Introduction
Gastric cancer (GC) is one of the most common
malignancies globally, accounting for ~70,000 new cases and 650,000
mortalities per year (1,2). Despite advances in strategies for early
detection, the majority of patients still present with an advanced
disease at diagnosis. The prognosis of patients with advanced tumor
remains poor (3). Further decreases
in mortality rates would require improved treatment outcomes in
patients with advanced GC.
Chemotherapy is recognized as the most effective
treatment for patients with unresectable advanced or metastatic GC.
To date, multiple clinical trials have evaluated its efficacy and
safety (4–9). As well as for unresectable cases,
neoadjuvant chemotherapy may also be considered for potentially
resectable cases to further improve their outcomes. Several
previous studies have evaluated the potential usefulness of
neoadjuvant chemotherapy in locally advanced GC (10–14).
Regarding the response assessment to the
chemotherapy, an early evaluation during neoadjuvant chemotherapy
would be of interest for tailoring chemotherapy based on the
individual response. Correct identification of responding or
non-responding patients would be important for more appropriate
implementation, to avoid toxic and ineffective chemotherapy
(15–17).
Histopathological classification of regression is
considered to be standard criteria for response assessments to the
chemotherapy in GC. The Japanese classification of gastric
carcinoma (JCGC) defined histological classification of resected
specimens for an early response evaluation of neoadjuvant
chemotherapy (18). Previous studies
have evaluated the validity of histological evaluation of resected
specimens from patients with advanced GC receiving neoadjuvant
chemotherapy (19,20). Kurokawa et al (19) compared JCGC histological-based
evaluation with response evaluation criteria in solid tumors
(RECIST) as well as upper gastrointestinal (GI) X-ray or endoscopy
based response evaluation of primary lesions, using two different
cohorts. The results demonstrated the superiority of histological
evaluation compared with RECIST and upper GI X-ray or endoscopy
based response evaluation. Heger et al (21) performed histological based evaluations
using the scoring systems of Becker et al (20). They also performed computed tomography
(CT) and endoscopy-based response evaluation and confirmed a good
correlation among the three evaluation systems (21).
To evaluate the validity of JCGC histological
classification for an early response evaluation of neoadjuvant
chemotherapy in advanced GC, the JCGC histological based evaluation
was compared with CT-based response evaluation following 2 courses
of chemotherapy. The results demonstrated that histological-based
evaluation was superior to the CT-based response evaluation as an
independent prognostic predictor in advanced GC being treated with
neoadjuvant chemotherapy.
Patients and methods
Patients, survival and response
evaluation using different criteria
The studied population comprised of 78 Japanese
patients with advanced GC, receiving neoadjuvant chemotherapy from
April 2003 to September 2012 at the Fujita Health University
hospital (Toyoake, Japan) All GC cases were diagnosed
histologically and were classified according to Lauren's
classification (22). Detailed
information concerning anatomical location, macroscopic types,
depth, lymph node and other metastasis and peritoneal dissemination
was obtained according to the JCGC (18).
Using CT, the response to chemotherapy was assessed
following 2 courses of treatment (7–10 weeks following initial
administration, which varied across the different regimens). If
measurable lesions existed, RECIST was applied and cases were
classified into complete response (CR), partial response (PR),
stable disease (SD) and progressive disease (PD) (23). CR and PR were considered to be
responders according to RECIST. If RECIST was not applicable,
responders were defined as cases with a clear reduction of the
primary lesion in the CT images assessed by experienced physicians
with the consensus was taken as the final result. Information
concerning the upper GI X-ray or endoscopy-based response
evaluation of primary lesions was also available for all patients.
Upper GI X-ray or endoscopy-based responders were defined as PR or
CR in the JCGC criteria (18). The
assessment was performed by experienced physicians and the
consensus was taken as the final result. Those who were not
considered to be responders by CT and upper GI X-ray or
endoscopy-based evaluations were considered to be CT and upper GI
X-ray or endoscopy based non-responders, respectively.
All patients underwent gastrectomy with a D2 lymph
node dissection following 2 courses of chemotherapy.
Histological-based response evaluation of resected tumors was
performed by the senior pathologists at the Fujita Health
University hospital using Japanese Gastric Cancer Association
criteria (18), and all cases were
classified as Grade 0, 1a, 1b, 2 or 3. Patients were scored as
Grade 0 if there was no evidence of chemotherapeutic effect.
