Introduction
Bone is one of the most common sites of metastases
in patients with cancer (1). It is
reported that ~75% of women with advanced breast cancer develop
bone metastasis (2,3). Bone metastasis frequently results in
skeletal-related events, including severe bone pain, pathological
fraction, spinal cord compression and the requirement for surgery
or radiotherapy, which may be associated with decreased quality of
life and poor prognosis (1,2,4).
Current treatments for bone metastases include
bisphosphonates, denosumab, non-steroidal anti-inflammatory drugs
(NSAIDs) and analgesics, but each of them has certain limitations
(5). Bisphosphonates, which are
recommended for bone metastasis treatment, are associated with the
occasional development of osteonecrosis of the jaw (6). Denosumab is superior to zoledronic acid
in reducing skeletal-related events in patients with bone
metastasis, but hypocalcemia occurs more frequently in patients
receiving denosumab (7). NSAIDs are
frequently considered to be more efficacious in reducing bone
cancer pain compared with other pain states, but they are
associated with gastrointestinal injury and myocardial infarction
(5,8).
In this regard, alternative drugs that are able to assist the
treatment for bone metastasis are required.
Osteolytic bone metastases are considered to derive
from a ‘vicious cycle’ of progressive interactions between tumor
cells and the bone microenvironment (9). In this microenvironment, large
quantities of cytokines and mediators, which are released from
tumor cells, osteocytes and degraded bone matrix promote the
process of bone resorption (1,10,11).
Iguratimod (T-614), a novel disease-modifying
anti-rheumatic drug, has exhibited anti-rheumatic effects through
suppression of the production of inflammatory cytokines, including
tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, IL-8
and IL-17, and immunoglobulins, as well as inhibiting the
activation of nuclear factor-kappa B (NF-κB) (12,13). As
cytokines are involved in the process of bone metastasis, the
present study evaluated the hypothesis that iguratimod may protect
against cancer-induced bone pain and bone destruction, potentially
via anti-inflammatory effects in a rat model. The findings may have
the potential to rapidly translate into treatment strategies for
patients with bone metastasis.
Materials and methods
Animals
Female Wistar rats (180–200 g, Tongji Hospital,
Huazhong University of Science and Technology, Wuhan, China) were
maintained at a temperature of 22±1°C under a 12-h/12-h light-dark
cycle regime with free access to food and water. All experimental
protocols were approved by the Medical Ethics Committee of Huazhong
University of Science and Technology and were performed according
to the ethical guidelines of the National Institutes of Health
Guide for Care and Use of Laboratory Animals.
Preparation of carcinoma cells
Walker 256 rat mammary gland carcinoma cells were
provided by the Department of Anesthesiology at Tongji Hospital
(Huazhong University of Science and Technology, Wuhan, China) and
cultured at 37°C, in an atmosphere containing 5% CO2 in
RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) containing 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
(Wuhan Boster Biological Technology, Ltd., Wuhan, China). The cells
were rinsed twice with calcium-and magnesium-free PBS solution and
collected by centrifuging the medium for 5 min at 200 × g. The
pellet was subsequently re-suspended in PBS solution and the
concentration was adjusted to 8×106 cells/ml using a
hemocytometer. The cell suspension was maintained on ice until
inoculation.
Bone cancer pain model
The procedure was performed as previously described
(14,15). Briefly, the rats were completely
anesthetized with 10% chloral hydrate (3 ml/kg, intraperitoneal)
and placed in the supine position. The left leg was shaved and the
skin was disinfected with 7% iodine. The top half of the tibia was
exposed with minimum damage. A 23-gauge needle was inserted into
the intramedullary canal of tibia, 7 mm distal to the epiphyseal
growth plate below the knee joint. Then the needle was removed and
replaced with a 25 µl Hamilton syringe containing the cells (10 µl,
8×104 cells) or vehicle (PBS solution). Following slow
injection and 3 min retention, the Hamilton syringe was removed and
the drilled hole was immediately sealed with bone wax. The site was
thoroughly washed with sterile deionized water and infiltrated with
gentamicin. The muscle and skin were finally sutured and
disinfected. The rats were returned to their room cages following
regaining consciousness.
Drug treatments
Iguratimod was provided by Simcere Pharmaceutical
Group (Nanjing, China). The drug was suspended in 0.5%
methylcellulose solution. Iguratimod (daily dose 5 or 20 mg/kg) or
vehicle (0.5% methylcellulose solution) was administered orally
once daily from day 11 after the tumor cell inoculation (day 0) for
7 days (16).
Mechanical allodynia test
Each rat was tested for mechanical allodynia prior
to the injection of cancer cells or sham, and again on days 4, 8,
12 and 16 post-surgery. Animals were placed in individual plastic
boxes with a metal mesh floor and allowed to habituate for 30 min
prior to tests. Mechanical paw withdrawal threshold was measured by
an ascending series of von Frey filaments (0.6, 1.0, 1.4, 2.0, 4.0,
6.0, 8.0, 10.0 and 15.0 g; Stoelting, Wood Dale, IL, USA) as
previously reported (14,17,18). The
filaments were applied perpendicular to mid-plantar surface of the
left hind paw. Each hair was held for ~1–2 sec with a 10 sec
interval and was applied 5 times per filament. The test was
initiated with the application of the 2.0 g hair and the positive
response was defined as a quick withdrawal or paw flinching.
Whenever a positive response was performed, the next lowest hair
was applied and whenever a negative response occurred, a higher
hair was applied. The paw withdrawal frequency (PWF) to each
monofilament was calculated from five applications. Paw withdrawal
threshold (PWT) was considered the force at which PWF≥60%; 15 g was
recorded as the PWT if PWF<60% to all filaments (18).
