Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-associated mortality worldwide. It has been
reported to have the second lowest five-year survival rate of all
tumor types in China (1). A number of
patients with early HCC did not have any clinical symptoms.
Patients with early liver cancer with radical resection may have a
better survival rate. Advanced liver cancer prognosis is very poor
(2) Thus, early detection and
monitoring of HCC is critical for successful clinical therapy.
α-fetoprotein (AFP) has been considered useful in the screening and
early diagnosis of HCC. Although in one study >60% of patients
with HCC had an AFP level of above 400 ng/ml (3), up to 50% of patients with HCC may have
an AFP level below 20 ng/ml. Therefore, AFP should not be used as
the only method to screen for HCC (4). Due to the lack of effective early
detection methods, HCC patients have frequently been diagnosed at
an advanced stage or have progressed rapidly following therapy
(5).
There have been numerous advances in the
identification of potential biomarkers for HCC. Tat-interacting
protein 30 (HTATIP2/TIP30), also called CC3, has been identified as
a transcriptional cofactor that enhances Tat-mediated transcription
(6).
The purpose of the current study was to measure
serum levels of HTATIP2/TIP30, and determine its potential
diagnostic and prognostic ability in patients with HCC.
Patients and methods
Patients
A total of 114 subjects, including 52 HCC patients
diagnosed by histopathology, of whom 67% had cirrhosis, were
enrolled in the study. A further 62 cases were enrolled in a
control group consisting of 32 healthy individuals who did not have
HCC, and 30 patients with liver cirrhosis without HCC. All the
subjects were admitted to the Hunan Provincial People's Hospital,
Changsha, China, between September 2013 and December 2013. Prior to
therapy, computed tomography and ultrasound scans, as well as
biochemical and serological parameters were obtained. Three
milliliters of blood were taken from each patient. Patients who
were diagnosed with HCC by histopathology or clinical parameters
were included in this study. Patients who were diagnosed with
benign hepatic tumors were excluded. This study was approved by the
Local Human Ethics Committee of the Ministry of Health in China.
Blood and tissue were obtained after the patients provided written
informed consent.
The clinical characteristics of the study population
are shown in Table I. Patients were
scored according to the Child-Pugh classification system (1972).
The clinical biochemical indexes used in the Child-Pugh
classification system were as follows: Hepatic encephalopathy
(grade), nothing (1 points), 1–2 (2 points), 3–4 (3 points);
ascites, light (1 point), moderate (2 points) and severe (3
points); total bilirubin (µmol/l), <34 (1 point), 34–51 (2
points), >51 (3 points); albumin (g/l), >35 (1 point), 28–35
(2 points), <28 (3 points); and prothrombin time (sec), <4 (1
point), 4–6 (2 points), >6 (3 points). These scores were
subsequently added to yield the final classification score: Class
A, 5–6 points; class B, 7–9 points; and class C: ≥10 points
(7).
 | Table I.Baseline characteristics of
samples. |
Table I.
Baseline characteristics of
samples.
| Characteristics | Hepatocellular
carcinoma patients | Controls |
|---|
| Number of
patients | 52 | 62 |
|
Male/female (%) | 45 (86.5%)/7
(13.4%) | 30/32 |
| Age, years | 54±12 | 43±15 |
| Tumor size (cm) | 5.6±4.9 | – |
| Tumor number
(single/multiple) (%) | 22 (42.3%)/30
(57.6%) | – |
| TNM score
(I/II/III/IV) (%) | 7 (13.46%)/21
(40.38%)/14 (26.92%)/10 (19.23%) | – |
| Child-Pugh score
(A/B/C) (%) | 37 (71.15%)/10
(19.23%)/5 (9.61%) | – |
| Portal vein
thrombosis (Y/N/Unclear), n (%) | 7 (19.23%)/42
(80.87%)/3 (5.76%) | – |
| Lymph node
metastasis (Y/N/Unclear), n (%) | 7 (19.23%)/43
(80.87%)/2 (3.84%) | – |
| Distantmetastasis
(Y/N), n (%) | 10 (19.23%)/42
(80.87%) | – |
| Viral infection
(Y/N), n (%) | 40 (76.90%)/12
(23.07%) | 6 (9.6%) |
|
HBsAg-positive | 39 | 6 |
| HCV
Ab-positive | 1 | 0 |
| BUN (mmol/l) | 6.22±4.29 | 5.69±2.67 |
| Creatinine
(µmol/l) | 81.70±61.70 | 56.30±30.80 |
Blood sampling
Blood samples were collected and centrifuged at
3,200 × g for 10 min at 4°C. The sera were kept at −80°C until the
biochemical measurements for HTATIP2/TIP30 evaluation were
conducted.
Biochemical analyses
HTATIP2/TIP30 levels were determined using a
commercially available ELISA kit (Cusabio Biotech, Wuhan, China;
cat. no. CSB-E14917H) (8). For this
kit, the minimum detectable level of human HTATIP2/TIP30 was 0.16
ng/ml. The sensitivity of this assay, or the lower limit of
detection, was defined as the lowest protein concentration that
could be differentiated from zero. The intra-assay precision
(precision within an assay) had a coefficient of variation
percentage (CV%) <8%, and the inter-assay precision (precision
between assays) was CV% <10%) according to the kit protocol.
Immunohistochemistry studies
HTATIP2/TIP30 levels were determined by
immunohistochemistry using HTATIP2/TIP30 (Abgent Biotech Co, Ltd.,
Suzhou, China; AP14762a) concentrated rabbit anti-human
HTATIP2/TIP3 monoclonal antibody, 1:200, for 1 h at room
temperature. The percentage of the number of positive cells and the
total number of cells were determined. Staining intensity was
scored as follows: 0 (negative), 1 (low), 2 (moderate) or 3
(strong). The number of positive cells was scored as follows:
<10%, 11–25%, 26–50% and 51–100% were assigned 0, 1, 2 or 3
points, respectively. The two scores were multiplied, and the mean
value of five visual fields was obtained. The total scores were 0–3
(−); 4–5 (+); 6–8 (++) or 9 (+++).
