Introduction
Ovarian cancer is the most lethal type of
gynecological cancer amongst females worldwide, responsible for
~125,000 cancer-associated mortalities annually (1,2). Ovarian
cancer is the seventh most common type of cancer and the fifth
leading cause of cancer-associated mortality amongst females
globally (3,4). The incidence rate of this disease is
high, with >225,000 females diagnosed with ovarian cancer
annually (5,6). Improvements have been made in the
treatment of this disease, including the combination of surgery,
radiation and chemotherapy with paclitaxel, gemcitabine and
cisplatin; however, the overall 5-year survival rate is <40%
(7). Recently, various platinum-based
treatment regimens have been administered for the treatment of
ovarian cancer (8), but no
improvement in patient survival has been observed (1) due to the acquisition of chemotherapeutic
drug resistance and tumor recurrence.
Flavonoids are naturally occurring phytochemicals
that are abundant in the majority of fresh fruits, grains, green
vegetables and certain traditional medicinal herbs (9,10). Almost
all chemically synthesized drugs currently used for cancer therapy
have a significant toxicity to normal cells (11); however, various naturally-occurring
flavonoids have demonstrated selective cytotoxicity in types of
human cancer cells, accompanied by minimal toxicity to normal cells
(10). Myricetin
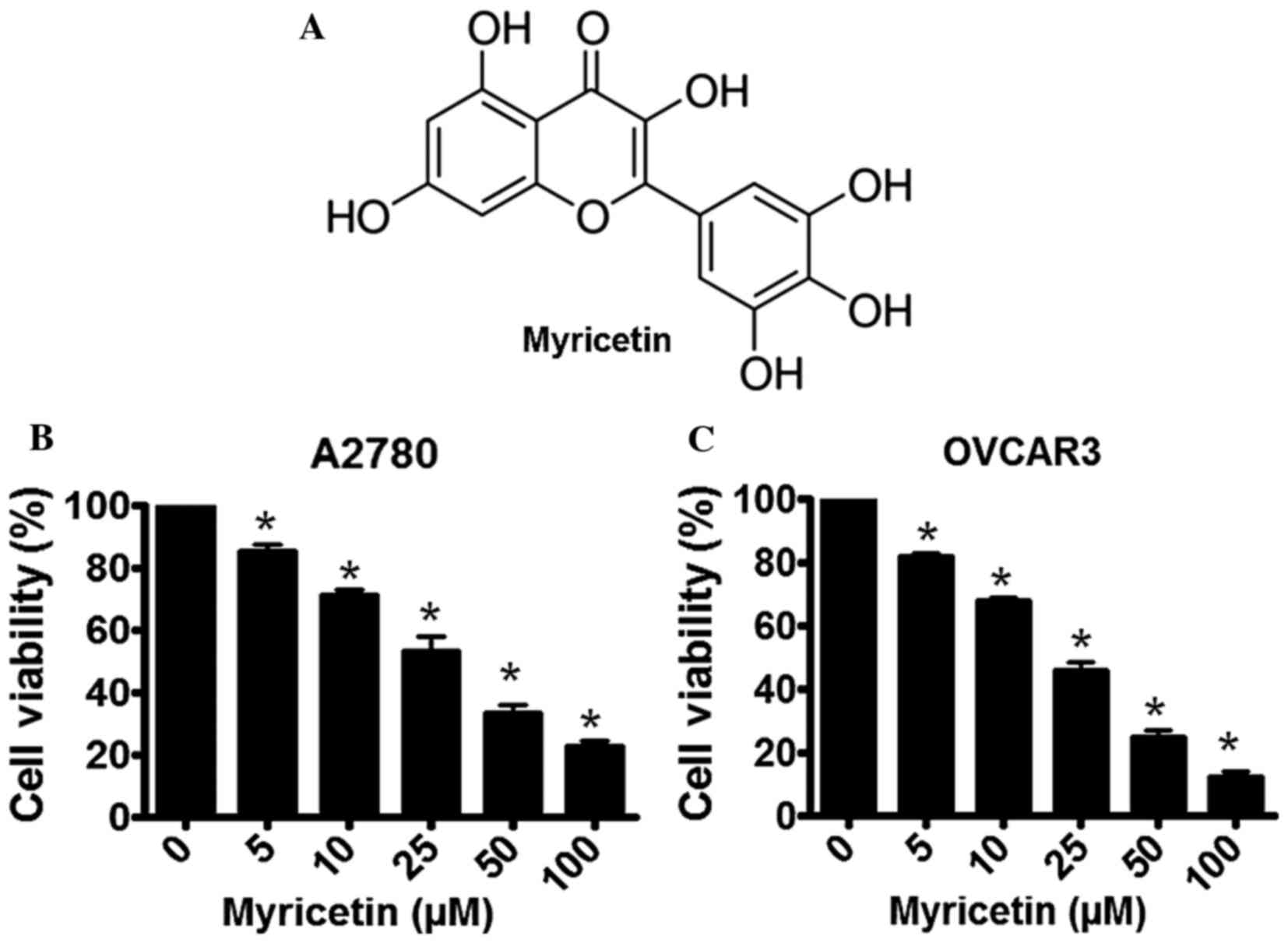
(3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4-chromenone; Fig. 1A) is a dietary flavonoid present in
fresh fruits (such as grapes, oranges, berries), vegetables
(including sweet potatoes, parsley, broad beans), herbs, tea and
wine (12,13). The physiological availability of
myricetin in certain natural products is significant, such as 24
mg/kg in grapes, 0.2–0.5 g/kg physical abundance in average food
and 0.8–1.6 g/kg in green tea (12).