Western blot analysis
Rats were sacrificed on day 17, 4–6 h after drug
treatments. The whole spinal cord at L2-L5 segments was quickly
removed and the total protein was extracted using
Trizol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Protein concentrations were measured using a bicinchoninic
acid assay kit (Beyotime Institue of Biotechnology, Guangzhou,
China), and protein samples were heated for 5 min at 100°C with
SDS-PAGE sample buffer (Wuhan Boster Biological Technology, Ltd.).
Subsequently, the equivalent amounts of protein samples (30 µg)
were separated by 10% SDS-PAGE electrophoresis and subsequently
transferred onto polyvinylidene difluoride membranes. The membranes
were blocked in 5% bovine serum albumin containing 0.1% Tween-20 at
room temperature for 1 h and incubated overnight at 4°C with
primary antibodies against phosphorylated extracellular
signal-related kinase (pERK) 1/2 (dilution, 1:1,000; #4370; Cell
Signaling Technology, Inc., Danvers, MA, USA), extracellular
signal-related kinase (ERK) 1/2 (dilution, 1:1,000; #9120; Cell
Signaling Technology, Inc.), c-Fos (dilution, 1:1,000; #sc-52;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or GAPDH
(dilution, 1:2,000; #PB0141, Wuhan Boster Biological Technology,
Ltd.). Subsequently, the membranes were washed in Tris-buffered
saline containing 0.1% Tween-20 and incubated with the secondary
antibody conjugated with horseradish peroxidase (dilution, 1:2,000;
#BA1054, Wuhan Boster Biological Technology, Ltd) for 1 h at room
temperature. Membranes were visualized with Pierce Super Signal
West Pico Chemiluminescent Substrate (Pierce; Thermo Fisher
Scientific, Inc.). Images were captured with the ChemiDoc™ XRS+
imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
protein expression was normalized to GAPDH or total proteins
presented in the corresponding lane on the membrane using Image Lab
software, version 5.1 (Bio-Rad Laboratories, Inc.).
X-ray test
Left hind limbs were collected from cadavers and
assessed radiologically on day 17 prior to decalcification and
histological staining. Hind limbs were exposed to an X-ray source
for 80.0 msec at 55 kV (DR-F, GE Hualun Medical Systems, Beijing,
China). Radiological scores to evaluate the bone destruction of
each tibia were determined based on blind analysis of radiographs,
using a previously published system (19,20). All
scores are associated with the tibia (bone): 0, normal bone
structure without any sign of deterioration; 1, small radiolucent
lesions in the proximal epiphysis (<3), close to the site of the
injection; 2, increased number of radiolucent lesions (>3) loss
of medullary bone; 3, loss of medullary bone, plus erosion of the
cortical bone; 4, full thickness unicortical bone loss; 5, full
thickness bicortical bone loss and displaced fractures (19,20).
Histological staining
For histological staining, rat tibiae were gently
separated and fixed in 4% paraformaldehyde for 2 days. Following
decalcification in 10% EDTA for 2 weeks, the tibiae were embedded
and stained with Harris' hematoxylin and eosin (H&E) to
determine cancer cell infiltration. Subsequently, decalcified
slices were stained using tartrate-resistant acid phosphatase
(TRAP) according to the manufacturer's protocol (Nanjing Jiancheng
Biological Engineering Research Institute, Nanjing, China).
Osteoclasts were defined as TRAP-positive cells containing ≥3
nuclei, as counted under a light microscope (TE2000; Nikon
Corporation, Tokyo, Japan). Five randomly selected fields under
×400 magnification were examined to count TRAP (+) cells in each
group. Analysis was performed in a blinded fashion.
Quantitative analysis of plasma IL-6
level
Rats' blood was obtained using left ventricular
puncture with syringes containing heparin on day 17 post-surgery.
Plasma was separated following centrifugation. The quantitation of
IL-6 in plasma was performed using the rat IL-6 ELISA kit according
to the manufacturer's protocol (Dakewe Biotech Co., Ltd., Beijing,
China).
Statistical analysis
Statistical analysis was performed using SPSS
software, version 19.0 (IBM SPSS, Armonk, NY, USA). All data are
expressed as the mean ± standard error of the mean. Statistical
analyses between two samples were performed using the Student's
t-test. Statistical comparison of more than two groups was
performed using one-way analysis of variance followed by a Tukey
test. Data from the behavior test and X-ray scores were analyzed
across treatment groups using a Kruskal-Wallis nonparametric
analysis of variance test. P<0.05 was considered to indicate a
statistically significant difference.
