Ionizing radiation is used as a primary treatment
for a number of types of cancer. In total, >40% of patients with
cancer depend on radiotherapy for the treatment of their disease
(1). The major goal of radiotherapy
in oncology is to enhance the cytotoxic effects on the tumor while
minimizing injury to the neighboring normal tissues. In the last
several decades, radiotherapeutic technologies have advanced by
improvements in engineering and computing, resulting in the
development of intensity-modulated radiotherapy, image-guided
radiotherapy and stereotactic radiotherapy (1–3). In
addition, spatial-localizing techniques and radiation-fractionated
therapy have improved the rate of satisfactory therapeutic outcomes
by enabling the repair of radiation-induced damage and regeneration
of damaged and fresh cells. Positive therapeutic outcomes may be
accomplished by the development of selective radioadjuvants,
including radiosensitizers and radioprotectors (4–6). In spite
of the improvement in radiotherapeutic modalities, surrounding
normal tissues are also affected during radiotherapy, with local
tissue damage, mucositis and general weakness occurring (7–9). These
undesired side effects should be managed and minimized by
treatments of adjuvant compounds. Various agents have been proposed
owing to their radioprotective mechanisms and therapeutic effects
(10–13). However, as numerous chemical compounds
exhibit high toxicity, only a limited number have been investigated
in clinical trials. Sulfhydryls have been considered the most
promising radioprotectors. Among numerous sulfhydryl compounds,
only amifostin (WR-2721, Ethyol®) has been approved by
the US Food and Drug Administration for radiotherapeutic treatment
of head and neck cancer (14,15). Further potential candidates for
radiotherapeutic adjuvants are expected to decrease the harmful
effects of radiation on normal cells.
Previous studies have been conducted to identify
traditional oriental medicines (TOMs) that may serve as potent
radioprotectors due to their safety and low toxicity, which have
been confirmed by empirical testing and their clinical use in Asian
countries (16–20). These compounds have been reported to
have antioxidant properties, which may neutralize radiation-induced
damage, including oxidative stress caused by reactive oxygen
species (ROS). TOMs may regulate abnormal redox signaling by acting
as proton donors, reducing agents and metal chelators, consequently
exhibiting antioxidant activities. TOMs have also been reported to
exhibit anti-inflammatory, pro-survival and anti-cytotoxic
activities in animal systems, indicating that they have high
potential to serve as radioprotectors. The present review attempts
to evaluate the roles of TOMs in decreasing the radiological
effects and summarizes the results of studies of radiation and
medicinal plants, which may enhance the effects of radiotherapy by
protecting normal tissues from radiation-induced damage.
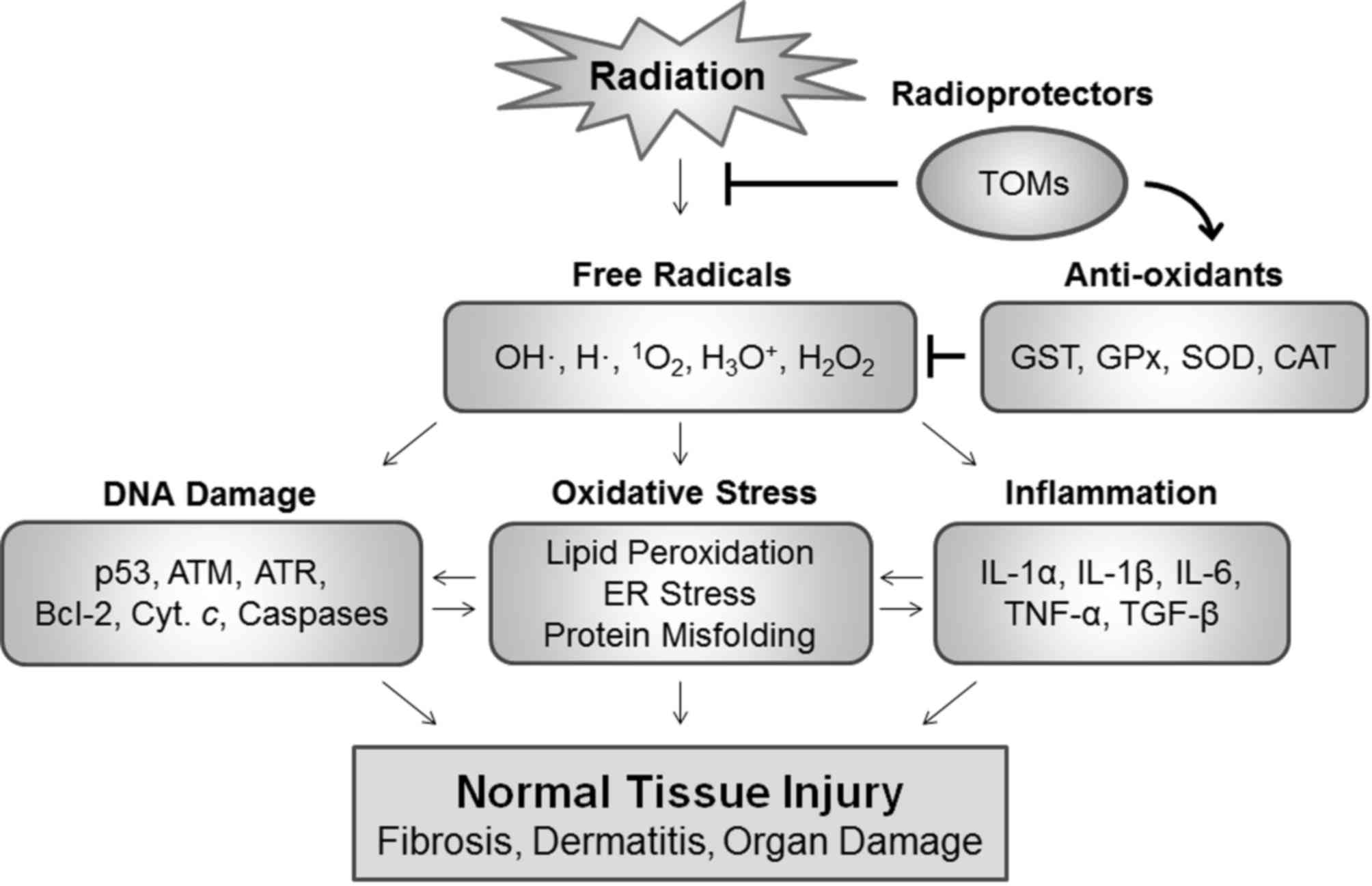
Ionizing radiation generates electrically charged
ions and possesses energy to induce ionization. This energy passes
through the cells in the tissues, and may destroy cancer cells or
induce damage to the genome, resulting in cell death. Cells may be
affected by ionizing radiation directly or indirectly (21). The radiation is able to directly
damage biological macromolecules leading to protein malfunction,
lipid peroxidation and genetic alterations, including mutations,
base lesions and DNA strand breaks. The radiation also causes the
formation of ROS including OH•, H•,
1O2, H3O+ and
H2O2 through the interaction with water
molecules (19,22). When these ROS interact with