Statistical analyses
SPSS for Windows version 17.0 (SPSS, Inc., Chicago,
IL, USA) was used for statistical calculations. The results were
expressed as the means ± standard deviation. Analysis of variance
(ANOVA) and multiple comparisons were used to determine significant
differences in HTATIP2/TIP30 levels between the healthy controls
and HCC groups and subgroups (I–II stage and III–IV stage). ROC
analysis was used to detect the optimal cut-off points for
separating the healthy control group from the HCC group, its
subgroups with metastasis and without metastasis (III–IV stage and
I–II stage), and I–II stage patients from healthy controls.
Correlations between HTATIP2/TIP30 and clinical characteristics
were assessed using Spearman correlation coefficients. Logistic
regression analysis results established a combined model of
HTATIP2/TIP30 and AFP.
Results
Serum levels of HTATIP2/TIP30
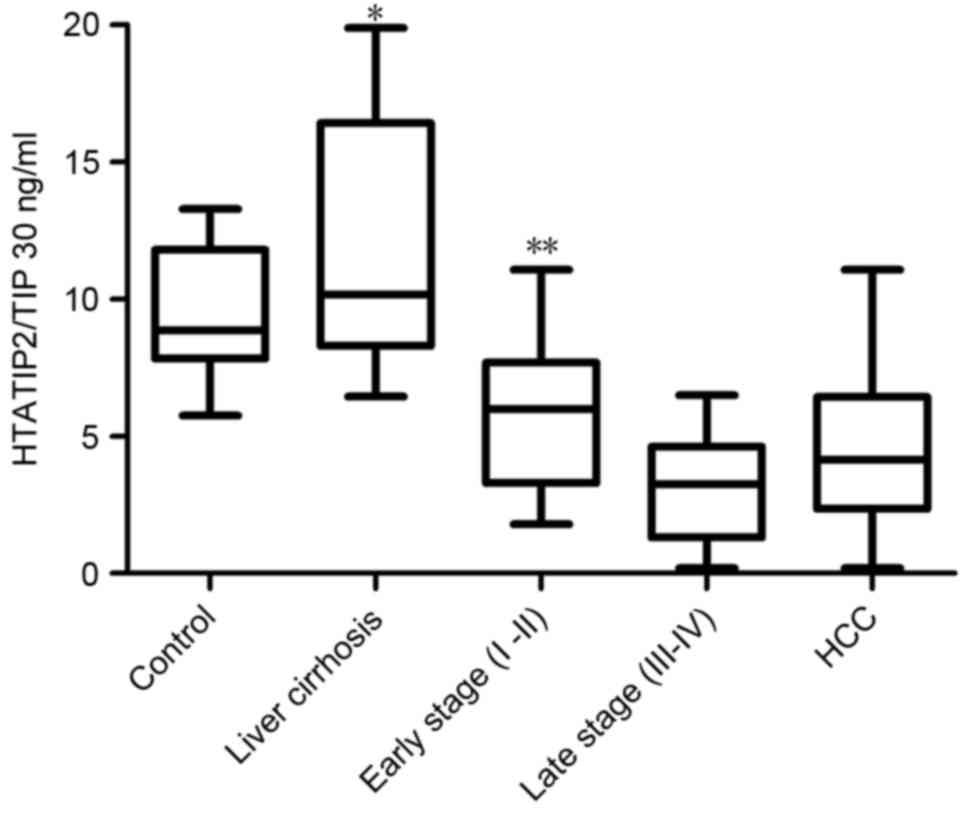
HTATIP2/TIP30 levels were significantly lower in the
HCC group (mean, 4.50±2.63 ng/ml; range, 2.35–6.41 ng/ml) compared
with those of the control group (mean, 9.50±2.04 ng/ml; range,
7.82–11.79 ng/ml; P<0.001; Fig.
1). For the subgroups, the serum levels of HTATIP2/TIP30 were
significantly lower in the late stages (III–IV) (mean, 3.02±1.79
ng/ml; range, 2.26–3.77 ng/ml) than in the early stages (I–II)
(mean, 5.78±2.59 ng/ml; range, 4.77–6.78 ng/ml; P=0.001; Fig. 1) as determined by ANOVA and multiple