Results
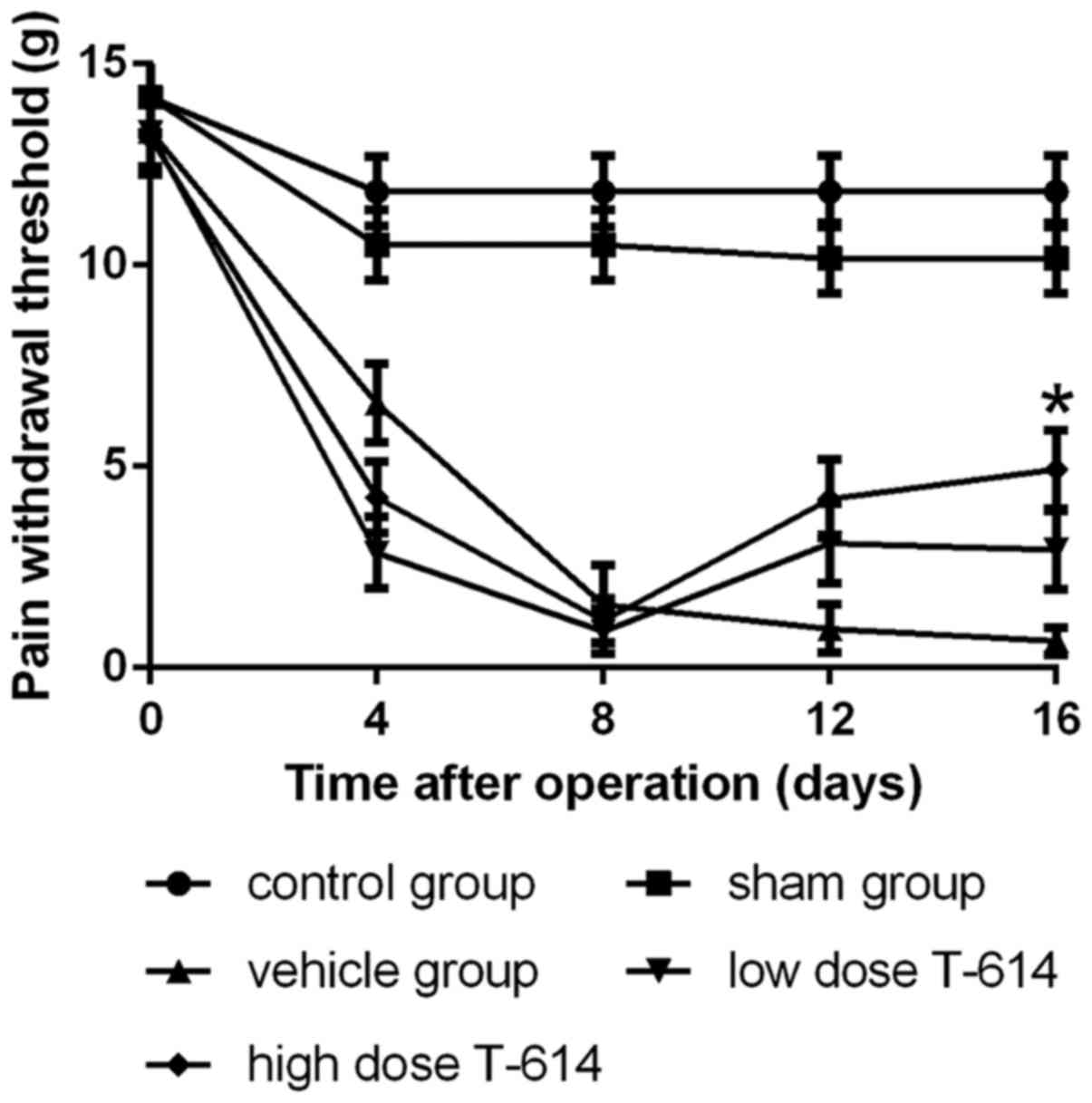
Analgesic effects of iguratimod
The analgesic effect of iguratimod was investigated
using animal models. Fig. 1 reveals
that iguratimod significantly improved the pain withdrawal
threshold of the left hind paw in dose-dependent manner.
The mechanical PWT of each rat was tested prior to
injections and every 4 days following the surgery. The PWT of
tumor-free rats remained at a high level throughout the test, but
the PWT of tumor-bearing rats decreased from day 4 post-surgery and
reached a low level at day 8. Iguratimod and vehicle were
administered to groups from days 11–17. From day 12, the PWT of
rats with iguratimod exhibited an upward trend and it increased
more significantly in the high dose group (20 mg/kg), whereas the
PWT of tumor-bearing rats treated with vehicle maintained a
downward trend. On day 16, the PWT of rats in the high dose group
was significantly higher compared with the vehicle group (4.60±0.98
vs. 0.80±0.33 g; P<0.05). Low dose iguratimod (5 mg/kg) also
exhibited a degree of analgesic effect, but the difference in PWT
between the vehicle group and the low dose group at day 16 was not
statistically significant.