biomolecules, secondary free radicals may be produced, leading to
cytotoxic events (23,24).
The majority of radiation-induced DNA damage results
from short-lived primary free radicals produced by radiation and
secondary free radicals produced by the interaction between primary
radicals and biomolecules. In addition, ROS may act as signaling
molecules to activate various cellular signaling pathways,
including pro-inflammatory, pro-survival and pro-apoptotic
responses (22,25,26).
Exposure of cells to ionizing radiation results in oxidative stress
and DNA damage, with subsequent activation of p53 and ataxia
telangiectasia mutated involved in damage-response signaling and
cell death signaling. In addition, ROS may induce loss of
mitochondrial membrane potential, which consequently leads to the
release of mitochondrial pro-apoptotic proteins, including
cytochrome c, second mitochondria-derived activator of
caspases and apoptosis-inducing factor (25,27,28). These
pro-apoptotic proteins may subsequently translocate into the
cytoplasm and the nucleus to activate mitochondria-dependent
apoptosis signaling. Radiation-induced inflammation is regarded as
a critical side effect.
Various free radicals may stimulate an inflammatory
response through the induction of cytokines and chemokines, which
generates long-lived free radicals, leading to chronic damage
(26,29). Various cytokines including
transforming growth factor-β1 (TGF-β1), tumor necrosis factor-α
(TNF-α) and interleukins (e.g. IL-1α, IL-1β, IL-6 and IL-12) that
are upregulated by radiation exposure cause radiation-induced
inflammation in normal tissues. Chronic inflammation occurring as a
late effect of radiation is primarily responsible for the induction
of fibrosis, which is an irreversible disease (29–31). In
this setting, sufficient amounts of antioxidants as radioprotectors
may neutralize the toxic effects of these free radicals and protect
normal tissues against ROS-induced damage during radiation exposure
(Fig. 1). Positive therapeutic
outcomes may be accomplished by scavenging ROS in normal tissues
and/or increasing the cytotoxic activities of these free radicals
in tumors.
TOMs are typically multi-plant formulas that have
long been used to treat diseases in Asian countries (32–34). These
natural herbal products have developed through practical testing
and improvement over thousands of years. Numerous people around the
world currently depend on TOMs for improving their quality of life.
In addition, investigation of TOMs provides fundamental knowledge
essential for modern drug development. However, a major problem
associated with TOMs is limited understanding of their underlying
molecular mechanisms of action, which has restricted their
widespread application to public healthcare. TOM products are
frequently used to treat various symptoms without considering their
action mechanisms or disease-causing mechanisms. Therefore,
numerous researchers have focused on how each TOM acts in a
biological system and what signaling pathways are associated with
these phytochemicals. Investigations to elucidate the underlying
molecular mechanisms of TOMs have led to the establishment of
proper medication protocols and development of promising drugs with
decreased side effects. A number of medicinal plants utilized for
the treatment of a number of ROS-related diseases including
rheumatoid arthritis, cancer, aging and other inflammatory diseases
have been identified to exhibit antioxidative, anti-inflammatory,
antimicrobial and immunostimulatory activities (16–20). These
results indicate that plants may include specific compounds that
may protect against radiation-mediated damage as well, which is
closely associated with ROS-induced damage.