The therapeutic potential of myricetin has
previously been established and it is considered to have various
pathophysiological properties, including antioxidant,
cytoprotective, antiviral, antimicrobial, antiplatelet and
anticancer properties (12,14). Myricetin has also been indicated to be
significantly effective in the treatment of various types of
cancers, including prostate cancer, hepatocellular carcinoma,
gastric cancer and human squamous cell carcinoma (12,15–18).
Myricetin may also increase the chemotherapeutic potential of
certain anticancer drugs by sensitizing the target cancer cells to
chemotherapy (19,20). As the currently available conventional
chemotherapeutic agents have not been observed to improve the
overall survival rate of patients with ovarian cancer, novel drug
regimens are required in order to combat the disease. In the
present study, the anticancer effects of myricetin were evaluated
in the A2780 and OVCAR3 ovarian cancer cell lines. It was also
investigated whether myricetin is able to sensitize these ovarian
cancer cells to the frequently used chemotherapeutic drugs
paclitaxel and cisplatin.
Materials and methods
Cell culture and maintenance
The A2780 and OVCAR3 human ovarian cancer cells were
purchased from ATCC (Manassas, VA, USA) and cultured in RPMI-1640
medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented
with 10% fetal bovine serum (Sigma-Aldrich; Merck KGaA), penicillin
(100 U/ml; Sigma-Aldrich; Merck KGaA) and streptomycin (100 µg/ml;
Sigma-Aldrich; Merck KGaA), at 37°C in a humidified chamber.
Cell viability assay
Cell viability was determined spectrophotometrically
using an MTT assay. A2780 and OVCAR3 cells were seeded into 96-well
plates at a density of 1×104 cells/ml. The cells were
cultured to ~70% confluency and exposed to various concentrations
of myricetin (0, 5, 10, 25, 50 and 100 µM; Sigma-Aldrich; Merck
KGaA) for 48 h, at 37°C, under the described cell culture
conditions. Following treatment, MTT (Sigma-Alrich; Merck KGaA)
solution was added to each well at a concentration of 2 mg/ml and
incubated at 37°C for 2 h. The formazan that formed was dissolved
using DMSO and the absorbance was measured at 570 nm using a
microplate reader and Multiskan EX software, v3.4 (Multiskan EX,
Lab systems, Helsinki, Finland).
Apoptosis assay
Role of apoptosis in myricetin-induced cytotoxicity
in A2780 and OVCAR3 cells was determined using a single
stranded-DNA Apoptosis ELISA kit (EMD Millipore, Billerica, MA,
USA) (21). Cultured A2780 and OVCAR3
cells (1×104 cells/ml) were treated with 25 µM myricetin
for 48 h at 37°C, and induction of apoptosis in untreated (control)
and myricetin-treated cells were determined according to the
manufacturer's protocol. The extent of apoptosis was determined
from the absorbance measured at 405 nm using a microplate reader
and Multiskan EX software, v3.4 (Multiskan EX, Lab systems).
Evaluation of cell migration using a
Boyden chamber assay
The migratory properties of A2780 and OVCAR3 cells
in the presence and absence of myricetin were determined using a
Boyden chamber assay with track-etched polyethylene terephthalate
membranes and an 8.0-µm diameter pore size (Corning Incorporated,
Corning, NY, USA). A2780 and OVCAR3 cells (1×104
cells/ml) were treated with various concentrations of myricetin (0,
10 and 25 µM) for 48 h under the described cell culture conditions
and the migration assay was performed according to the
manufacturer's protocol.