comparison analysis.
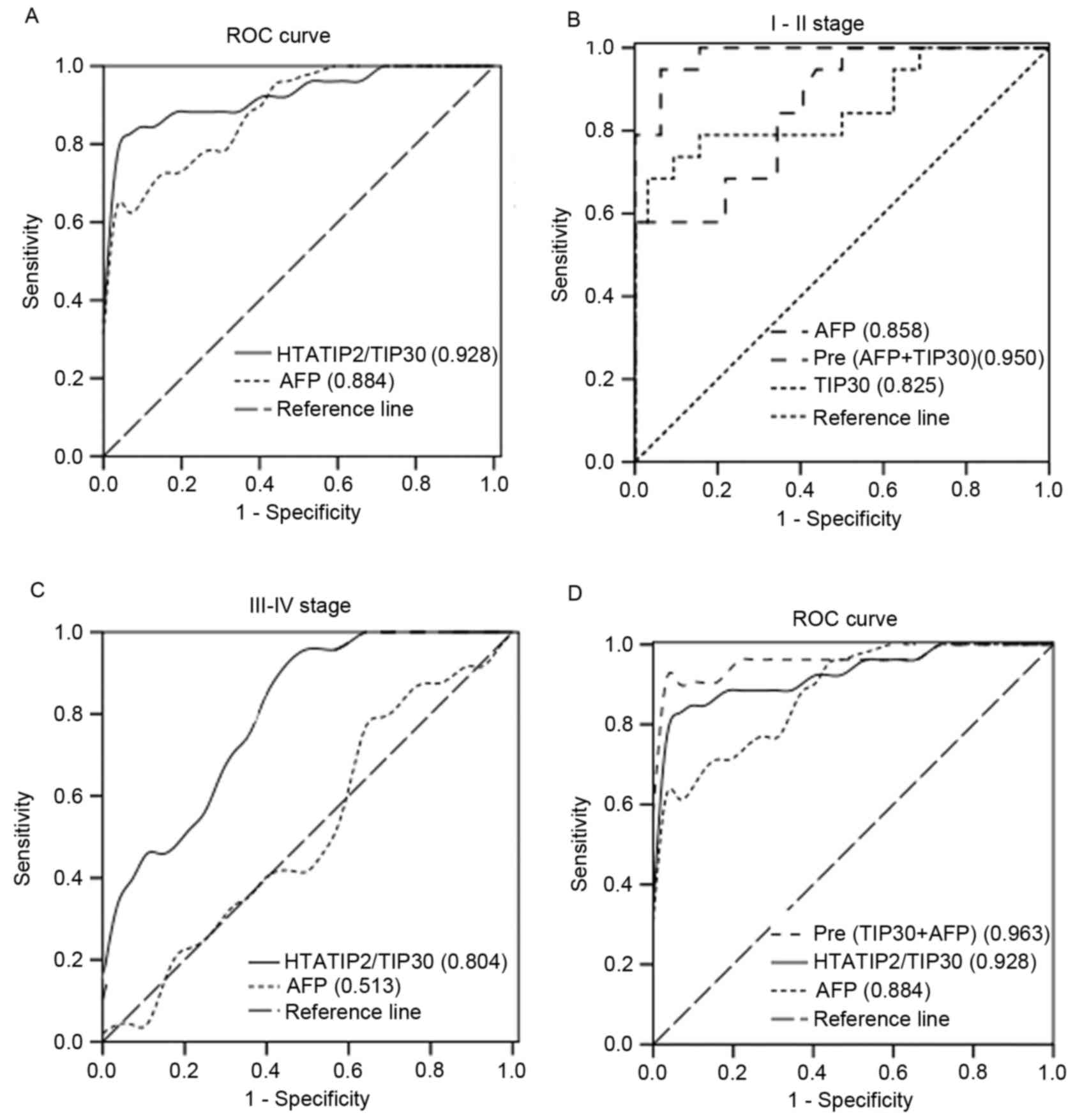
ROC curves for HTATIP2/TIP30 and
AFP
ROC analysis was used to detect the optimal cut-off
points of HTATIP2/TIP30 (7.27 ng/ml) and AFP (32.4 ng/ml) for
discrimination between HCC patients and healthy individuals. The
areas under the curve (AUCs) of HTATIP2/TIP30 and AFP were 0.928
(0.928±0.027, P<0.001) and 0.884 (0.884±0.035, P<0.001),
respectively (Fig. 2A). ROC analysis
was also used to detect the optimal cut-off points of HTATIP2/TIP30
(8.98 ng/ml) and AFP (2.15 ng/ml) for discrimination between the
I–II stage group and the control group. When combined, the AUC was
greatly increased (0.950) (Fig. 2B).
The HTATIP2/TIP30 AUC was greater than that of AFP allowing
discrimination between the III–IV group and the I–II group
(Fig. 2C). ROC analysis of the
logistic regression model of combined HTATIP2/TIP30 and AFP
demonstrated greater sensitivity and specificity than either marker
alone (Fig. 2D). ROC AUCs for
combined HTATIP2/TIP30 and AFP and those for HTATIP2/TIP30 and AFP
individually are shown in Table II,
Comparison of sensitivity and specificity for HTATIP2/TIP30 and AFP
among patients with HCC and control and all stages of HCC are shown
in Table III.
 | Table II.Comparison of receiver operating
characteristic AUCs for HTATIP2/TIP30 and AFP among patients with
all stages of HCC. |