As a number of types of TOM essentially consist of a
multi-plant formula, these compounds may exert synergistic effects
that single-active ingredients do not. Such multi-formula medicines
may potentiate multi-target approaches to treat complex diseases
including cancer caused by abnormal signaling pathways associated
with a number of key molecules at the same time (35–37). Their
functions may result from an isolated single constituent as well as
a combinational effect from crosstalk of a number of constituents
in the same plant or multiple plant complexes. For synergistic
effects, considerable toxicity produced by major active ingredients
of a certain plant may be neutralized by other ingredients in the
plant without disrupting therapeutic activities. Therefore, the
focus has been on the evaluation of radioprotective efficacy from
whole extracts of a plant to isolated constituents based on the
anti-cytotoxic activity against radiation-induced damage.
A variety of TOMs have been reported to contain
antioxidant phytochemicals and to have the potential to function in
a radioprotective role in various systems (16–20). The
most appropriate approach to screening candidates as potential
radioprotectors is to assess whether an active constituent or a
number of compounds with synergistic effects have antioxidative,
anti-inflammatory, antimicrobial or immunostimulatory activities.
Table I presents a summary of TOMs
that exhibit radioprotective activities.
It is clear that a number of TOMs exhibit a variety
of biological activities with respect to radioprotective roles.
However, only a limited number of TOMs have been studied, and
clinical trials of the majority of TOMs have not yet been
conducted. Although these medicinal plants have been utilized in
numerous Asian countries, validation of their quality, chemical
contents and underlying molecular mechanism of action is imperative
prior to initiating clinical trials for application to current
therapeutic systems. Indiscreet variations composed of similar
agents have appeared due to a lack of regulation of these plant
products. As environmental factors including soil content, water,
temperature and acidity differ, different specimens of the same
plant species frequently have differential amounts of the bioactive
ingredients, leading to inconsistent outcomes of biological
activities. Further studies are required to identify the bioactive
constituents in TOMs responsible for specific properties including
radioprotection. For preclinical assays and clinical application,
it is important that the exact formula of TOMs or their active
constituents be determined with specified preparation methods and
that they be tested with strict validation and verification in
accordance with modern scientific standards. Overall, further
intensive studies of these medicinal plants are necessary to verify
their potential for use as radioprotectors and application in
further clinical trials.
|
1
|
Ahmad SS, Duke S, Jena R, Williams MV and
Burnet NG: Advances in radiotherapy. BMJ. 345:e77652012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schaue D and McBride WH: Opportunities and
challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol.
12:527–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baskar R, Lee KA, Yeo R and Yeoh KW:
Cancer and radiation therapy: Current advances and future
directions. Int J Med Sci. 9:193–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Copp RR, Peebles DD, Soref CM and Fahl WE:
Radioprotective efficacy and toxicity of a new family of aminothiol
analogs. Int J Radiat Biol. 89:485–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim W, Youn H, Kwon T, Kang J, Kim E, Son
B, Yang HJ, Jung Y and Youn B: PIM1 kinase inhibitors induce
radiosensitization in non-small cell lung cancer cells. Pharmacol
Res. 70:90–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang J, Kim E, Kim W, Seong KM, Youn H,
Kim JW, Kim J and Youn B: Rhamnetin and cirsiliol induce
radiosensitization and inhibition of epithelial-mesenchymal
transition (EMT) by miR-34a-mediated suppression of Notch-1
expression in non-small cell lung cancer cell lines. J Biol Chem.
288:27343–27357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trotti A, Bellm LA, Epstein JB, Frame D,
Fuchs HJ, Gwede CK, Komaroff E, Nalysnyk L and Zilberberg MD:
Mucositis incidence, severity and associated outcomes in patients
with head and neck cancer receiving radiotherapy with or without
chemotherapy: A systematic literature review. Radiother Oncol.
66:253–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Furby A, Behin A, Lefaucheur JP, Beauvais
K, Marcorelles P, Mussini JM, Bassez G, Créange A, Eymard B and
Pénisson-Besnier I: Late-onset cervicoscapular muscle atrophy and
weakness after radiotherapy for Hodgkin disease: A case series. J
Neurol Neurosurg Psychiatry. 81:101–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Radvansky LJ, Pace MB and Siddiqui A:
Prevention and management of radiation-induced dermatitis,
mucositis, and xerostomia. Am J Health Syst Pharm. 70:1025–1032.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y and Okunieff P: Radiation and
third-generation chemotherapy. Hematol Oncol Clin North Am.