Western blot analysis
Cultured (at 37°C) A2780 and OVCAR3 cells were
treated with 25 µM of myricetin. Total protein from the cell
extracts was isolated in ice-cold lysis buffer consisting of 150 mM
NaCl, 20 mM Tris-HCl, 1% NP-40, 20 µg/ml leupeptin, 20 µg/ml
aprotinin, 1 mM ortho-vanadate and 2 mM PMSF, pH 7.4. After
treatment, cells were incubated in the lysis buffer at 4°C for 30
min and the cell suspension was subsequently centrifuged at 2,000 ×
g for 15 min at 4°C. Protein concentration was estimated using a
Bradford reagent (Sigma-Alrich; Merck KGaA). Protein from each cell
sample (~50 µg) was loaded into each well and separated by 10%
SDS-PAGE, and then transferred to a polyvinylidene difluoride
membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membrane was blocked in Super Block blocking buffer (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 1 h at room temperature and
subsequently incubated with various primary antibodies, including
rabbit polyclonal anti-Bax (dilution, 1:500; N20, #sc-493; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit polyclonal
anti-Bcl-2 (dilution, 1:500; N-19, sc-7382; Santa Cruz
Biotechnology, Inc.), rabbit monoclonal anti-cleaved caspase-3
(Asp175) antibody (dilution, 1:1,000; #9661; Cell Signaling
Technology, Inc., Danvers, MA, USA), mouse monoclonal anti-Mdr-1
antibody (dilution, 1:500; G-1; sc-13131, Santa Cruz Biotechnology,
Inc.). After blocking membranes were washed three times in 0.25%
TBST buffer for 10 min and incubated with secondary antibodies,
including goat anti-mouse immunoglobulin G (IgG)-horseradish
peroxidase (HRP; #sc-2005; Santa Cruz Biotechnology, Inc.) and
mouse anti-rabbit IgG-HRP (#sc-2357; Santa Cruz Biotechnology,
Inc.) for 30 min at room temperature. The secondary antibodies were
used at a dilution of 1:10,000. Images of the membranes were
captured using the Super Signal ULTRA Chemiluminescent Substrate
(Pierce; Thermo Fisher Scientific, Inc.) using a KODAK Image
Station 4000 (Kodak, Rochester, NY, USA).
Statistical analysis
The data were analyzed using GraphPad Prism version
4.0 (GraphPad Software, Inc., La Jolla, CA, USA). All data are
presented as the mean ± standard deviation. Statistically
significant differences between the groups were determined using a
