/NCBI
|
|
25
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hotta H, Ross AH, Huebner K, Isobe M,
Wendeborn S, Chao MV, Ricciardi RP, Tsujimoto Y, Croce CM and
Koprowski H: Molecular cloning and characterization of an antigen
associated with early stages of melanoma tumor progression. Cancer
Res. 48:2955–2962. 1988.PubMed/NCBI
|
|
27
|
Lin D, Kamsteeg EJ, Zhang Y, Jin Y,
Sterling H, Yue P, Roos M, Duffield A, Spencer J, Caplan M and Wang
WH: Expression of tetraspan protein CD63 activates protein-tyrosine
kinase (PTK) and enhances the PTK-induced inhibition of ROMK
channels. J Biol Chem. 283:7674–7681. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshida T, Ebina H and Koyanagi Y:
N-linked glycan-dependent interaction of CD63 with CXCR4 at the
Golgi apparatus induces downregulation of CXCR4. Microbiol Immunol.
53:629–635. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sauer G, Kurzeder C, Grundmann R,
Kreienberg R, Zeillinger R and Deissler H: Expression of
tetraspanin adaptor proteins below defined threshold values is
associated with in vitro invasiveness of mammary carcinoma cells.
Oncol Rep. 10:405–410. 2003.PubMed/NCBI
|
|
30
|
Radford KJ, Thorne RF and Hersey P:
Regulation of tumor cell motility and migration by CD63 in a human
melanoma cell line. J Immunol. 158:3353–3358. 1997.PubMed/NCBI
|
|
31
|
Jung KK, Liu XW, Chirco R, Fridman R and
Kim HR: Identification of CD63 as a tissue inhibitor of
metalloproteinase-1 interacting cell surface protein. EMBO J.
25:3934–3942. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tominaga N, Hagiwara K, Kosaka N, Honma K,
Nakagama H and Ochiya T: RPN2-mediated glycosylation of tetraspanin
CD63 regulates breast cancer cell malignancy. Mol Cancer.
13:1342014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takino T, Miyamori H, Kawaguchi N, Uekita
T, Seiki M and Sato H: Tetraspanin CD63 promotes targeting and
lysosomal proteolysis of membrane-type 1 matrix metalloproteinase.
Biochem Biophys Res Commun. 304:160–166. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rubinstein E, Le Naour F,
Lagaudrière-Gesbert C, Billard M, Conjeaud H and Boucheix C: CD9,
CD63, CD81, and CD82 are components of a surface tetraspan network
connected to HLA-DR and VLA integrins. Eur J Immunol. 26:2657–2665.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Berditchevski F, Zutter MM and Hemler ME:
Characterization of novel complexes on the cell surface between
integrins and proteins with 4 transmembrane domains (TM4 proteins).
Mol Biol Cell. 7:193–207. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Berditchevski F, Bazzoni G and Hemler ME:
Specific association of CD63 with the VLA-3 and VLA-6 integrins. J
Biol Chem. 270:17784–17790. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Skubitz KM, Campbell KD, Iida J and
Skubitz AP: CD63 associates with tyrosine kinase activity and
CD11/CD18 and transmits an activation signal in neutrophils. J
Immunol. 157:3617–3626. 1996.PubMed/NCBI
|
|
38
|
Radford KJ, Thorne RF and Hersey P: CD63
associates with transmembrane 4 superfamily members, CD9 and CD81,
and with beta 1 integrins in human melanoma. Biochem Biophys Res
Commun. 222:13–18. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Si Z and Hersey P: Expression of the
neuroglandular antigen and analogues in melanoma. CD9 expression
appears inversely related to metastatic potential of melanoma. Int
J Cancer. 54:37–43. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zucker S and Vacirca J: Role of matrix
metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis
Rev. 23:101–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tania M, Khan MA and Fu J: Epithelial to
mesenchymal transition inducing transcription factors and
metastatic cancer. Tumour Biol. 35:7335–7342. 2014. View Article : Google Scholar : PubMed/NCBI
|