Patients were scored as Grade 1a if viable tumor cells remained in
<2/3 of the tumorous area. Patients were scored as Grade 1b if
viable tumor cells remained in >1/3 but <2/3 of the tumorous
area. Patients were scored as Grade 2 if viable tumor cells
remained in <1/3 of the tumorous area. Patients were scored as
Grade 3 if no viable tumor cells remained in the section where the
tumor was thought to have been located at the pretreatment
assessment (18). The evaluation was
performed using multiple sections of hematoxylin and eosin staining
of paraffin-embedded sections (4 µm) of the resected specimen to
avoid the influence of tumor heterogeneity. Based on this
histological assessment, Grade 1b, 2 and 3 (viable tumor cells
remaining in <2/3 of the tumorous area) cases were defined as
histological responders, and all others were considered to be
histological non-responders. Overall survival (OS) was defined as
the time from the start of initial administration of chemotherapy
to the date of cancer-associated mortality. If cancer-associated
mortality had not occurred, the OS was censored on the last date
the patient was known to be alive. Progression-free survival (PFS)
was defined as the time of initial administration of chemotherapy
to tumor progression or cancer-associated mortality. Patients with
no confirmation of progression or cancer-associated mortality were
censored at the date of the last objective tumor assessment. The
Ethical Review Board of the Fujita Health University School of
Medicine (Toyoake, Japan) approved the protocol, and written
informed consent was obtained from all participating subjects.
Statistical analysis
Categorical variables among the two groups were
assessed using the two-tailed Fisher's exact test. A κ coefficient
value was calculated to assess the consistency of different
response evaluations to chemotherapy. OS and PFS among the two
groups were assessed using the Kaplan-Meier method and the Log rank
test. P<0.05 was considered to indicate a statistically
significant difference. Multivariate survival analysis using Cox's
regression model was also performed for calculating hazard ratios
(HR), 95% confidence intervals (CI) and a C index with adjustment
for clinicopathological factors.
Results
Clinicopathological characteristics of subjects and
information about the treatment are listed in Table I and II, respectively. The majority of the cases
underwent tegafur/gimeracil/oteracil based preoperative
chemotherapy as the first line treatment (n=76; 96%). OS and PFS
were assessed among all cases. The median OS and median PFS in all
cases were 34.0 and 20.3 months, respectively. Although all
patients were considered to be operable following two courses of
chemotherapy, distant metastatic lesions were identified in 13
cases during surgery (Table I).
 | Table I.Clinicopathological features of
patients with gastric cancer. |
Table I.
Clinicopathological features of
patients with gastric cancer.
| Clinicopathological
feature | Value |
|---|
| Number of
patients | 78 |
| Median
age (range) | 68 (39–83) |
| Gender |
|
| Female n
(%) | 21 (26.9) |
| Male n
(%) | 57 (73.1) |
| Location |
|
| Upper n
(%) | 18 (23.1) |
| Middle n
(%) | 35 (44.9) |
| Lower n
(%) | 25 (32.1) |
| Histology |
|
|
Intestinal n (%) | 35 (44.9) |
| Diffuse n
(%) | 35 (44.9) |
| Mixed n
(%) | 8 (10.2) |
| Morphology |
|
| Type1 n
(%) | 4 (5.1) |
| Type2 n
(%) | 25 (32.1) |
| Type3 n
(%) | 44 (56.4) |
| Type4 n
(%) | 5 (6.4) |
| Staging |
|
| II n
(%) | 33 (42.3) |
| III n
(%) | 34 (43.6) |
| IV n
(%) | 11 (14.1) |
| Depth |
|
| T2 n
(%) | 17 (21.8) |
| T3 n
(%) | 16 (20.5) |
| T4 n
(%) | 45 (57.7) |
| Lymph node
metastasis |
|
| N0 n
(%) | 23 (29.5) |
| N1 n
(%) | 17 (21.8) |
| N2 n
(%) | 20 (25.6) |
| N3 n
(%) | 18 (23.1) |
| Distant
metastasis |
|
|
Peritoneal dissemination n
(%) | 8 (10.3) |
| Liver
metastasis n (%) | 3 (3.8) |
| Other
metastasis n (%) | 2 (2.6) |
 | Table II.Information concerning the treatment
of gastric cancer. |
Table II.