Table II.
Comparison of receiver operating
characteristic AUCs for HTATIP2/TIP30 and AFP among patients with
all stages of HCC.
| Serum marker |
HTATIP2/TIP30AUC | AFP AUC | HTATIP2/TIP30 plus
AFP AUC |
|---|
| HCC and
control | 0.928 | 0.884 | 0.963 |
| I–II stage and
control | 0.825 | 0.858 | 0.950 |
 | Table III.Comparison of sensitivity and
specificity for HTATIP2/TIP30 and AFP among patients with all
stages of HCC. |
Table III.
Comparison of sensitivity and
specificity for HTATIP2/TIP30 and AFP among patients with all
stages of HCC.
|
| HTATIP2/TIP30
(%) | AFP (%) |
|---|
|
|
|
|
|---|
| Groups | Sensitivity | Specificity | Youden | Sensitivity | Specificity | Youden |
|---|
| HCC and
control | 84.6 | 93.7 | 78.3 | 61.5 | 100.0 | 61.5 |
| I–II stage and
control | 88.2 | 50.0 | 38.2 | 94.1 |
59.4 | 53.3 |
| III–IV and I–II
stages | 91.7 | 51.7 | 43.4 | 79.2 |
35.7 | 14.9 |
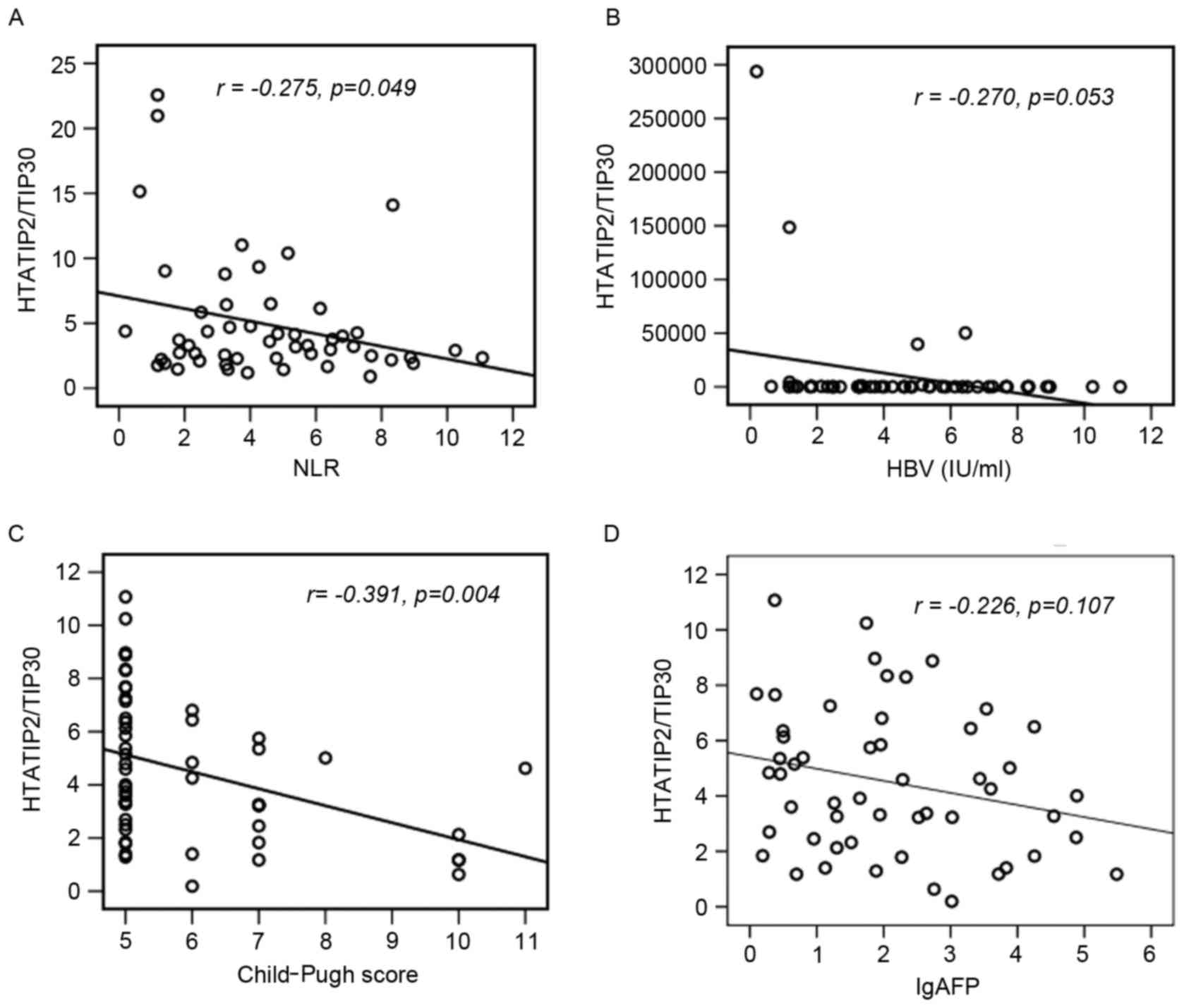
Correlation between clinical
characteristics and HTATIP2/TIP30
Correlations between HTATIP2/TIP30 levels, tumor
size, hepatitis B virus (HBV) DNA loads, neutrophil-lymphocyte
ratios (NLR), Child-Pugh scores and AFP levels were analyzed.
HTATIP2/TIP30 levels demonstrated a statistically significantly
negative correlation with the NLR (r=−0.275, P=0.049; Fig. 3A), HBV DNA loads (r=−0.270, P=0.053;
Fig. 3B) and Child-Pugh scores
(r=−0.391, P=0.004; Fig. 3C), but