18:55–80. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prouillac C, Vicendo P, Garrigues JC,
Poteau R and Rima G: Evaluation of new thiadiazoles and
benzothiazoles as potential radioprotectors: Free radical
scavenging activity in vitro and theoretical studies (QSAR, DFT).
Free Radic Biol Med. 46:1139–1148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim W, Seong KM and Youn B:
Phenylpropanoids in radioregulation: Double edged sword. Exp Mol
Med. 43:323–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang HJ, Youn H, Seong KM, Yun YJ, Kim W,
Kim YH, Lee JY, Kim CS, Jin YW and Youn B: Psoralidin, a dual
inhibitor of COX-2 and 5-LOX, regulates ionizing radiation
(IR)-induced pulmonary inflammation. Biochem Pharmacol. 82:524–534.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brizel DM, Wasserman TH, Henke M, Strnad
V, Rudat V, Monnier A, Eschwege F, Zhang J, Russell L, Oster W and
Sauer R: Phase III randomized trial of amifostine as a
radioprotector in head and neck cancer. J Clin Oncol. 18:3339–3345.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hensley ML, Schuchter LM, Lindley C,
Meropol NJ, Cohen GI, Broder G, Gradishar WJ, Green DM, Langdon RJ
Jr, Mitchell RB, et al: American society of clinical oncology
clinical practice guidelines for the use of chemotherapy and
radiotherapy protectants. J Clin Oncol. 17:3333–3355. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arora R, Gupta D, Chawla R, Sagar R,
Sharma A, Kumar R, Prasad J, Singh S, Samanta N and Sharma RK:
Radioprotection by plant products: Present status and future
prospects. Phytother Res. 19:1–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weiss JF and Landauer MR: Protection
against ionizing radiation by antioxidant nutrients and
phytochemicals. Toxicology. 189:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jagetia GC: Radioprotective potential of
plants and herbs against the effects of ionizing radiation. J Clin
Biochem Nutr. 40:74–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sagar SM: Can the therapeutic gain of
radiotherapy be increased by concurrent administration of Asian
botanicals? Integr Cancer Ther. 9:5–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kma L: Plant extracts and plant-derived
compounds: Promising players in a countermeasure strategy against
radiological exposure. Asian Pac J Cancer Prev. 15:2405–2425. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nambiar D, Rajamani P and Singh RP:
Effects of phytochemicals on ionization radiation-mediated
carcinogenesis and cancer therapy. Mutat Res. 728:139–157. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bourgier C, Levy A, Vozenin MC and Deutsch
E: Pharmacological strategies to spare normal tissues from
radiation damage: Useless or overlooked therapeutics? Cancer
Metastasis Rev. 31:699–712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maurya DK, Devasagayam TP and Nair CK:
Some novel approaches for radioprotection and the beneficial effect
of natural products. Indian J Exp Biol. 44:93–114. 2006.PubMed/NCBI
|
|
24
|
Kuntic VS, Stanković MB, Vujic ZB, Brborić
JS and Uskoković-Marković SM: Radioprotectors-the evergreen topic.
Chem Biodivers. 10:1791–1803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Valerie K, Yacoub A, Hagan MP, Curiel DT,
Fisher PB, Grant S and Dent P: Radiation-induced cell signaling:
Inside-out and outside-in. Mol Cancer Ther. 6:789–801. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Multhoff G and Radons J: Radiation,
inflammation, and immune responses in cancer. Front Oncol.
2:582012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verheij M: Clinical biomarkers and imaging
for radiotherapy-induced cell death. Cancer Metastasis Rev.
27:471–480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fogg VC, Lanning NJ and Mackeigan JP:
Mitochondria in cancer: At the crossroads of life and death. Chin J
Cancer. 30:526–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Robbins ME and Zhao W: Chronic oxidative
stress and radiation-induced late normal tissue injury: A review.
Int J Radiat Biol. 80:251–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paun A, Kunwar A and Haston CK: Acute
adaptive immune response correlates with late radiation-induced
pulmonary fibrosis in mice. Radiat Oncol. 10:452015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giridhar P, Mallick S, Rath GK and Julka
PK: Radiation induced lung injury: Prediction, assessment and
management. Asian Pac J Cancer Prev. 16:2613–2617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim HU, Ryu JY, Lee JO and Lee SY: A
systems approach to traditional oriental medicine. Nat Biotechnol.
33:264–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheung F: TCM: Made in China. Nature.
480:S82–S83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumar H, Song SY, More SV, Kang SM, Kim
BW, Kim IS and Choi DK: Traditional Korean East Asian medicines and
herbal formulations for cognitive impairment. Molecules.
18:14670–14693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu Z: Modernization: One step at a time.
Nature. 480:S90–S92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schmidt BM, Ribnicky DM, Lipsky PE and
Raskin I: Revisiting the ancient concept of botanical therapeutics.