Information concerning the treatment
of gastric cancer.
| Variable | n (%) |
|---|
| Agent |
|
|
S-1+CDDP | 71 (91.0) |
| S-1 | 4 (5.1) |
|
Others | 3 (3.8) |
| Response to
chemotherapy (CT)a |
|
|
Responder | 25 (32.5) |
|
Non-responder | 52 (67.5) |
| Response to
chemotherapy (upper GI X-ray or endoscopy) |
|
|
Responder | 53 (67.9) |
|
Non-responder | 25 (32.1) |
| Response to
chemotherapy (histological grade) |
|
| 0 | 2 (2.6) |
| 1a | 47 (60.3) |
| 1b | 16 (20.5) |
| 2 n | 11 (14.1) |
| 3 n | 2 (2.6) |
Response rates using three different criteria are
listed in Table II. A total of 25
(32%) and 53 (68%) cases were considered to be CT and upper GI
X-ray or endoscopy based responders, respectively. Concerning
histological grade, 2 (2.6%), 47 (60.3%), 16 (20.5%), 11 (14.1%),
and 2 (2.6%) cases were considered to be Grade 0, 1a, 1b, 2 and 3,
respectively. A total of 29 cases (37%) were considered to be
histological responders (Table II).
The κ coefficient values were initially calculated to assess the
consistency of histological evaluations and the other two criteria.
It was demonstrated that there were low consistencies among the
histological evaluation and CT (κ=0.13; Table III) or the histological evaluation
and upper GI X-ray or endoscopy (κ=0.17; Table III). CT-based evaluation was not
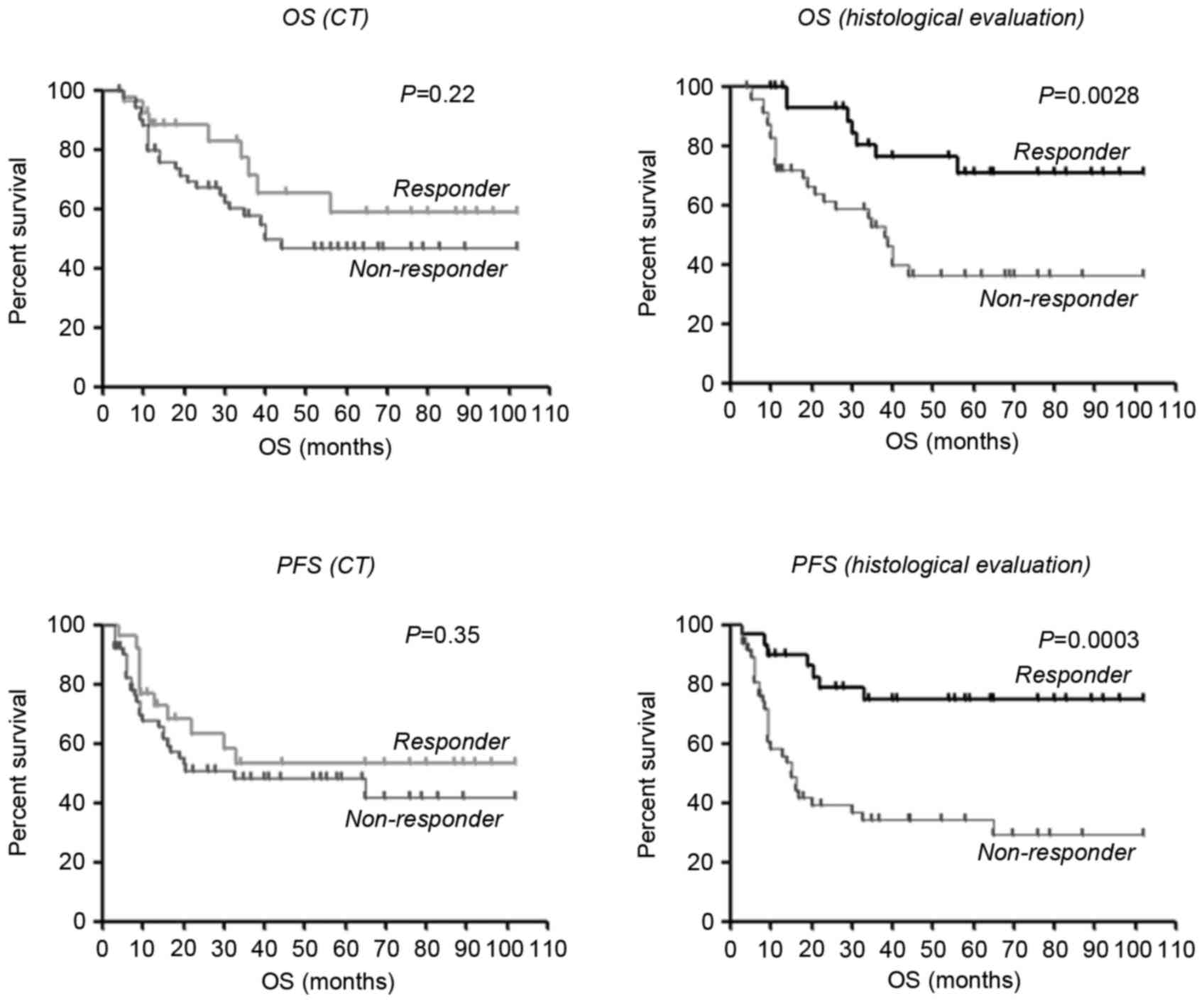
significantly associated with OS (P=0.22) or PFS (P=0.35) by the
log-rank test. On the other hand, histological evaluation was
significantly associated with OS and PFS (P=0.0028 and P=0.0003,
respectively) by the log-rank test (Fig.
1).
 | Table III.κ coefficient value assessing the
concordance among histological grade, CT and upper GI X-ray or
endoscopy. |
Table III.
κ coefficient value assessing the
concordance among histological grade, CT and upper GI X-ray or
endoscopy.