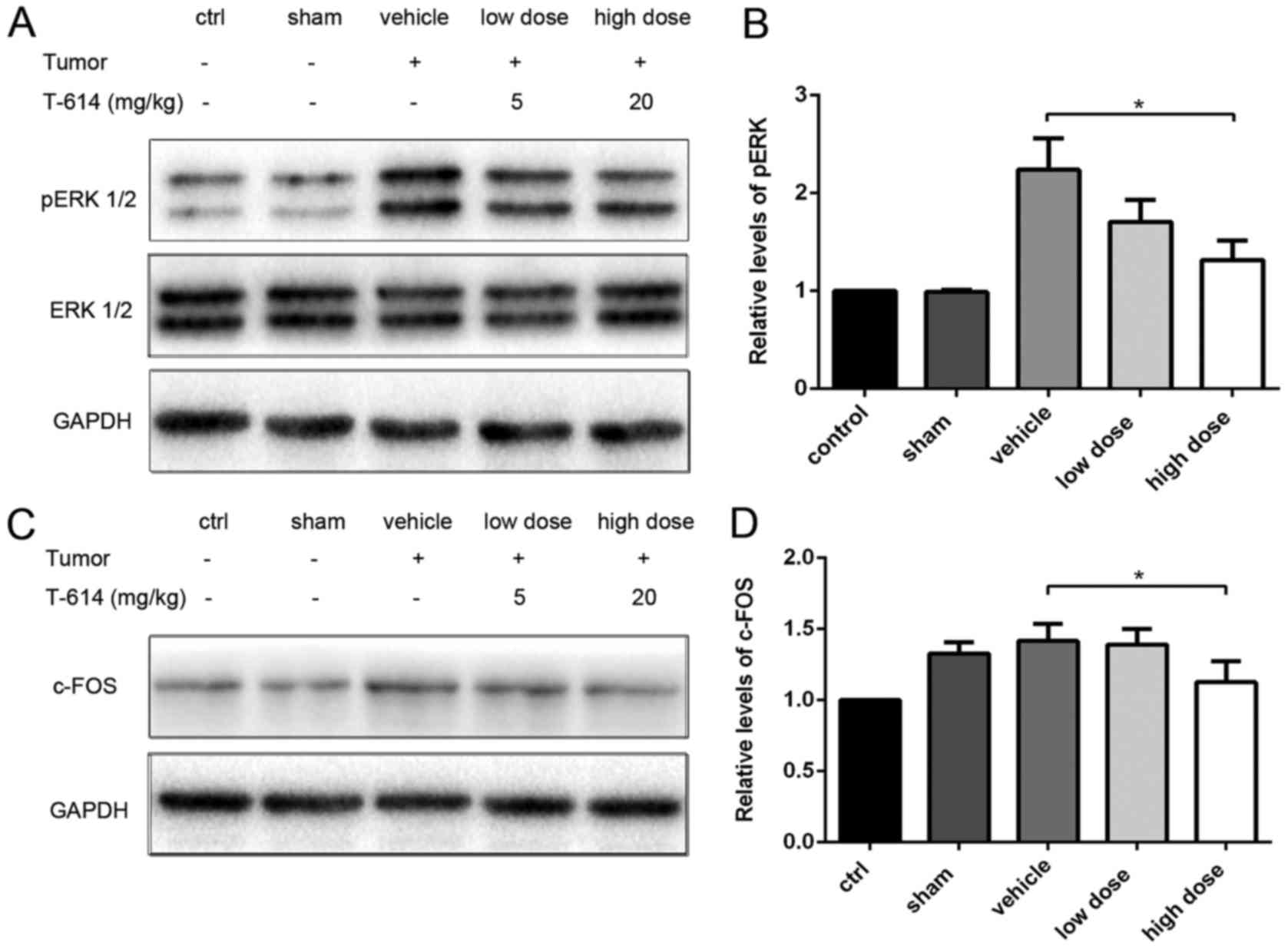
Effects of iguratimod on pERK and
c-Fos expression in spinal cord
To determine whether bone cancer pain was mitigated
by iguratimod, proteins that are associated with bone cancer pain
in the spinal cord were investigated. As pERK and c-Fos are markers
for neuronal activation and central sensitization, the protein
levels of pERK1/2 and c-Fos in the spinal cord were evaluated using
western blot analysis (21–24). As presented in Fig. 2, the protein levels of spinal pERK1/2
were increased in tumor-bearing rats at day 17, whereas the levels
were lower in cancer-free rats. Furthermore, the pERK1/2 levels
declined in a dose-dependent manner when iguratimod was
administered and the difference between the vehicle group and high
dose group was statistically significant. No significant effect was
observed on the total ERK1/2 levels. The same trend in c-Fos levels
in the spinal cords was detected and the difference between the
vehicle group and high dose group was also statistically
significant (Fig. 2). Alterations in
pERK1/2 and c-Fos were concordant with the trends in mechanical
PWT.
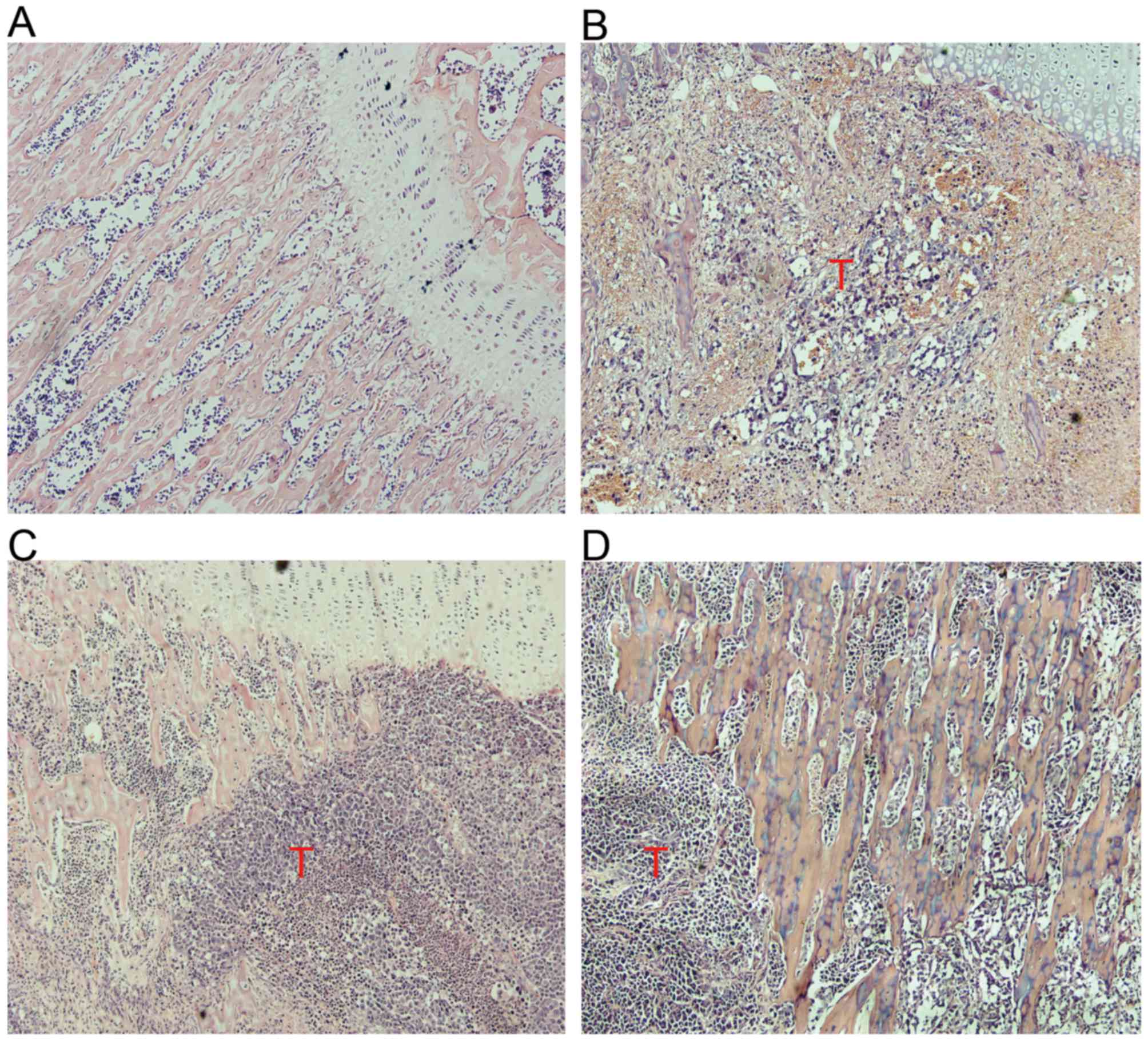
Effect of iguratimod on bone
resorption
A total of 17 days after injection of Walker 256 rat
mammary cancer cells into the intramedullary space of the rat