Nat Chem Biol. 3:360–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barabasi AL, Gulbahce N and Loscalzo J:
Network medicine: A network-based approach to human disease. Nat
Rev Genet. 12:56–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cao W, Li XQ, Wang X, Li T, Chen X, Liu SB
and Mei QB: Characterizations and anti-tumor activities of three
acidic polysaccharides from Angelica sinensis (Oliv.) Diels. Int J
Biol Macromol. 46:115–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang X, Zhao Y, Zhou Y, Lv Y, Mao J and
Zhao P: Component and antioxidant properties of polysaccharide
fractions isolated from Angelica sinensis (OLIV.) DIELS. Biol Pharm
Bull. 30:1884–1890. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Y, Duan JA, Qian D, Guo J, Song B and
Yang M: Assessment and comparison of immunoregulatory activity of
four hydrosoluble fractions of Angelica sinensis in vitro on the
peritoneal macrophages in ICR mice. Int Immunopharmacol.
10:422–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie CH, Zhang MS, Zhou YF, Han G, Cao Z,
Zhou FX, Zhang G, Luo ZG, Wu JP, Liu H, et al: Chinese medicine
Angelica sinensis suppresses radiation-induced expression of
TNF-alpha and TGF-beta1 in mice. Oncol Rep. 15:1429–1436.
2006.PubMed/NCBI
|
|
42
|
Han G, Zhou YF, Zhang MS, Cao Z, Xie CH,
Zhou FX, Peng M and Zhang WJ: Angelica sinensis down-regulates
hydroxyproline and Tgfb1 and provides protection in mice with
radiation-induced pulmonary fibrosis. Radiat Res. 165:546–552.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao L, Wang Y, Shen HL, Shen XD, Nie Y,
Wang Y, Han T, Yin M and Zhang QY: Structural characterization and
radioprotection of bone marrow hematopoiesis of two novel
polysaccharides from the root of Angelica sinensis (Oliv.) Diels.
Fitoterapia. 83:1712–1720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee JG, Hsieh WT, Chen SU and Chiang BH:
Hematopoietic and myeloprotective activities of an acidic Angelica
sinensis polysaccharide on human CD34+ stem cells. J
Ethnopharmacol. 139:739–745. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu C, Li J, Meng FY, Liang SX, Deng R, Li
CK, Pong NH, Lau CP, Cheng SW, Ye JY, et al: Polysaccharides from
the root of Angelica sinensis promotes hematopoiesis and
thrombopoiesis through the PI3K/AKT pathway. BMC Complement Altern
Med. 10:792010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen XP, Li W, Xiao XF, Zhang LL and Liu
CX: Phytochemical and pharmacological studies on Radix Angelica
sinensis. Chin J Nat Med. 11:577–587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Anand P, Thomas SG, Kunnumakkara AB,
Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K,
Priyadarsini IK, Rajasekharan KN and Aggarwal BB: Biological
activities of curcumin and its analogues (Congeners) made by man
and Mother Nature. Biochem Pharmacol. 76:1590–1611. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hatcher H, Planalp R, Cho J, Torti FM and
Torti SV: Curcumin: From ancient medicine to current clinical
trials. Cell Mol Life Sci. 65:1631–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nemavarkar P, Chourasia BK and Pasupathy
K: Evaluation of radioprotective action of compounds using
Saccharomyces cerevisiae. J Environ Pathol Toxicol Oncol.
23:145–151. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pal A and Pal AK: Radioprotection of
turmeric extracts in bacterial system. Acta Biol Hung. 56:333–343.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jagetia GC: Radioprotection and
radiosensitization by curcumin. Adv Exp Med Biol. 595:301–320.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nada AS, Hawas AM, Nel D Amin, Elnashar MM
and Abd Elmageed ZY: Radioprotective effect of Curcuma longa
extract on gamma-irradiation-induced oxidative stress in rats. Can
J Physiol Pharmacol. 90:415–423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aravindan N, Madhusoodhanan R, Ahmad S,
Johnson D and Herman TS: Curcumin inhibits NFkappaB mediated
radioprotection and modulate apoptosis related genes in human
neuroblastoma cells. Cancer Biol Ther. 7:569–576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Qian Y, Ma J, Guo X, Sun J, Yu Y, Cao B,
Zhang L, Ding X, Huang J and Shao JF: Curcumin enhances the
radiosensitivity of U87 cells by inducing DUSP-2 up-regulation.
Cell Physiol Biochem. 35:1381–1393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Goel A and Aggarwal BB: Curcumin, the
golden spice from Indian saffron, is a chemosensitizer and
radiosensitizer for tumors and chemoprotector and radioprotector
for normal organs. Nutr Cancer. 62:919–930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Srinivasan M, Prasad N Rajendra and Menon
VP: Protective effect of curcumin on gamma-radiation induced DNA
damage and lipid peroxidation in cultured human lymphocytes. Mutat
Res. 611:96–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Inano H and Onoda M: Radioprotective
action of curcumin extracted from Curcuma longa LINN: Inhibitory
effect on formation of urinary 8-hydroxy-2′-deoxyguanosine,
tumorigenesis, but not mortality, induced by gamma-ray irradiation.