paired Student's two-tailed t-test. P<0.05 was considered to
indicate a statistically significant result.
Results
Myricetin induces cytotoxicity in
ovarian cancer cells
Treatment of the A2780 and OVCAR3 human ovarian
cancer cells with various concentration of myricetin resulted in
the induction of cytotoxicity. Following a 48-h incubation, a
gradual loss of cell viability was observed in myricetin-treated
A2780 cells, with the IC50 concentration determined to be ~25 µM
(Fig. 1B). A similar inhibition of
cellular growth was observed in myricetin-treated OVCAR3 cells
following a 48-h incubation period, with the IC50 concentration
calculated as ~25 µM (Fig. 1C).
Myricetin induces apoptosis in ovarian
cancer cells
To elucidate the mechanisms underlying
myricetin-induced cytotoxicity in ovarian cancer cells, the
involvement of apoptosis in myricetin-treated cells was
investigated. The induction of apoptosis was evaluated using the
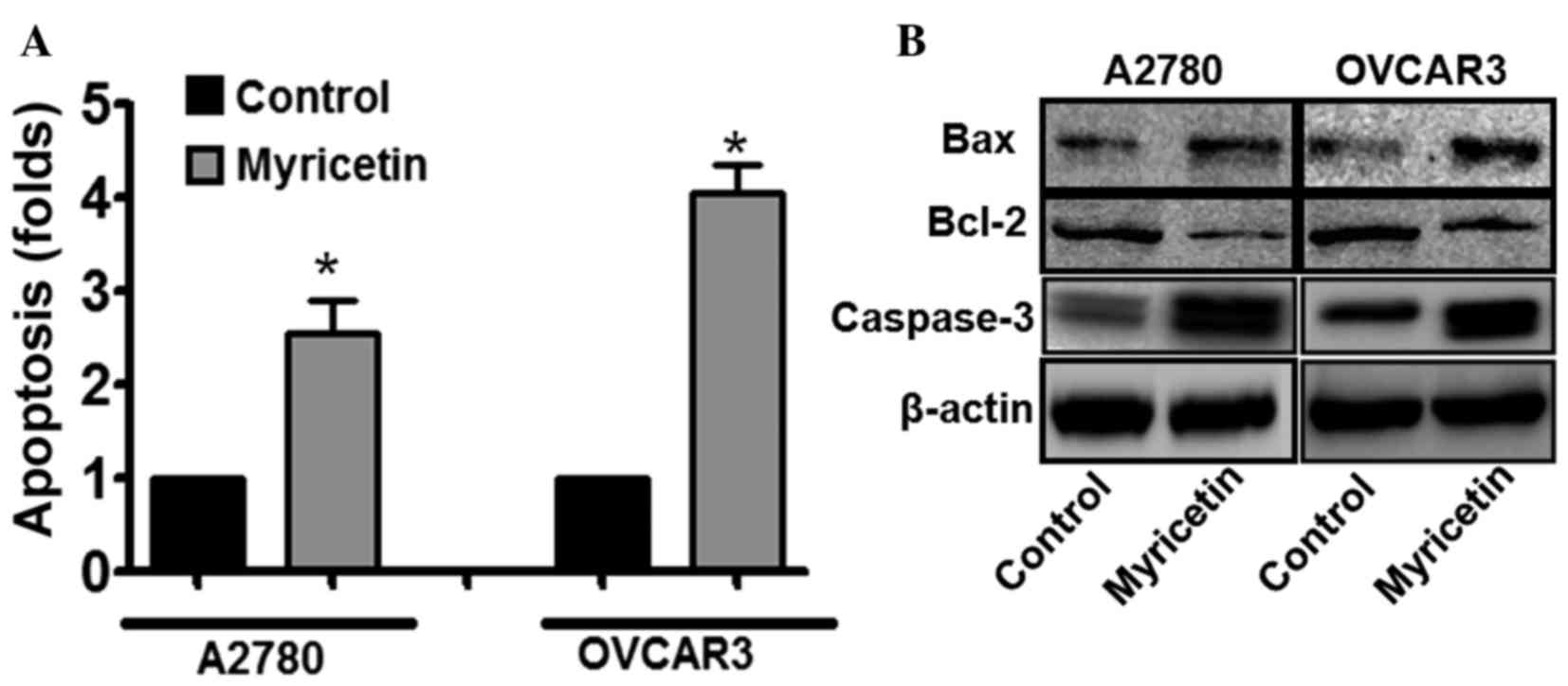
single-stranded DNA Apoptosis ELISA kit. Myricetin treatment was
observed to induce apoptosis in A2780 and OVCAR3 cells. In A2780
cells treated with 25 µM myricetin, an ~2.5-fold increase in the
apoptotic signal was observed, compared with untreated cells,
whereas under similar treatment conditions, the apoptotic signal
was increased by ~4-fold in OVCAR3 cells compared with untreated
cells (*P<0.05, control vs. myricetin-treated cells; Fig. 2A). The results suggest that apoptosis
is involved in myricetin-induced cytotoxicity in certain ovarian
cancer cells.
Furthermore, it was also revealed that the
expression of the pro-apoptotic protein B-cell lymphoma-2
(Bcl-2)-associated X-protein (BAX) was significantly upregulated,
and the expression of the anti-apoptotic protein Bcl-2 was
downregulated, in myricetin-treated ovarian cancer cells compared
with untreated cells (*P<0.05, control vs. myricetin-treated
cells; Fig. 2B). Cleaved caspase-3 is
central to the intrinsic apoptotic signaling pathway, and was also
revealed to be upregulated in myricetin-treated cells compared with
untreated cells (*P<0.05, control vs. myricetin-treated cells).
Therefore, the results suggested that apoptosis has an important
role in myricetin-induced cell death.