were not associated with AFP (r=−0.226, P=0.107; Fig. 3D).
Logistic regression models to predict
HCC
Using logistic regression analysis of HTATIP2/TIP30
and AFP levels, odds ratio values were calculated to be 0.488 and
1.043, with a 95% confidence interval (CI) of 0.331–0.719 and
0.998–1.089, respectively (Table
IV). In the ROC analysis of the logistic model for
HTATIP2/TIP30 and AFP, the AUC (0.963, Fig. 2B) of the combination of HTATIP2/TIP30
and AFP had a greater sensitivity of 90.6%, and specificity of
90.4%, compared with the values of the two indicators alone.
 | Table IV.Logistic regression model by
HTATIP2/TIP30 and AFP. |
Table IV.
Logistic regression model by
HTATIP2/TIP30 and AFP.
| Predictor | P-value | OR | 95% CI |
|---|
| HTATIP2/TIP30 | <0.001 | 0.488 | 0.331–0.719 |
| AFP |
0.059 | 1.043 | 0.998–1.089 |
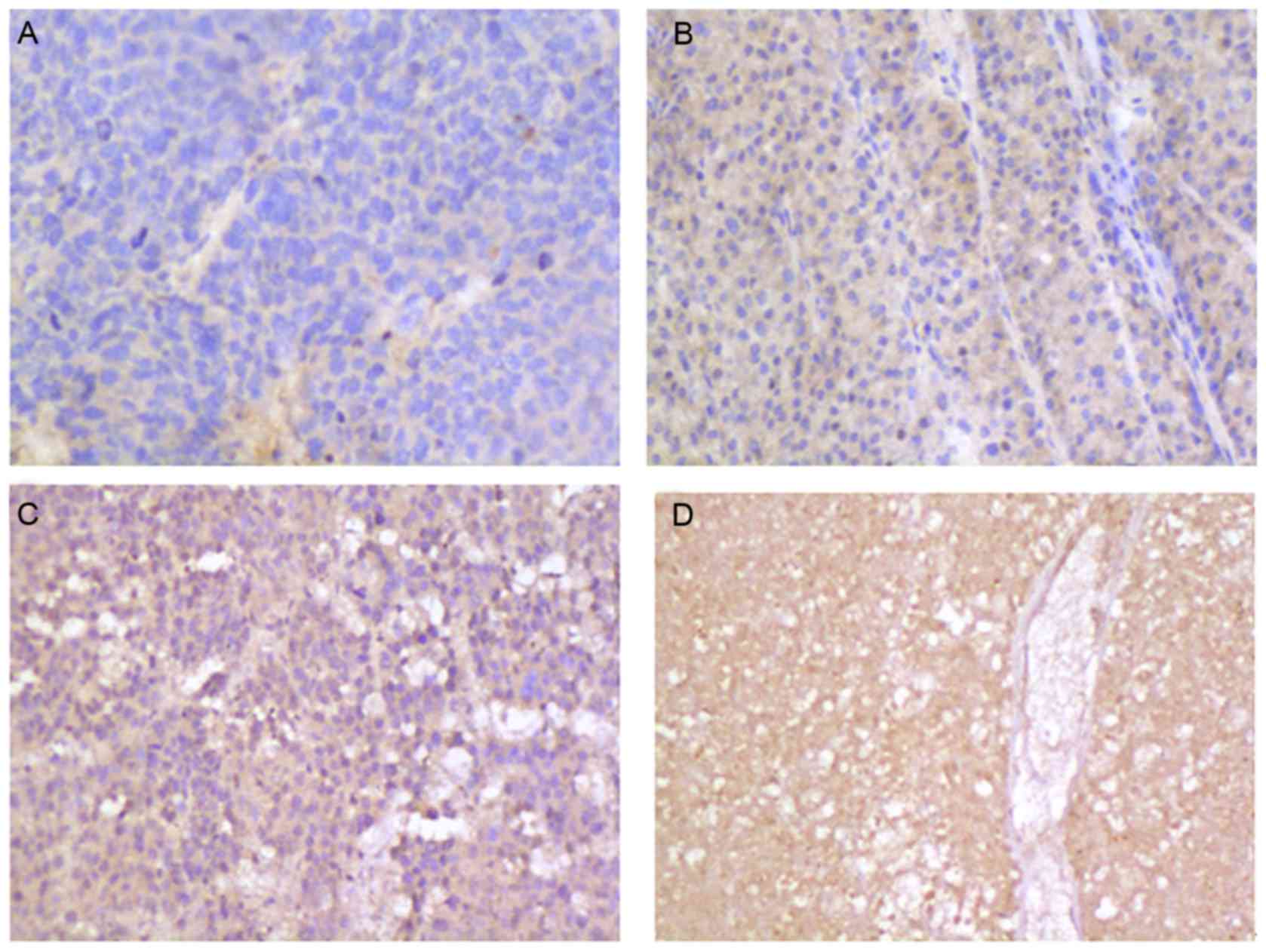
HTATIP2/TIP30 levels detected by
immunohistochemistry
HTATIP2/TIP30 levels were measured in HCC cells. No
HTATIP2/TIP30 was observed in 25 patients (25/52, 48.07%; Fig. 4A), a low level was observed in 12
patients (12/52, 23.07%; Fig. 4B), a
medium level in 9 patients (9/52, 17.30%; Fig. 4C), and a high level in 4 patients
(4/52, 7.69%; Fig. 4D). HTATIP2/TIP30
levels in serum demonstrated a statistically significant positive
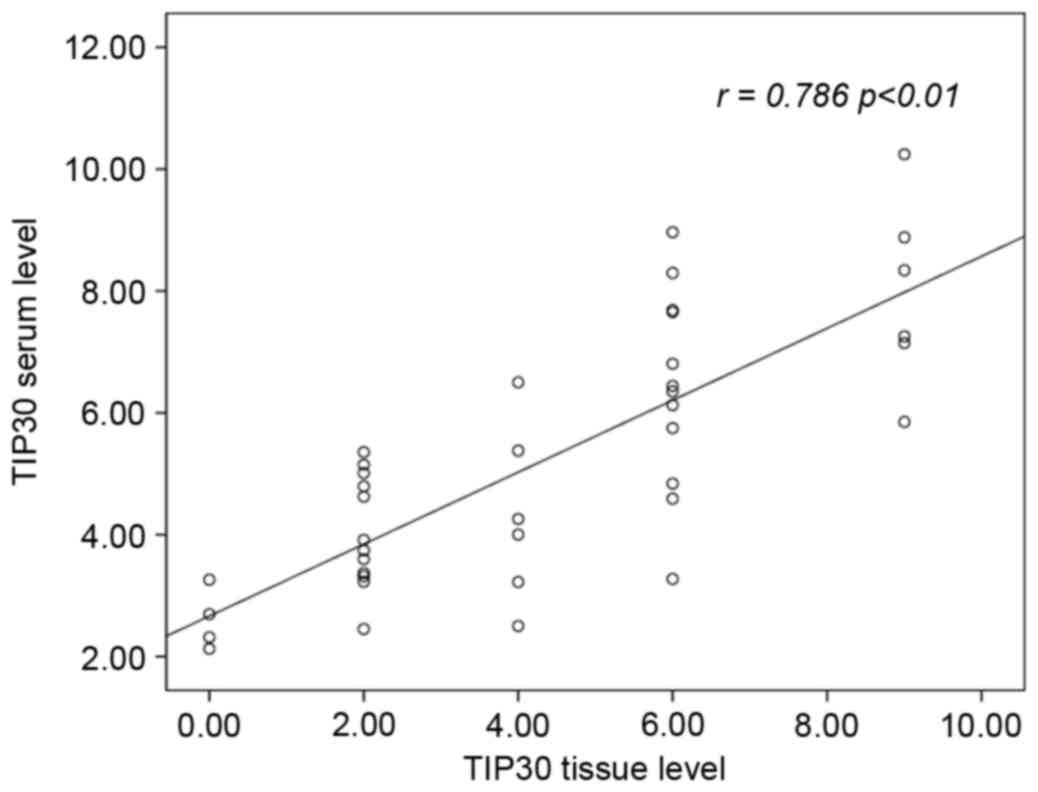
correlation with tissue levels (r= 0.768, P<0.01; Fig. 5).