tibia, tumor growth and bone resorption were observed in
tumor-bearing tibiae. Serial sections stained with H&E
demonstrated that cancer cells grew invasively in the bone marrow
cavity and the trabecular bone was damaged significantly in the
vehicle group at day 17 post-surgery. However, trabecular bone
destruction was lighter and some normal trabecular bone was
observed in rats treated with iguratimod. Bone resorption was not
observed in the sham group (Fig.
3).
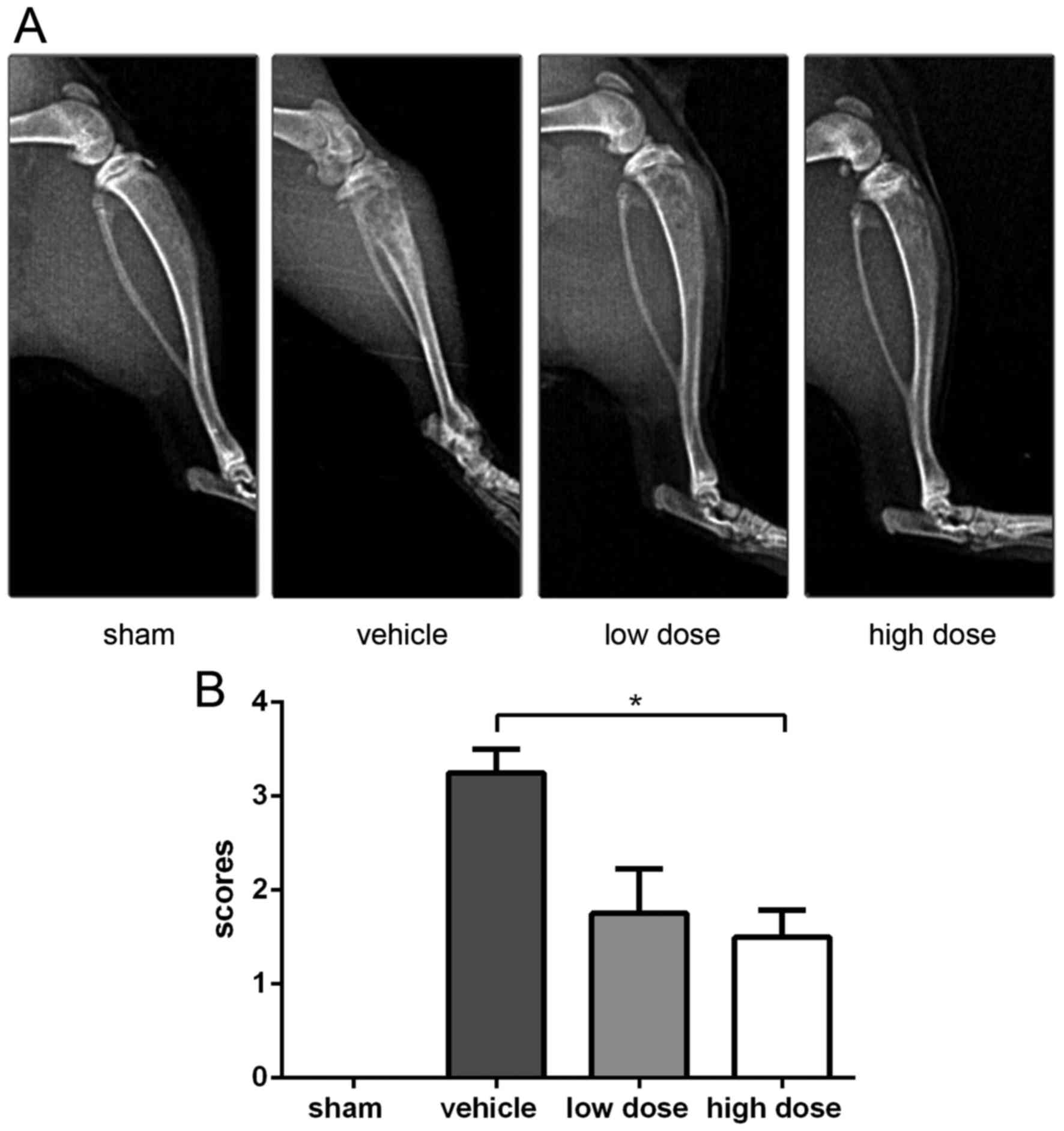
X-ray tests were conducted for each left hind paw at
day 17 after inoculation and the scores of bone destruction were
calculated. As presented in Fig. 4,
varying degrees of bone destruction were detected in the proximal
tibiae of tumor-bearing rats, whereas these phenomena were not
observed in the sham group. Rats from the vehicle group presented
multiple radiation translucent areas due to medullary bone loss, as
well as bilateral cortical defects. However, fewer radiolucent
lesions were detected in rats treated with iguratimod, and
bicortical bone loss was less common compared with the vehicle
group; however, unicortical defects may still exist. According to
the scores calculated from each group, the scores of the high dose
group were significantly lower than those of the vehicle group
(1.6±0.50 vs. 3.33±0.58, respectively).