Int J Radiat Oncol Biol Phys. 53:735–743. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lee JC, Kinniry PA, Arguiri E, Serota M,
Kanterakis S, Chatterjee S, Solomides CC, Javvadi P, Koumenis C,
Cengel KA and Christofidou-Solomidou M: Dietary curcumin increases
antioxidant defenses in lung, ameliorates radiation-induced
pulmonary fibrosis, and improves survival in mice. Radiat Res.
173:590–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jelveh S, Kaspler P, Bhogal N, Mahmood J,
Lindsay PE, Okunieff P, Doctrow SR, Bristow RG and Hill RP:
Investigations of antioxidant-mediated protection and mitigation of
radiation-induced DNA damage and lipid peroxidation in murine skin.
Int J Radiat Biol. 89:618–627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chan PC, Xia Q and Fu PP: Ginkgo biloba
leave extract: Biological, medicinal, and toxicological effects. J
Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 25:211–244.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jacobs BP and Browner WS: Ginkgo biloba: A
living fossil. Am J Med. 108:341–342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yirmibesoglu E, Karahacioglu E, Kilic D,
Lortlar N, Akbulut G and Omeroglu S: The protective effects of
Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: An
experimental study. Clin Exp Dermatol. 37:387–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sener G, Kabasakal L, Atasoy BM, Erzik C,
Velioğlu-Oğünç A, Cetinel S, Gedik N and Yeğen BC: Ginkgo biloba
extract protects against ionizing radiation-induced oxidative organ
damage in rats. Pharmacol Res. 53:241–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Attia A, Rapp SR, Case LD, D'Agostino R,
Lesser G, Naughton M, McMullen K, Rosdhal R and Shaw EG: Phase II
study of Ginkgo biloba in irradiated brain tumor patients: Effect
on cognitive function, quality of life, and mood. J Neurooncol.
109:357–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Suleyman H, Gumustekin K, Taysi S, Keles
S, Oztasan N, Aktas O, Altinkaynak K, Timur H, Akcay F, Akar S, et
al: Beneficial effects of Hippophae rhamnoides L. on nicotine
induced oxidative stress in rat blood compared with vitamin E. Biol
Pharm Bull. 25:1133–1136. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cheng J, Kondo K, Suzuki Y, Ikeda Y, Meng
X and Umemura K: Inhibitory effects of total flavones of Hippophae
rhamnoides L on thrombosis in mouse femoral artery and in vitro
platelet aggregation. Life Sci. 72:2263–2271. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zeb A: Important therapeutic uses of sea
buckthorn (Hippophae): A Review. J Biol Sci. 4:687–693. 2004.
View Article : Google Scholar
|
|
68
|
Goel HC, Prasad J, Singh S, Sagar RK,
Kumar IP and Sinha AK: Radioprotection by a herbal preparation of
Hippophae rhamnoides, RH-3, against whole body lethal irradiation
in mice. Phytomedicine. 9:15–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Agrawala PK and Adhikari JS: Modulation of
radiation-induced cytotoxicity in U 87 cells by RH-3 (a preparation
of Hippophae rhamnoides). Indian J Med Res. 130:542–549.
2009.PubMed/NCBI
|
|
70
|
Gupta V, Bala M, Prasad J, Singh S and
Gupta M: Leaves of Hippophae rhamnoides prevent taste aversion in
gamma-irradiated rats. J Diet Suppl. 8:355–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shukla SK, Chaudhary P, Kumar IP, Samanta
N, Afrin F, Gupta ML, Sharma UK, Sinha AK, Sharma YK and Sharma RK:
Protection from radiation-induced mitochondrial and genomic DNA
damage by an extract of Hippophae rhamnoides. Environ Mol Mutagen.
47:647–656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sureshbabu AV, Barik TK, Namita I and
Kumar I Prem: Radioprotective properties of Hippophae rhamnoides
(sea buckthorn) extract in vitro. Int J Health Sci (Qassim).
2:45–62. 2008.PubMed/NCBI
|
|
73
|
Goel HC, Kumar IP, Samanta N and Rana SV:
Induction of DNA-protein cross-links by Hippophae rhamnoides:
Implications in radioprotection and cytotoxicity. Mol Cell Biochem.
245:57–67. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kumar IP, Namita S and Goel HC: Modulation
of chromatin organization by RH-3, a preparation of Hippophae
rhamnoides, a possible role in radioprotection. Mol Cell Biochem.