Myricetin inhibits the migratory
properties of ovarian cancer cells
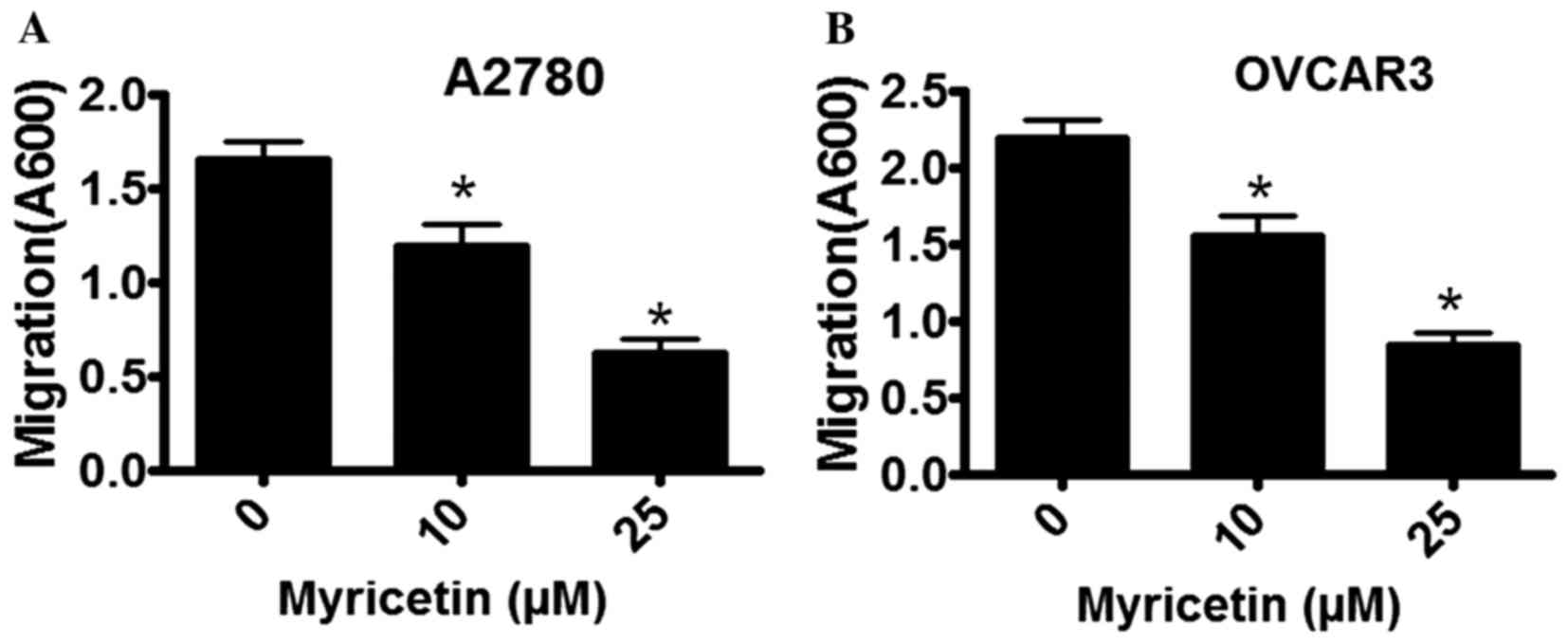
The migratory properties of A2780 and OVCAR3 cells
were determined in the absence and presence of myricetin using a
Boyden Chamber assay. Cell migration was observed to be markedly
inhibited by myricetin, in a dose-dependent manner. In A2780 cells
that were treated with 10 µM myricetin the extent of migration was
inhibited by ~18%, whereas in the presence of 25 µM myricetin
migratory activity was inhibited by ~60% compared with untreated
cells (*P<0.05, control vs. myricetin-treated cells; Fig. 3A). A similar inhibitory pattern was
observed in myricetin-treated OVCAR3 cells. In the presence of 10
µM myricetin, OVCAR3 cell migration was inhibited by ~28%, and in
the presence of 25 µM myricetin it was inhibited by ~65% compared
with untreated cells (*P<0.05, control vs. myricetin-treated
cells).
Myricetin enhances the
chemotherapeutic potential of paclitaxel in ovarian cancer cells by
targeting MDR-1
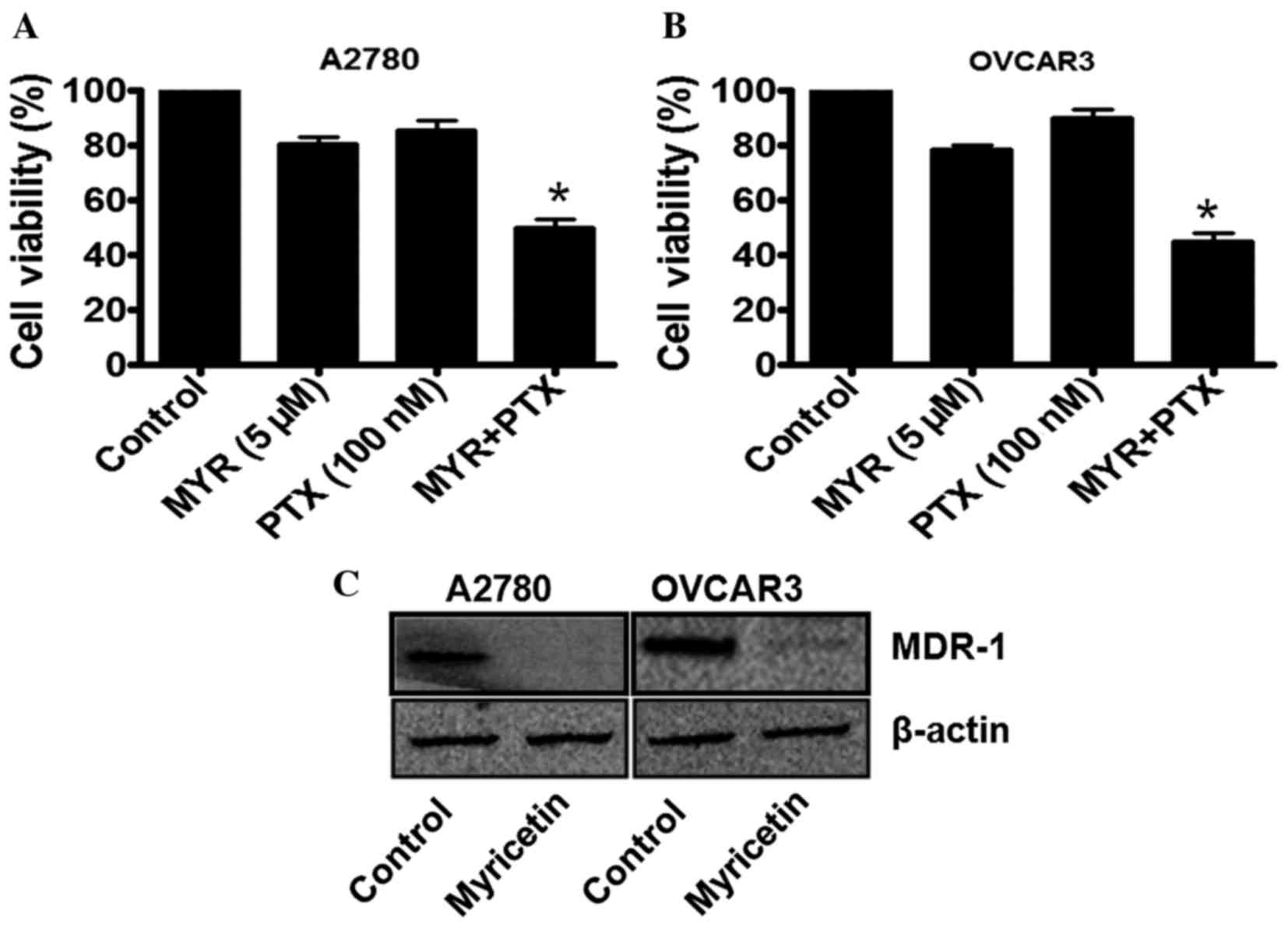
In order to investigate whether myricetin is able to
enhance the chemotherapeutic potential of paclitaxel (PTX), the
ovarian cancer cells were treated with a sub-lethal concentration
of myricetin (5 µM) for 48 h, and then incubated with a sub-lethal
concentration of paclitaxel (100 nM). As previously demonstrated
(Fig. 1), 5 µM myricetin induces
<25% inhibition of cell viability in A2780 and OVCAR3 cells. By
contrast, 100 nM paclitaxel was not observed to induce significant
cytotoxicity in the two cell types. However, when the
myricetin-treated cells were further incubated with 100 nM