| Variable | κ coefficient
value |
|---|
| Histological grade
vs. CT | 0.13 |
| Histological grade
vs. upper |
|
| GI X-ray or
endoscopy | 0.17 |
To evaluate the independent prognostic factors
associated with OS, multivariate survival analysis using Cox's
regression model was performed. For this analysis, all
clinicopathological factors including sex, age, anatomical
location, macroscopic and histologic types, depth, information
about metastasis, staging and response to treatment were included.
This analysis demonstrated that histological non-response (HR=3.97,
95% CI=1.31–11.99, C index=0.65; Table
IV) and upper GI X-ray or endoscopy based non-response
(HR=10.1, 95% CI=3.68–27.73, C index=0.73; Table IV) were independent prognostic
factors for predicting worse OS, while intestinal histology was
associated with improved OS (HR=0.30, 95% CI=0.09–0.997, C
index=0.59; Table IV). No other
factors were demonstrated to be associated with OS by this analysis
(data not shown).
 | Table IV.Multivariate survival analysis using
Cox's regression model for adjustment of clinicopathological
factors. |
Table IV.
Multivariate survival analysis using
Cox's regression model for adjustment of clinicopathological
factors.
| Variable | HR (95%CI) | P-value | C index |
|---|
| Histological grade
(non-responder) | 3.97
(1.31–11.99) | 0.015 | 0.65 |
| Upper GI X-ray or
endoscopy (non-responder) | 10.10
(3.68–27.73) | <0.0001 | 0.73 |
| Histology
(intestinal type) | 0.30
(0.09–0.997) | 0.049 | 0.59 |
Discussion
The present study has demonstrated that the results
of histological based evaluation are a good prognostic predictor
for advanced GC receiving neoadjuvant chemotherapy. RECIST is the
most widely accepted criteria for evaluating the response to
chemotherapy, but it requires the presence of a measurable lesion.