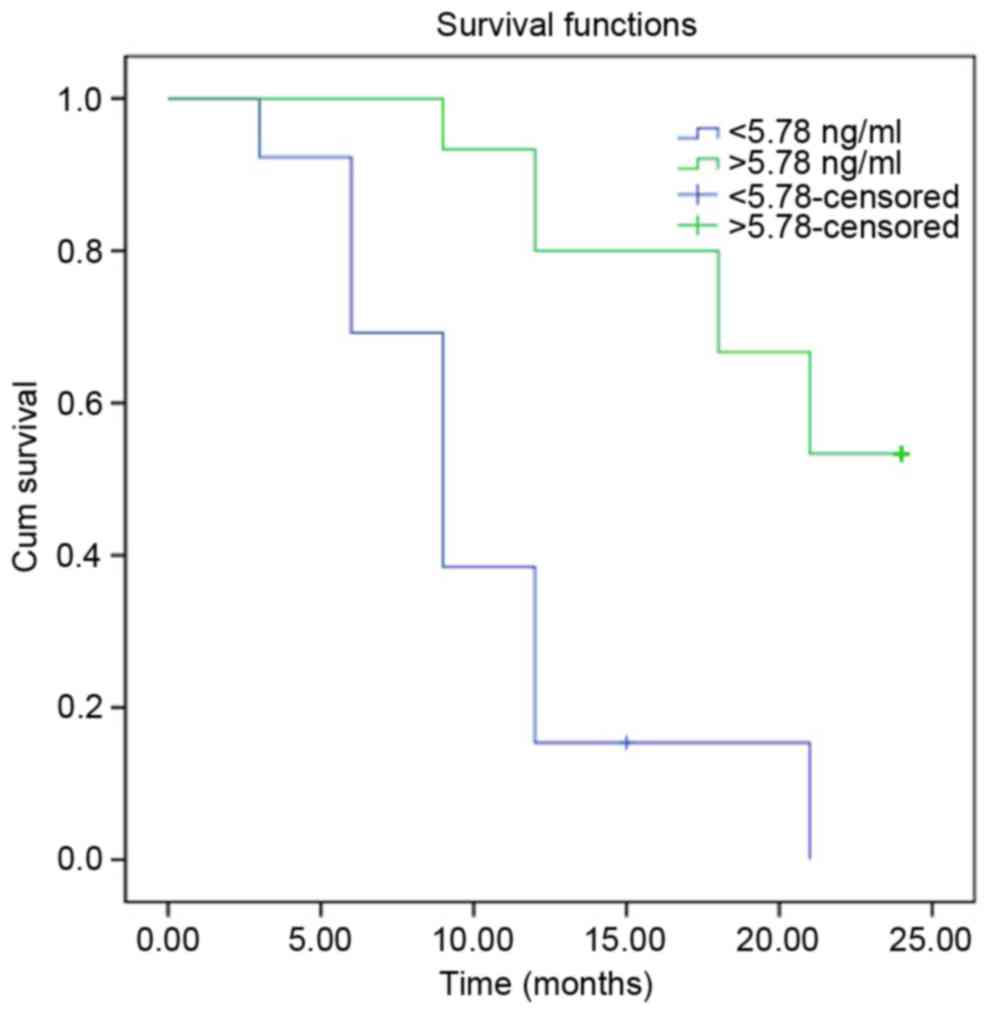
Log-rank analysis of I–II stage HCC
cases
Patients with stage I–II HCC were followed up for a
median period of 14 months, for a range of 3–24 months. Log-rank
analysis of the recurrence-free survival time of the HCC (I–II
stage) group demonstrated that for cases with HTATIP2/TIP30
>5.71 ng/ml, the time was significantly longer than for the
control group (HTATIP2/TIP30 <5.78 ng/ml) (P<0.001; Fig. 6). The current findings suggest that
HTATIP2/TIP30 levels may be an effective biomarker for the
treatment, diagnosis and estimation of prognosis for HCC.
Discussion
Downregulation of HTATIP2/TIP30 has been observed in
various cancer types including colon cancer, breast cancer,
neuroblastoma, melanoma, ovarian cancer and lung cancer (9–11). Tong
et al revealed that downregulation of HTATIP2/TIP30 promoted
metastasis, particularly in lung cancer (12). In fact, the roles of HTATIP2/TIP30
have been reported in particular with regard to controlling tumor
suppression. It has also been demonstrated that the expression of
the gene was involved in apoptosis and metastasis (11,12).
Studies have indicated that there is a signal pathway facilitated
by HTATIP2/TIP30 and its associated factors (10). Other studies have demonstrated that
the anti-metastatic properties of HTATIP2/TIP30 are due in part to
the inhibition of antigenic properties of tumor cells, and the
predisposition of tumor cells to apoptosis (13–15).
Previous studies of HTATIP2/TIP30 in HCC have
revealed that osteopontin and possibly other Ets-1 target genes
that are known to be involved in tumor metastasis are regulated by
HTATIP2/TIP30. The mechanism for metastasis has been demonstrated
to involve low levels of HTATIP2/TIP30 in HCC cell lines (16). It has been reported that the
HTATIP2/TIP30 gene may eradicate its native tumor-suppressor
activity and gain oncogenic activity partially through upregulation
of N-cadherin, thereby potentiating the pathogenesis of HCC
(17). Epigenetic silencing of
HTATIP2/TIP30 gene expression by CpG island DNA hypermethylation is
associated with poor prognosis in patients with HCC (18). T1406N in carbamoyl-phosphate synthase
1 and S197R in HTATIP2/TIP30 were observed to be highly associated
with HCC progression (19). There
have been a limited number of clinical studies on the status of
HTATIP2/TIP30 in HCC, and no studies of HTATIP2/TIP30 levels in HCC
patient serum.
The HTATIP2/TIP30 gene was independently identified
by differential display analysis of mRNA from a highly metastatic
human small cell lung carcinoma (SCLC) cell line when compared with
less metastatic SCLC cell lines (20). In addition, deletion of one or both
alleles of HTATIP2/TIP30 was associated with spontaneous
development of HCC and other tumors in mice. The fact that a
deficiency in HTATIP2/TIP30 was associated with tumorigenesis,
invasion and metastasis suggests that HTATIP2/TIP30 may act as a
tumor suppressor (21).
There has been significant progress in the
development of anti-cancer agents, including tyrosine kinase
inhibitors, angiogenesis inhibitors and agents that interact with
the cell cycle and cell death (apoptosis) (21). HTATIP2/TIP30 is an evolutionarily
conserved gene that is expressed ubiquitously in human tissues and
certain tumor tissues. The protein exhibits serine threonine kinase
activity that could phosphorylate the carboxyl terminal domain of
RNA polymerase II in a Tat-dependent manner (22). Therefore, it is essential to evaluate
its potential value.