paclitaxel, a marked reduction in cell viability was observed
(Fig. 4A and B). The combination
therapy of myricetin and paclitaxel was, therefore, effective in
these cell lines, causing an ~50% loss of cell viability (Fig. 4A and B).
As MDR-1 expression in ovarian cancer cells has been
indicated to be associated with paclitaxel-resistance, the status
of MDR-1 in the myricetin-treated cells was further investigated
using western blot analysis. It was observed that, in the
myricetin-treated A2780 and OVCAR3 cells, MDR-1 expression was
significantly downregulated compared with untreated cells
(*P<0.05, control vs. myricetin-treated cells; Fig. 4C), which may be associated with the
enhancement of paclitaxel efficacy in certain ovarian cancer
cells.
Discussion
Amongst all the gynecological malignancies, ovarian
cancer is responsible for the majority of these types of
cancer-associated mortalities globally, often due to a late-stage
diagnosis and a poor prognosis (1–8). The
currently available conventional therapies for ovarian cancer
include surgery, or combined chemotherapy with cisplatin,
paclitaxel and various other drugs (5–7). Although
there has been an improvement in the overall survival rate in
recent years the overall 5-year survival-rate is <40% (7), and the majority of patients with ovarian
cancer suffer from a recurrent, progressive disease due to the
acquisition of a resistance phenotype towards the conventional
chemotherapeutic agents (1,6,7). Due to
the heterogeneous nature of ovarian tumors (1), the development of defined and novel
therapeutic regimens for this disease has been a challenge.