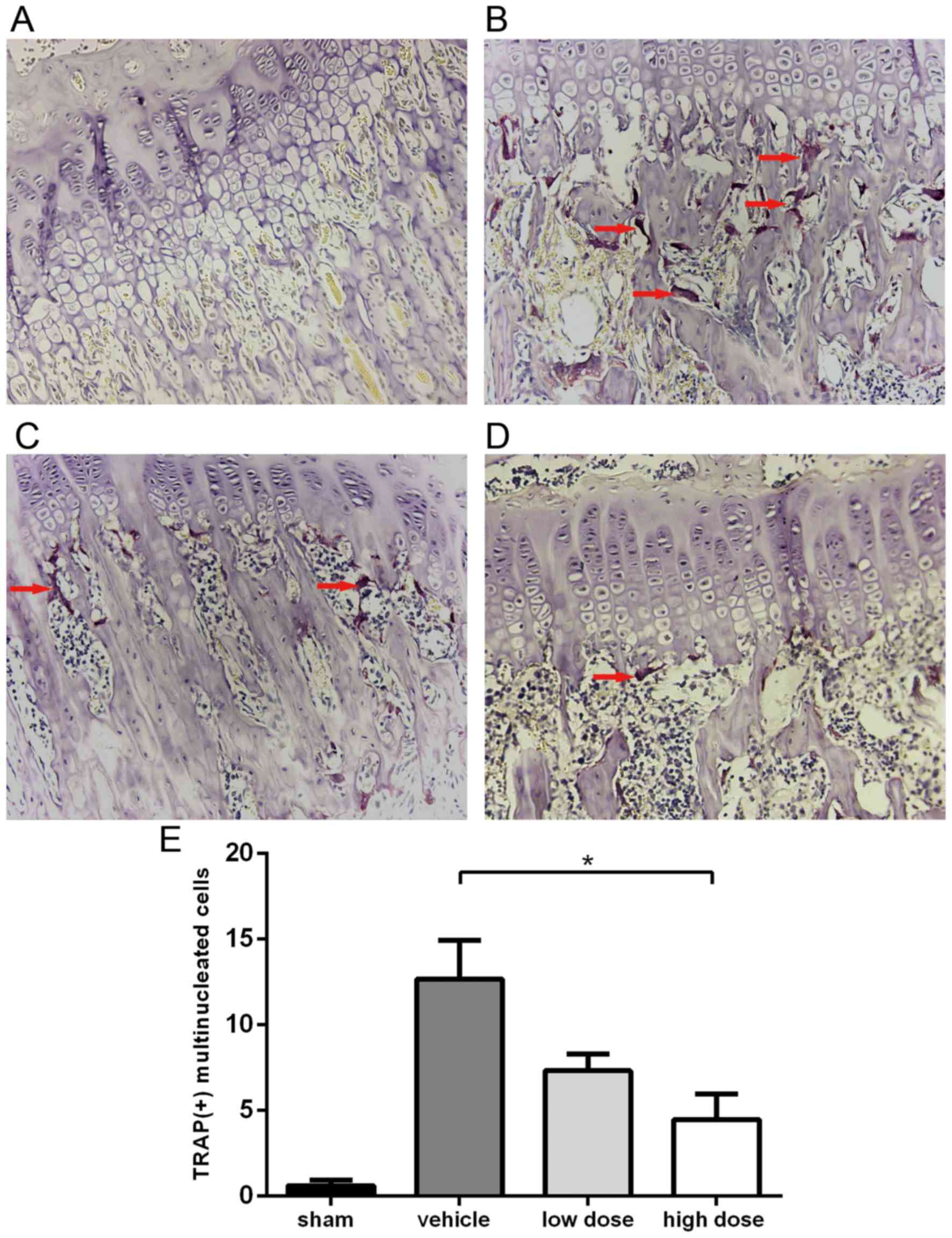
As bone destruction is associated with the increased
activity of osteoclasts, the activation of osteoclasts was detected
using TRAP staining. As observed under the microscope, osteoclasts
were claret-colored multinucleated cells primarily distributed
along the edges of trabeculae in the tibia metaphysis. As presented
in Fig. 5, osteoclasts were rarely
identified in the tibiae of the sham group. However, the number of
TRAP (+) multinucleated cells was significantly increased in the
vehicle group. In rats treated with iguratimod, the activity of
osteoclasts appeared weaker than that of the vehicle group,
resulting in fewer TRAP (+) multinucleated cells being identified
in stained sections. Five fields were randomly selected under ×400
magnification, and TRAP (+) cells were counted for each group. The
number of activated osteoclasts in the high dose group was
significantly lower than that in the vehicle group (4.47±2.61 vs.