In RECIST, primary gastric tumors are regarded as non-target
lesions (23). Since resectable GC
usually does not have a measurable lesion, it may be difficult to
apply RECIST, in particular for cases receiving neoadjuvant
chemotherapy. In the present study, for the cases in which RECIST
were not applicable, clear reduction of primary or metastatic
lesions in the images of CT were considered to be responders. The
results demonstrated that histological based evaluation was
superior to CT-based response evaluation for the prediction of
prognosis, by univariate and multivariate analysis. These results
suggested that CT-based evaluation may not effectively assess the
response of locally advanced GC to neoadjuvant chemotherapy, and
that histological based evaluation of primary tumor may be more
suitable for a precise response assessment of neoadjuvant
chemotherapy. The prognostic influence of histological response has
been demonstrated in locally advanced GC undergoing neoadjuvant
chemotherapy (19). Heger et
al (21) demonstrated a
correlation between histological, CT and endoscopy based response
evaluations. In the results of the present study, however, there
were low consistencies among these evaluations. The differences
observed in these results may be due to patient constitution and
the different time points selected for response evaluation. The
response assessment of CT and endoscopy were earlier in the study
of Heger et al (21) than the
present study.
Multivariate survival analysis demonstrated that
histological-based non-response was an independent prognostic
factor for predicting worse OS. However, the association of upper
GI X-ray or endoscopy based non-responders was stronger, which was
different to the results of a previous study. Kurokawa et al
(19) compared JCGC
histological-based evaluation with RECIST as well as GI X-ray or
endoscopy based evaluation. The results demonstrated the
superiority of histological evaluation compared with the other two
evaluations. However, two different cohorts were investigated by
Kurokawa et al (19), thus it
was not possible to assess the direct correlation of the three
evaluations.
The low consistency among GI X-ray or endoscopy and
histology indicated that objective changes judged in these
evaluations may be different. For example, changes observed during
GI X-ray or endoscopy may be early morphological changes, as
indicated by a decrease in metabolic activity (16,24) or
transient tissue reactions, which were less compared with the
resected specimens. However, it is also expected that histological
response evaluation provides important information since it
directly evaluates the influence of chemotherapy on cancer
cells.
As histological evaluation is less invasive and more
objective compared with GI X-ray or endoscopy, it may be
recommended for all cases. Precise assessment of responses to
chemotherapy would be of great interest for tailoring chemotherapy
based on the individual response. As histological response was
associated with long-term outcomes of patients with GC, it would be
useful for identification of responding or non-responding patients
to avoid toxic and ineffective chemotherapy (15–17).
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
CT
|
computed tomography
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
References
|
1
|
Ferlay J, Bray F, Parkin DM and Pisani P:
Gobocan 2000: Cancer incidence and mortality worldwide (IARC Cancer
Bases No. 5). Lyon: IARC Press; 2001
|
|
2
|
Lau M, Le A and El-Serag HB: Noncardia
gastric adenocarcinoma remains an important and deadly cancer in
the United States: Secular trends in incidence and survival. Am J
Gastroenterol. 101:2485–2492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nashimoto A, Akazawa K, Isobe Y, Miyashiro
I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I, et
al: Gastric cancer treated in 2002 in Japan: 2009 annual report of
the JGCA nationwide registry. Gastric Cancer. 16:1–27. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boku N, Yamamoto S, Fukuda H, Shirao K,
Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H, et
al: Fluorouracil versus combination of irinotecan plus cisplatin
versus S-1 in metastatic gastric cancer: A randomised phase 3
study. Lancet Oncol. 10:1063–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamaguchi K, Sawaki A, Doi T, Satoh T,
Yamada Y, Omuro Y, Nishina T, Boku N, Chin K, Hamamoto Y, et al:
Efficacy and safety of capecitabine plus cisplatin in Japanese
patients with advanced or metastatic gastric cancer: Subset
analyses of the AVAGAST study and the ToGA study. Gastric Cancer.