Previous clinical studies have demonstrated that numerous
chemotherapy drugs, including 5-fluorouracil, cyclophosphamide,
dactinomycin and vincristine, have failed to inhibit tumor growth
and progression in patients with ovarian cancer (22,23).
However, members of the taxane group of drugs, including
paclitaxel, which acts as a mitotic poison and targets the cellular
microtubule network, administered as monotherapy or in combination
with cisplatin have emerged as a potentially effective therapeutic
regimen against ovarian cancer (23,24).
Previous studies have indicated that
MDR1/P-glycoprotein has an important role in the exclusion of drugs
from tumor cells (24,25). MDR-1 is a 170-kDa membrane-localized
phospho-glycoprotein encoded by the MDR1 gene, which acts as
an ATP-dependent efflux pump and is associated with the decreased
accumulation of chemotherapy drugs within cancer cells (26,27).
Numerous studies have revealed that the overexpression of MDR-1 in
aggressive ovarian cancer cells is associated with the development
of resistance to paclitaxel treatment (28,29).
Therefore, it is essential to screen novel drug candidates that may
target MDR-1 in ovarian cancer cells, and also to enhance the
chemotherapeutic potential of currently available anticancer
drugs.
In the current study, it was demonstrated that the
naturally occurring dietary flavonoid myricetin efficiently
inhibits the proliferation of A2780 and OVCAR3 ovarian cancer
cells, with the simultaneous induction of apoptosis. It was also
observed that myricetin is able to inhibit the migratory capacity
of ovarian cancer cells. Following an evaluation of the cytotoxic
effect of myricetin in ovarian cancer cells, it was investigated
whether myricetin enhances the sensitivity of ovarian cancer cells
to paclitaxel treatment. It was observed that paclitaxel activity
was significantly increased in the cells that were pre-treated with
a sub-lethal concentration of myricetin. The results suggest that
myricetin is able to enhance the chemotherapeutic potential of
paclitaxel, in addition to increasing the efficacy of paclitaxel at
lower concentrations. In order to investigate the underlying
mechanisms by which myricetin increases the cytotoxic potential of
paclitaxel, the status of MDR-1 in myricetin-treated cells was
determined. Treatment of A2780 and OVCAR3 cells with myricetin
induced a marked downregulation of MDR-1, which may explain the
enhancement of paclitaxel efficacy in ovarian cancer cells. In
conclusion, the results suggest that that myricetin monotherapy may
be a potentially effective therapeutic agent for the treatment of
patients with ovarian cancer.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vecchione A, Belletti B, Lovat F, Volinia
S, Chiappetta G, Giglio S, Sonego M, Cirombella R, Onesti EC,
Pellegrini P, et al: A microRNA signature defines chemoresistance
in ovarian cancer through modulation of angiogenesis. Proc Natl
Acad Sci USA. 110:pp. 9845–9850. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nam MS, Jung DB, Seo KH, Kim BI, Kim JH,
Kim JH, Kim B, Baek NI and Kim SH: Apoptotic effect of sanggenol L
via caspase activation and inhibition of NF-kB signaling in ovarian
cancer cells. Phytother Res. 30:90–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ivanov S, Ivanov S and Khadzhiolov N:
Ovarian tumours-accuracy of frozen section diagnosis. Akush Ginekol
(Sofiia). 44:11–13. 2005.(In Bulgarian).
|
|
6
|
Saika K and Sobue T: Cancer statistics in
the world. Gan To Kagaku Ryoho. 40:2475–2480. 2013.(In Japanese).
PubMed/NCBI
|
|
7
|
Edwards SJ, Barton S, Thurgar E and Trevor
N: Topotecan, pegylated liposomal doxorubicin hydrochloride,
paclitaxel, trabectedin and gemcitabine for advanced recurrent or
refractory ovarian cancer: A systematic review and economic
evaluation. Health Technol Assess. 19:1–480. 2015. View Article : Google Scholar
|
|
8
|
Bookman MA, Brady MF, McGuire WP, Harper
PG, Alberts DS, Friedlander M, Colombo N, Fowler JM, Argenta PA, de
Geest K, et al: Evaluation of new platinum-based treatment regimens
in advanced-stage ovarian cancer: A Phase III Trial of the
gynecologic cancer intergroup. J Clin Oncol. 27:1419–1425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sak K: Site-specific anticancer effects of
dietary flavonoid quercetin. Nutr Cancer. 66:177–193. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sak K: Cytotoxicity of dietary flavonoids
on different human cancer types. Pharmacogn Rev. 8:122–146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta S, Afaq F and Mukhtar H: Selective
growth-inhibitory, cell-cycle deregulatory and apoptotic response
of apigenin in normal versus human prostate carcinoma cells.