12.67±3.95, respectively).
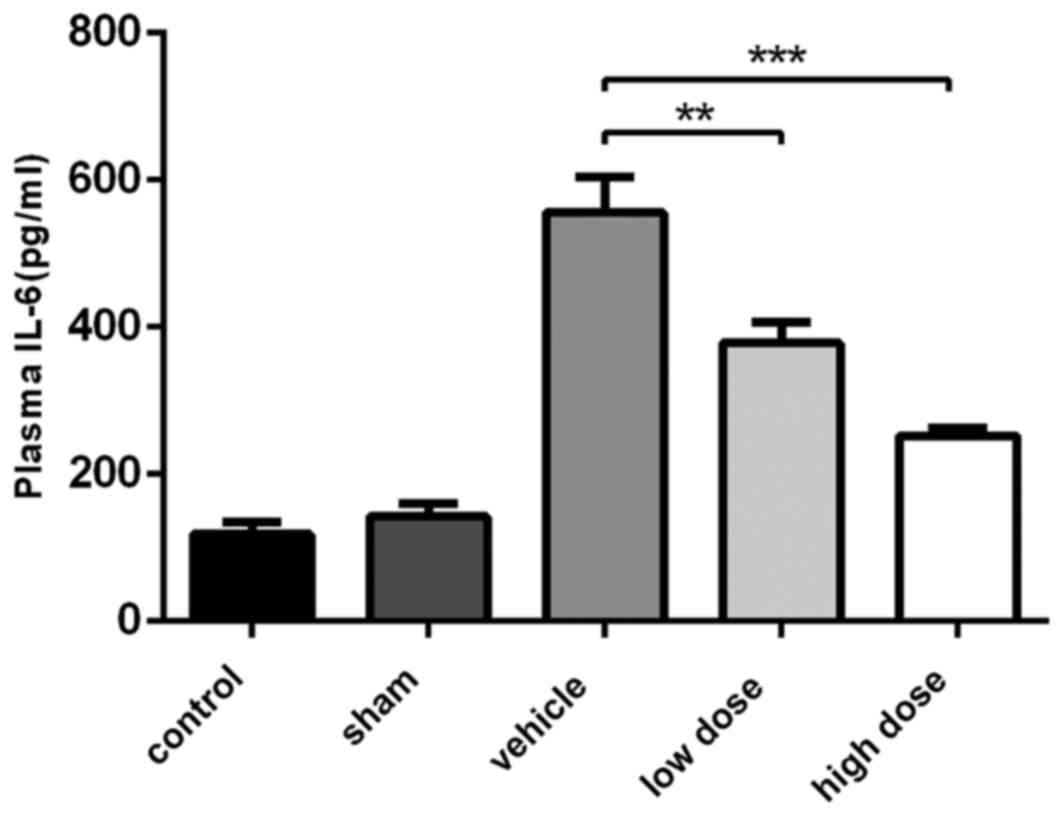
Effect of iguratimod on plasma levels
of IL-6
The plasma IL-6 levels of rats in each group were
detected following drug treatment using ELISA analysis. As
presented in Fig. 6, plasma IL-6
levels of rats in the vehicle group were highly increased compared
with those in the sham group (394.76±36.67 vs. 128.93±30.35 pg/ml,
respectively). The IL-6 levels in rats treated with iguratimod were
decreased in a dose-dependent manner compared with those in the
vehicle group (249.10±31.73 vs. 394.76±36.67 pg/ml; 198.09±33.73
vs. 394.76±36.67 pg/ml, respectively) and were correlated
positively with the changes in mechanical allodynia and bone
destruction in individual animals.
Discussion
The Walker 256 rat mammary carcinoma cell-induced
bone cancer pain model has been extensively utilized to elucidate
the underlying mechanisms for cancer-induced bone pain. In the
current study, the anti-nociceptive effect of iguratimod was
investigated in this rat model.
The mechanical PWT was used to detect the analgesic
effect of iguratimod. In the vehicle-treated animals, the PWT
declined throughout the study, but when iguratimod was
administered, the PWT exhibited an upward trend. The changes in the
expression levels of biomarkers in the spinal cord associated with
bone cancer pain were coincident with those of mechanical PWT. The
current study also revealed that iguratimod reduced the bone
destruction resulting from cancer cell invasion, using X-ray
analysis and TRAP staining. As the plasma IL-6 levels of rats
declined in the iguratimod-treated groups, the present study
hypothesizes that the efficacy of iguratimod may be associated with
its anti-inflammatory effects.
Typically, when bone metastasis occurs, crosstalk
between the tumor cells and the bone microenvironment drives a
vicious cycle of tumor growth and bone destruction (25–27). In
this microenvironment, tumor cells and their associated stromal
cells, as well as osteocytes, release large quantities of factors
including TNF-α, IL-6, bradykinin, endothelins, cannabinoids,
granulocyte-macrophage colony-stimulating factor, nerve growth
factor, and parathyroid hormone-related protein (10,28–30).