16:175–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koizumi W, Nakayama N, Tanabe S, Sasaki T,
Higuchi K, Nishimura K, Takagi S, Azuma M, Ae T, Ishido K, et al: A
multicenter phase II study of combined chemotherapy with docetaxel,
cisplatin and S-1 in patients with unresectable or recurrent
gastric cancer (KDOG 0601). Cancer Chemother Pharmacol. 69:407–413.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okines AF, Norman AR, McCloud P, Kang YK
and Cunningham D: Meta-analysis of the REAL-2 and ML17032 trials:
Evaluating capecitabine-based combination chemotherapy and infused
5-fluorouracil-based combination chemotherapy for the treatment of
advanced oesophago-gastric cancer. Ann Oncol. 20:1529–1534. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamaguchi T, Shirao K, Ohtsu A, Hyodo I,
Arai Y, Takiuchi H, Fujii H, Yoshida M, Saito H, Denda T, et al: A
phase II study of biweekly mitomycin C and irinotecan combination
therapy in patients with fluoropyrimidine-resistant advanced
gastric cancer: A report from the Gastrointestinal oncology group
of the Japan clinical oncology group (JCOG0109-DI Trial). Gastric
Cancer. 14:226–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kodera Y, Ishiyama A, Yoshikawa T,
Kinoshita T, Ito S, Yokoyama H, Mochizuki Y, Ito H, Tsuburaya A,
Sakamoto J, et al: A feasibility study of postoperative
chemotherapy with S-1 and cisplatin (CDDP) for gastric carcinoma
(CCOG0703). Gastric Cancer. 13:197–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuburaya A, Nagata N, Cho H, Hirabayashi
N, Kobayashi M, Kojima H, Munakata Y, Fukushima R, Kameda Y,
Shimoda T, et al: Phase II trial of paclitaxel and cisplatin as
neoadjuvant chemotherapy for locally advanced gastric cancer.
Cancer Chemother Pharmacol. 71:1309–1314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirakawa M, Sato Y, Ohnuma H, Takayama T,
Sagawa T, Nobuoka T, Harada K, Miyamoto H, Sato Y, Takahashi Y, et
al: A phase II study of neoadjuvant combination chemotherapy with
docetaxel, cisplatin, and S-1 for locally advanced resectable
gastric cancer: Nucleotide excision repair (NER) as potential
chemoresistance marker. Cancer Chemother Pharmacol. 71:789–797.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshikawa T, Rino Y, Yukawa N, Oshima T,
Tsuburaya A and Masuda M: Neoadjuvant chemotherapy for gastric
cancer in Japan: A standing position by comparing with adjuvant
chemotherapy. Surg Today. 44:11–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ott K, Weber WA, Lordick F, Becker K,
Busch R, Herrmann K, Wieder H, Fink U, Schwaiger M and Siewert JR:
Metabolic imaging predicts response, survival, and recurrence in
adenocarcinomas of the esophagogastric junction. J Clin Oncol.
24:4692–46988. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weber WA, Ott K, Becker K, Dittler HJ,
Helmberger H, Avril NE, Meisetschläger G, Busch R, Siewert JR,
Schwaiger M and Fink U: Prediction of response to preoperative
chemotherapy in adenocarcinomas of the esophagogastric junction by
metabolic imaging. J Clin Oncol. 19:3058–3065. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lordick F, Ott K, Krause BJ, Weber WA,
Becker K, Stein HJ, Lorenzen S, Schuster T, Wieder H, Herrmann K,
et al: PET to assess early metabolic response and to guide
treatment of adenocarcinoma of the oesophagogastric junction: The
MUNICON phase II trial. Lancet Oncol. 8:797–805. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Japanese Gastric Cancer Association, .
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurokawa Y, Shibata T, Sasako M, Sano T,
Tsuburaya A, Iwasaki Y and Fukuda H: Validity of response
assessment criteria in neoadjuvant chemotherapy for gastric cancer
(JCOG0507-A). Gastric Cancer. 17:514–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Becker K, Mueller JD, Schulmacher C, Ott
K, Fink U, Busch R, Böttcher K, Siewert JR and Höfler H:
Histomorphology and grading of regression in gastric carcinoma
treated with neoadjuvant chemotherapy. Cancer. 98:1521–1530. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heger U, Bader F, Lordick F, Burian M,
Langer R, Dobritz M, Blank S, Bruckner T, Becker K, Herrmann K, et
al: Interim endoscopy results during neoadjuvant therapy for
gastric cancer correlate with histopathological response and
prognosis. Gastric Cancer. 17:478–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
23
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ott K, Fink U, Becker K, Stahl A, Dittler
HJ, Busch R, Stein H, Lordick F, Link T, Schwaiger M, et al:
Prediction of response to preoperative chemotherapy in gastric
carcinoma by metabolic imaging: Results of a prospective trial. J
Clin Oncol. 21:4604–4610. 2003. View Article : Google Scholar : PubMed/NCBI
|