238:1–9. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Rai MK: In vitro evaluation of medicinal
plant extracts against Pestalotiopsis mangiferae. Hindustan
Antibiot Bull. 38:53–56. 1996.PubMed/NCBI
|
|
76
|
Gupta SK, Prakash J and Srivastava S:
Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a
medicinal plant. Indian J Exp Biol. 40:765–773. 2002.PubMed/NCBI
|
|
77
|
Singh S, Majumdar DK and Rehan HM:
Evaluation of anti-inflammatory potential of fixed oil of Ocimum
sanctum (Holybasil) and its possible mechanism of action. J
Ethnopharmacol. 54:19–26. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Devi PU: Radioprotective, anticarcinogenic
and antioxidant properties of the Indian holy basil, Ocimum sanctum
(Tulasi). Indian J Exp Biol. 39:185–190. 2001.PubMed/NCBI
|
|
79
|
Uma Devi P and Ganasoundari A:
Radioprotective effect of leaf extract of Indian medicinal plant
Ocimum sanctum. Indian J Exp Biol. 33:205–208. 1995.PubMed/NCBI
|
|
80
|
Monga J, Sharma M, Tailor N and Ganesh N:
Antimelanoma and radioprotective activity of alcoholic aqueous
extract of different species of Ocimum in C(57)BL mice. Pharm Biol.
49:428–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Subramanian M, Chintalwar GJ and
Chattopadhyay S: Antioxidant and radioprotective properties of an
Ocimum sanctum polysaccharide. Redox Rep. 10:257–264. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Uma Devi P, Ganasoundari A, Rao BS and
Srinivasan KK: In vivo radioprotection by ocimum flavonoids:
Survival of mice. Radiat Res. 151:74–78. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Uma Devi P, Ganasoundari A, Vrinda B,
Srinivasan KK and Unnikrishnan MK: Radiation protection by the
ocimum flavonoids orientin and vicenin: Mechanisms of action.
Radiat Res. 154:455–460. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nayak V and Devi PU: Protection of mouse
bone marrow against radiation-induced chromosome damage and stem
cell death by the ocimum flavonoids orientin and vicenin. Radiat
Res. 163:165–171. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shin JY, Song JY, Yun YS, Yang HO, Rhee DK
and Pyo S: Immunostimulating effects of acidic polysaccharides
extract of Panax ginseng on macrophage function. Immunopharmacol
Immunotoxicol. 24:469–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Konoshima T, Takasaki M and Tokuda H:
Anti-carcinogenic activity of the roots of Panax notoginseng. II.
Biol Pharm Bull. 22:1150–1152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Jung CH, Seog HM, Choi IW, Choi HD and Cho
HY: Effects of wild ginseng (Panax ginseng C.A. Meyer) leaves on
lipid peroxidation levels and antioxidant enzyme activities in
streptozotocin diabetic rats. J Ethnopharmacol. 98:245–250. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lee YS, Chung IS, Lee IR, Kim KH, Hong WS
and Yun YS: Activation of multiple effector pathways of immune
system by the antineoplastic immunostimulator acidic polysaccharide
ginsan isolated from Panax ginseng. Anticancer Res. 17:323–331.
1997.PubMed/NCBI
|
|
89
|
Wang W, Shen H, Xie JJ, Ling J and Lu H:
Neuroprotective effect of ginseng against spinal cord injury
induced oxidative stress and inflammatory responses. Int J Clin Exp
Med. 8:3514–3521. 2015.PubMed/NCBI
|
|
90
|
Song JY, Han SK, Bae KG, Lim DS, Son SJ,
Jung IS, Yi SY and Yun YS: Radioprotective effects of ginsan, an
immunomodulator. Radiat Res. 159:768–774. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kim HJ, Kim MH, Byon YY, Park JW, Jee Y
and Joo HG: Radioprotective effects of an acidic polysaccharide of
Panax ginseng on bone marrow cells. J Vet Sci. 8:39–44. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Verma P, Jahan S, Kim TH and Goyal PK:
Management of radiation injuries by panax ginseng extract. J
Ginseng Res. 35:261–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bing SJ, Kim MJ, Ahn G, Im J, Kim DS, Ha
D, Cho J, Kim A and Jee Y: Acidic polysaccharide of Panax ginseng
regulates the mitochondria/caspase-dependent apoptotic pathway in
radiation-induced damage to the jejunum in mice. Acta Histochem.
116:514–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Koo HJ, Jang SA, Yang KH, Kang SC,
Namkoong S, Kim TH, do TT Hang and Sohn EH: Effects of red ginseng
on the regulation of cyclooxygenase-2 of spleen cells in whole-body
gamma irradiated mice. Food Chem Toxicol. 62:839–846. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kim SH, Son CH, Nah SY, Jo SK, Jang JS and
Shin DH: Modification of radiation response in mice by Panax
ginseng and diethyldithiocarbamate. In Vivo. 15:407–411.