It has been demonstrated that HTATIP2/TIP30 and
endothelial growth factor receptor (EGFR) have a close link in
signaling pathways in liver (8,21). As
reported previously, EGFR is central to the promotion of cell
growth, and has a role in the development of HCC (22). With the role of vascular endothelial
growth factor (VEGF) as a major antigenic factor in HCC, inhibiting
EGFR activity is an attractive method for anti-HCC treatment
(23,24). Zhang et al observed that
HTATIP2/TIP30 binding with endophilin B and acyl-CoA synthetase
long-chain family member 4 (ACSL4) forms a complex in the
cytoplasm. This is involved in the transport of EGFR (25). Following HTATIP2/TIP30-knockout,
EGF-induced EGFR degradation was inhibited, possibly due to the
fact that HTATIP2/TIP30, ACSL4 and endophilin B1 formed a complex
protein, which promoted EGFR from early endosomes into liposomal
sorting, and then accelerated the degradation of EGFR in liver
cells in mice and mammary cells (26). HTATIP2/TIP30 gene knockout cells could
not access early endosomes due to liposomal degradation, resulting
in the activation of EGFR and its downstream signaling, and
ultimately resulting in increased cell proliferation.
It was necessary to assess the HTATIP2/TIP30 gene
and evaluate its roles in the development of the HCC. In the
present study, it was observed that HTATIP2/TIP30 levels in the
serum samples of patients with HCC were significantly lower than
those of the normal control group. This protein was identified in
the cytoplasm of the tissue, and the level of protein was highly
consistent with the level in tissue. In addition, the current
results reveal that a much lower HTATIP2/TIP30 level was observed
in the HCC metastatic group than in the non-metastatic group. These
results all illustrate the potential value of the protein.
For further study of HTATIP2/TIP30, the usual
indicators of NLR, HBV viral load and Child-Pugh score were
combined to evaluate its diagnostic value. NLR has been evaluated
as a predictor of recurrence and survival in various malignancies.
Published data have also noted a correlation between elevated NLR
and a poorer prognosis in patients with HCC (27–29). The
number of HBV DNA copies present is associated with the risk of
liver cirrhosis and HCC worldwide (29,30).
Presently, ~80% of HCC patients have chronic HBV infection in China
(31,32). The Child-Pugh score has been widely
used for the prognosis of cirrhosis. A previous large systematic
review has revealed that the Child-Pugh score remains an essential
predictor (33). In the present
study, HTATIP2/TIP30 levels had a statistically significant
positive correlation with the NLR, HBV DNA load and Child-Pugh
score. HTATIP2/TIP30 levels also had a high sensitivity of 84.3% in
the present study, which is higher than that for AFP, alone or in
combination with des-gamma-carboxy prothrombin or AFP-L3 (78.3%)
recorded in a previous study (34).
These data indicate that HTATIP2/TIP30 levels alone or in
combination may be a useful biomarker for HCC.
In cases with AFP <400 ng/ml, among which ~30%
cases had lower HTATIP2/TIP30 levels, the average HTATIP2/TIP30
level was 4.518 ng/ml, and the ROC AUC of HTATIP2/TIP30 and AFP was
0.825 and 0.858, respectively. When combined, the AUC was 0.950.
Furthermore, the log-rank analysis of I–II stage HCC demonstrated
that patients with higher HTATIP2/TIP30 levels had significantly
longer recurrence-free survival times than the control group.
This study has limitations, including a relatively
small sample size. Further studies with more patients from multiple
centers are required to confirm these observations.
In conclusion, the present data have demonstrated
for the first time that serum HTATIP2/TIP30 levels are a valuable
biomarker, not only in the diagnosis of HCC, but also in monitoring
prognosis.
Acknowledgements
This study was supported by the Science and
Technology Department of Hunan Province Science and Technology Plan
(grant no. 2014FJ6066) and the Science and Technology Program of
Hunan Provincial Health Department (grant no. B2014-087).
References
|
1
|
Kudo M, Han KH, Kokudo N, Cheng AL, Choi
BI, Furuse J, Izumi N, Park JW, Poon RT and Sakamoto M: Liver
cancer working group report. Jpn J Clin Oncol. 40 Suppl 1:i19–i27.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yau T, Chan P, Epstein R and Poon RT:
Management of advanced hepatocellular carcinoma in the era of
targeted therapy. Liver Int. 29:10–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song P, Tobe RG, Inagaki Y, Kokudo N,
Hasegawa K, Sugawara Y and Tang W: The management of hepatocellular
carcinoma around the world: A comparison of guidelines from 2001 to
2011. Liver Int. 32:1053–1063. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farinati F, Marino D, de Giorgio M, Baldan
A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA,
Benvegnù L, et al: Diagnostic and prognostic role of
alpha-fetoprotein in hepatocellular carcinoma: Both or neither? Am
J Gastroenterol. 101:524–532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Villanueva A, Minguez B, Forner A, Reig M
and Llovet JM: Hepatocellular carcinoma: Novel molecular approaches
for diagnosis, prognosis and therapy. Annu Rev Med. 61:317–328.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiao H, Tao Y, Greenblatt J and Roeder RG:
A cofactor, TIP30, specifically enhances HIV-1 Tat-activated
transcription. Proc Natl Acad Sci USA. 95:pp. 2146–2151. 1998;
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morán S, Rodríguez-Leal G, Marín-López E,
Arista J, Poo JL, Vargas-Vorackova F, Kershenobich D and Uribe M:
Primary biliary cirrhosis: Clinical features and survival of a
mexican population. Rev Gastroenterol Mex. 61:212–219. 1996.(In
Spanish). PubMed/NCBI
|
|
8
|
Zhang C, Mori M, Gao S, Li A, Hoshino I,
Aupperlee MD, Haslam SZ and Xiao H: Tip30 deletion in MMTV-Neu mice
leads to enhanced EGFR signaling and development of estrogen
receptor-positive and progesterone receptor-negative mammary
tumors. Cancer Res. 70:10224–10233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ito M, Jiang C, Krumm K, Zhang X, Pecha J,
Zhao J, Guo Y, Roeder RG and Xiao H: TIP30 deficiency increases
susceptibility to tumorigenesis. Cancer Res. 63:8763–8767.
2003.PubMed/NCBI
|
|
10
|
Zhao J, Zhang X, Shi M, Xu H, Jin J, Ni H,
Yang S, Dai J, Wu M and Guo Y: TIP30 inhibits growth of HCC cell
lines and inhibits HCC xenografts in mice in combination with 5-FU.