Biochem Biophys Res Commun. 287:914–920. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Devi KP, Rajavel T, Habtemariam S, Nabavi
SF and Nabavi SM: Molecular mechanisms underlying anticancer
effects of myricetin. Life Sci. 142:19–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harnly JM, Doherty RF, Beecher GR, Holden
JM, Haytowitz DB, Bhagwat S and Gebhardt S: Flavonoid content of
U.S. fruits, vegetables, and nuts. J Agric Food Chem. 54:9966–9977.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ong CK, Nee S, Rambaut A, Bernard HU and
Harvey PH: Elucidating the population histories and transmission
dynamics of papillomaviruses using phylogenetic trees. J Mol Evol.
44:199–206. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boam T: Anti-androgenic effects of
flavonols in prostate cancer. Ecancermedicalscience.
9:5852015.PubMed/NCBI
|
|
16
|
Feng J, Chen X, Wang Y, Du Y, Sun Q, Zang
W and Zhao G: Myricetin inhibits proliferation and induces
apoptosis and cell cycle arrest in gastric cancer cells. Mol Cell
Biochem. 408:163–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iyer SC, Gopal A and Halagowder D:
Myricetin induces apoptosis by inhibiting P21 activated kinase 1
(PAK1) signaling cascade in hepatocellular carcinoma. Mol Cell
Biochem. 407:223–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maggioni D, Nicolini G, Rigolio R, Biffi
L, Pignataro L, Gaini R and Garavello W: Myricetin and naringenin
inhibit human squamous cell carcinoma proliferation and migration
in vitro. Nutr Cancer. 66:1257–1267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang H, Chen AY, Ye X, Li B, Rojanasakul
Y, Rankin GO and Chen YC: Myricetin inhibits proliferation of
cisplatin-resistant cancer cells through a p53-dependent apoptotic
pathway. Int J Oncol. 47:1494–1502. 2015.PubMed/NCBI
|
|
20
|
Yi JL, Shi S, Shen YL, Wang L, Chen HY,
Zhu J and Ding Y: Myricetin and methyl eugenol combination enhances
the anticancer activity, cell cycle arrest and apoptosis induction
of cis-platin against HeLa cervical cancer cell lines. Int J Clin
Exp Pathol. 8:1116–1127. 2015.PubMed/NCBI
|
|
21
|
Balint K, Xiao M, Pinnix CC, Soma A, Veres
I, Juhasz I, Brown EJ, Capobianco AJ, Herlyn M and Liu ZJ:
Activation of Notch1 signaling is required for
beta-catenin-mediated human primary melanoma progression. J Clin
Invest. 115:3166–3176. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tavassoli FA and Norris HJ: Sertoli tumors
of the ovary. A clinicopathologic study of 28 cases with
ultrastructural observations. Cancer. 46:2281–2297. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burton ER, Brady M, Homesley HD, Rose PG,
Nakamura T, Kesterson JP, Rotmensch J, Thigpen J Tate and Van Le L:
A phase II study of paclitaxel for the treatment of ovarian stromal
tumors: An NRG oncology/gynecologic oncology group study. Gynecol
Oncol. 140:48–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McGuire WP, Hoskins WJ, Brady MF, Kucera
PR, Partridge EE, Look KY, Clarke-Pearson DL and Davidson M:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alvarez M, Paull K, Monks A, Hose C, Lee
JS, Weinstein J, Grever M, Bates S and Fojo T: Generation of a drug
resistance profile by quantitation of mdr-1/P-glycoprotein in the
cell lines of the national cancer institute anticancer drug screen.
J Clin Invest. 95:2205–2214. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen H, Hao J, Wang L and Li Y:
Coexpression of invasive markers (uPA, CD44) and multiple
drug-resistance proteins (MDR1, MRP2) is correlated with epithelial
ovarian cancer progression. Br J Cancer. 101:432–440. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang B, Li S, Meng X, Shang H and Guan Y:
Inhibition of mdr1 by G-quadruplex oligonucleotides and reversal of
paclitaxel resistance in human ovarian cancer cells. Tumour Biol.
36:6433–6443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Wang J, Cai K, Jiang L, Zhou D,
Yang C, Chen J, Chen D and Dou J: Downregulation of gene MDR1 by
shRNA to reverse multidrug-resistance of ovarian cancer A2780
cells. J Cancer Res Ther. 8:226–231. 2012. View Article : Google Scholar : PubMed/NCBI
|