2001.PubMed/NCBI
|
|
96
|
Verma P, Sharma P, Parmar J, Sharma P,
Agrawal A and Goyal PK: Amelioration of radiation-induced
hematological and biochemical alterations in Swiss albino mice by
Panax ginseng extract. Integr Cancer Ther. 10:77–84. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Arun R, Prakash MV, Abraham SK and
Premkumar K: Role of Syzygium cumini seed extract in the
chemoprevention of in vivo genomic damage and oxidative stress. J
Ethnopharmacol. 134:329–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Muruganandan S, Srinivasan K, Chandra S,
Tandan SK, Lal J and Raviprakash V: Anti-inflammatory activity of
Syzygium cumini bark. Fitoterapia. 72:369–375. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
de Bona KS, Bellé LP, Sari MH, Thomé G,
Schetinger MR, Morsch VM, Boligon A, Athayde ML, Pigatto AS and
Moretto MB: Syzygium cumini extract decrease adenosine deaminase,
5′nucleotidase activities and oxidative damage in platelets of
diabetic patients. Cell Physiol Biochem. 26:729–738. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Jagetia GC, Baliga MS and Venkatesh P:
Influence of seed extract of Syzygium Cumini (Jamun) on mice
exposed to different doses of gamma-radiation. J Radiat Res.
46:59–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Srivastava S and Chandra D:
Pharmacological potentials of Syzygium cumini: A review. J Sci Food
Agric. 93:2084–2093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Jagetia GC and Baliga MS: Syzygium cumini
(Jamun) reduces the radiation-induced DNA damage in the cultured
human peripheral blood lymphocytes: A preliminary study. Toxicol
Lett. 132:19–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jagetia GC and Baliga MS: Evaluation of
the radioprotective effect of the leaf extract of Syzygium cumini
(Jamun) in mice exposed to a lethal dose of gamma-irradiation.
Nahrung. 47:181–185. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Baliga MS: Anticancer, chemopreventive and
radioprotective potential of black plum (Eugenia jambolana lam.).
Asian Pac J Cancer Prev. 12:3–15. 2011.PubMed/NCBI
|
|
105
|
Jagetia GC, Shetty PC and Vidyasagar MS:
Treatment of mice with leaf extract of jamun (Syzygium cumini Linn.
Skeels) protects against the radiation induced damage in the
intestinal mucosa of mice exposed to different doses of
gamma-radiation. Pharmacology online. 1:169–195. 2008.
|
|
106
|
Jagetia GC, Shetty PC and Vidyasagar MS:
Inhibition of radiation-induced DNA damage by jamun, Syzygium
cumini, in the cultured splenocytes of mice exposed to different
doses of γ-radiation. Integr Cancer Ther. 11:141–153. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Penna SC, Medeiros MV, Aimbire FS,
Faria-Neto HC, Sertié JA and Lopes-Martins RA: Anti-inflammatory
effect of the hydralcoholic extract of Zingiber officinale rhizomes
on rat paw and skin edema. Phytomedicine. 10:381–385. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sharma SS and Gupta YK: Reversal of
cisplatin-induced delay in gastric emptying in rats by ginger
(Zingiber officinale). J Ethnopharmacol. 62:49–55. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Young HY, Luo YL, Cheng HY, Hsieh WC, Liao
JC and Peng WH: Analgesic and anti-inflammatory activities of
[6]-gingerol. J Ethnopharmacol. 96:207–210. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Habib SH, Makpol S, Hamid NA Abdul, Das S,
Ngah WZ and Yusof YA: Ginger extract (Zingiber officinale) has
anti-cancer and anti-inflammatory effects on ethionine-induced
hepatoma rats. Clinics (Sao Paulo). 63:807–813. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ernst E and Pittler MH: Efficacy of ginger
for nausea and vomiting: A systematic review of randomized clinical
trials. Br J Anaesth. 84:367–371. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lien HC, Sun WM, Chen YH, Kim H, Hasler W
and Owyang C: Effects of ginger on motion sickness and gastric
slow-wave dysrhythmias induced by circular vection. Am J Physiol
Gastrointest Liver Physiol. 284:G481–G489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Du X, Pan H, Zhang C, Zhang H, Liu H, Chen
Z and Zeng X: Zingiber officinale extract modulates γ-rays-induced
immunosuppression in mice. J Med Plants Res. 4:1647–1655. 2010.
|
|
114
|
Jagetia GC, Baliga MS, Venkatesh P and
Ulloor JN: Influence of ginger rhizome (Zingiber officinale Rosc)
on survival, glutathione and lipid peroxidation in mice after
whole-body exposure to gamma radiation. Radiat Res. 160:584–592.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
115
|
Jagetia GC, Baliga M and Venkatesh P:
Ginger (Zingiber officinale Rosc.), a dietary supplement, protects
mice against radiation-induced lethality: Mechanism of action.
Cancer Biother Radiopharm. 19:422–435. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Sharma A, Haksar A, Chawla R, Kumar R,
Arora R, Singh S, Prasad J, Islam F, Arora MP and Sharma R Kumar:
Zingiber officinale Rosc. modulates gamma radiation-induced
conditioned taste aversion. Pharmacol Biochem Behav. 81:864–870.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Haksar A, Sharma A, Chawla R, Kumar R,
Arora R, Singh S, Prasad J, Gupta M, Tripathi RP, Arora MP, et al:
Zingiber officinale exhibits behavioral radioprotection against
radiation-induced CTA in a gender-specific manner. Pharmacol
Biochem Behav. 84:179–188. 2006. View Article : Google Scholar : PubMed/NCBI
|