Hepatology. 44:205–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumtepe Y, Halici Z, Sengul O, Kunak CS,
Bayir Y, Kilic N, Cadirci E, Pulur A and Bayraktutan Z: High serum
HTATIP2/TIP30 level in serous ovarian cancer as prognostic or
diagnostic marker. Eur J Med Res. 18:182013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong X, Li K, Luo Z, Lu B, Liu X, Wang T,
Pang M, Liang B, Tan M, Wu M, et al: Decreased TIP30 expression
promotes tumor metastasis in lung cancer. Am J Pathol.
174:1931–1939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo S, Jing W, Hu X, Zhou X, Liu L, Zhu M,
Yin F, Chen R, Zhao J and Guo Y: Decreased TIP30 expression
predicts poor prognosis in pancreatic cancer patients. Int J
Cancer. 134:1369–1378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi M, Yan SG, Xie ST and Wang HN:
Tip30-induced apoptosis requires translocation of Bax and involves
mitochondrial release of cytochrome c and Smac/DIABLO in
hepatocellular carcinoma cells. Biochim Biophys Acta. 1783:263–274.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao J, Chen J, Lu B, Dong L, Wang H, Bi
C, Wu G, Guo H, Wu M and Guo Y: TIP30 induces apoptosis under
oxidative stress through stabilization of p53 messenger RNA in
human hepatocellular carcinoma. Cancer Res. 68:4133–4141. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao J, Lu B, Xu H, Tong X, Wu G, Zhang X,
Liang A, Cong W, Dai J, Wang H, et al: Thirty-kilodalton
Tat-interacting protein suppresses tumor metastasis by inhibition
of osteopontin transcription in human hepatocellular carcinoma.
Hepatology. 48:265–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang C, Pecha J, Hoshino I, Ankrapp D and
Xiao H: TIP30 mutant derived from hepatocellular carcinoma
specimens promotes growth of HepG2 cells through up-regulation of
N-cadherin. Cancer Res. 67:3574–3582. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu B, Ma Y, Wu G, Tong X, Guo H, Liang A,
Cong W, Liu C, Wang H, Wu M, et al: Methylation of Tip30 promoter
is associated with poor prognosis in human hepatocellular
carcinoma. Clin Cancer Res. 14:7405–7412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song C, Wang F, Cheng K, Wei X, Bian Y,
Wang K, Tan Y, Wang H, Ye M and Zou H: Large-scale quantification
of single amino-acid variations by a variation-associated database
search strategy. J Proteome Res. 13:241–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shtivelman E: A link between metastasis
and resistance to apoptosis of variant small cell lung carcinoma.
Oncogene. 14:2167–2173. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Zhang C, Xing Y, Janicki JS,
Yamamoto M, Wang XL, Tang DQ and Cui T: Up-regulation of p27 (kip1)
contributes to Nrf2-mediated protection against angiotensin
II-induced cardiac hypertrophy. Cardiovasc Res. 90:315–324. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao H, Palhan V, Yang Y and Roeder RG:
TIP30 has an intrinsic kinase activity required for up-regulation
of a subset of apoptotic genes. EMBO J. 19:956–963. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berasain C and Avila MA: The EGFR
signalling system in the liver: From hepatoprotection to
hepatocarcinogenesis. J Gastroenterol. 49:9–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang P, Xu X, Wang L, Zhu B, Wang X and
Xia J: The role of EGF-EGFR signalling pathway in hepatocellular
carcinoma inflammatory microenvironment. J Cell Mol Med.
18:218–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Li A, Gao S, Zhang X and Xiao H:
The TIP30 protein complex, arachidonic acid and coenzyme A are
required for vesicle membrane fusion. PLoS One. 6:e212332011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li A, Zhang C, Gao S, Chen F, Yang C, Luo
R and Xiao H: TIP30 loss enhances cytoplasmic and nuclear EGFR
signaling and promotes lung adenocarcinogenesis in mice. Oncogene.
32:2273–2281, 2281e. 1–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shan SG, Gao YT, Xu YJ, Huang Y, Zhang Q,
Zhai DK, Li JB, Wang FM, Jing X, Du Z and Wang YJ: Gradually
increased Golgi protein 73 expression in the progression of benign
liver diseases to precancerous lesions and hepatocellular carcinoma
correlates with prognosis of patients. Hepatol Res. 43:1199–1210.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen TM, Lin CC, Huang PT and Wen CF:
Neutrophil-to-lymphocyte ratio associated with mortality in early
hepatocellular carcinoma patients after radiofrequency ablation. J
Gastroenterol Hepatol. 27:553–561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang ZL, Luo J, Chen MS, Li JQ and Shi M:
Blood neutrophil-to-lymphocyte ratio predicts survival in patients
with unresectable hepatocellular carcinoma undergoing transarterial
chemoembolization. J Vasc Interv Radiol. 22:702–709. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai F, Yano Y, Fukumoto T, Takebe A,
Tanaka M, Kuramitsu K, Anggorowati N, Rinonce HT, Widasari DI,
Saito M, et al: Quantification of pregenomic RNA and covalently
closed circular DNA in hepatitis B virus-related hepatocellular
carcinoma. Int J Hepatol. 2013:8492902013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang HI, Sherman M, Su J, Chen PJ, Liaw
YF, Iloeje UH and Chen CJ: Nomograms for risk of hepatocellular
carcinoma in patients with chronic hepatitis B virus infection. J
Clin Oncol. 28:2437–2444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mittal S and El-Serag HB: Epidemiology of
hepatocellular carcinoma: Consider the population. J Clin
Gastroenterol. 47 Suppl:S2–S6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zipprich A, Garcia-Tsao G, Rogowski S,
Fleig WE, Seufferlein T and Dollinger MM: Prognostic indicators of
survival in patients with compensated and decompensated cirrhosis.
Liver Int. 32:1407–1414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Colli A, Fraquelli M, Casazza G, Massironi
S, Colucci A, Conte D and Duca P: Accuracy of ultrasonography,
spiral CT, magnetic resonance and alpha-fetoprotein in diagnosing
hepatocellular carcinoma: A systematic review. Am J Gastroenterol.
101:513–523. 2006. View Article : Google Scholar : PubMed/NCBI
|