Although pain signals are processed in the nervous system, it is
considered that inflammatory mediators and cytokines released from
cancer cells, immunocytes, osteoclasts or injured tissues in the
local microenvironment are able to stimulate the nociceptor
terminals of peripheral afferent sensory neurons (8,30,31). The electrochemical signals converted
by local nociceptors are subsequently transmitted to the spinal
cord and the central nervous system, and pain sensitivity is
enhanced (31). Cytokines in the
microenvironment, including like IL-6, IL-1β and TNF-α have an
important role in driving bone cancer pain (32). They may directly interact with ion
channels and receptors on primary afferent nerves and activate
second messengers (protein kinase C, protein kinase A, ERK, c-Jun
N-terminal kinases, p38 and mitogen-activated protein kinases) in
neurons (33). The phosphorylation
states of the receptors and ion channels are subsequently altered
and the excitement threshold is reduced (33). When these cytokines affect the primary
afferent nerve chronically, transcription factors like cAMP
response element binding protein, signal transducer and activator
of transcription (STAT) and activating transcription factor-3 may
be activated by second messengers and the expression levels of
neurotransmitters, peptides and ion channel proteins may be altered
(33). This leads to sensitization of
the peripheral and central nervous system and results in continued
and aggravated pain (33). At
present, bone cancer pain is considered to be a mixed-mechanism
pain state involving inflammatory, neuropathic, ischemic and
cancer-specific mechanisms (34).
Anti-inflammatory drugs such as NSAIDs are commonly used as
adjuvant drugs to stronger analgesics, so that patients may achieve
improved analgesic effects (34).
Iguratimod is a novel disease-modifying anti-rheumatic drug.
Numerous studies have revealed that, when treated with iguratimod,
the levels of inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-8 and
IL-17) are declined in arthritis rats, and their arthritis symptoms
are also relieved (12,16,35). The
current study therefore hypothesized that its anti-inflammatory
effects may also enable it to alleviate bone cancer pain. According
to the data in the present study, iguratimod significantly reduced
the mechanical pain of tumor-bearing rats in a dose-dependent
manner, and the plasma IL-6 levels were also declined in rats
treated with iguratimod. This is consistent with the theoretical
hypothesis that iguratimod alleviates bone cancer pain by affecting
the vicious circle via exerting anti-inflammatory effects. The
present findings identified a potential additional beneficial
effect of iguratimod in treatment of bone cancer pain.
Iguratimod also demonstrated the effect of
protecting against bone destruction in the current study. It is
considered that increased activity of osteoclasts induced by tumor
cells is the main underlying mechanism responsible for bone
destruction (1). According to the
data in the present study, osteoclasts were markedly activated in
tumor-bearing rats while typical medullary bone loss and cortical
defects were also detected in them. Cytokines are reported to be a
contributor to the activation of osteoclast precursors (30). For example, IL-6, which is primarily
produced by stromal cells in the bone microenvironment, is a strong
stimulator of osteoclast formation (36), and enhances bone resorption in
numerous ways. Firstly, it induces the production of receptor
activator of nuclear factor κB ligand (RANKL) by bone marrow
mesenchymal cells and osteoblasts via the IL-6/STAT3 signaling
pathway. Osteoclast differentiation and maturation are therefore
increased, resulting in the binding of RANKL to its receptor RANK.
Secondly, IL-6 increases the expression levels of several proteins
that aggravate bone degradation, such as parathyroid
hormone-related protein, IL-8, RANKL and cyclooxygenase-2 (COX-2)
in tumor cells. Thirdly, IL-6 imbalances bone homeostasis towards
excessive degradation by inhibiting Wnt-mediated osteogenesis and
downregulating the synthesis of genes including type II collagen
and aggrecan (37,38). Other cytokines, such as TNF-α, IL-1β
and IL-8 also serve an important role in bone degradation (10). The present study therefore
hypothesized that the anti-inflammatory effects of iguratimod may
impact the bone destruction induced by bone metastasis. The current
study detected the effects of iguratimod on bone destruction using
X-rays and histological staining, and the extent of bone
destruction was reduced in rats treated with iguratimod.
As aforementioned, anti-inflammatory therapy
(primarily using NSAIDs) is considered to aid the relief of bone
cancer pain (5). However, traditional
non-selective NSAIDs are associated with gastrointestinal
ulceration, renal dysfunction and impaired platelet aggregation
(8,39). Using COX-2 selective inhibitors may
reduce the risk of gastrointestinal bleeding, but this advantage
appears to reduce after 6 months of treatment, and there is also an
increased risk of cardiovascular events with prolonged use of COX-2
inhibitors (39). Previous studies
demonstrated that iguratimod reduced the expression levels of
cytokines, potentially by suppressing NF-κB activation without
interfering with IκBα degradation (40). Previous clinical trials revealed that
in rheumatoid arthritis patients who received iguratimod for 52
weeks, the adverse events were principally mild or moderate in
severity, and its long-term use is safe (13,41–43).
According to the present study, when treated with iguratimod, not
only is the mechanical allodynia of tumor-bearing rats relieved,
but the bone destruction is also alleviated. As iguratimod is
well-tolerated for long-term use, the current findings may provide
important new insights into the treatment of bone metastasis
symptoms.
In conclusion, the present study first demonstrated
the effects of iguratimod on bone cancer pain and bone destruction
in a rat model, but there were certain limitations. Firstly, the
underlying mechanisms of iguratimod to alleviate bone cancer pain
have not been extensively examined. However, future studies focus
on conducting in vitro studies to further investigate these
mechanisms, and the data have not yet been published. Secondly,
larger-scale experiments are required to verify these effects.
Finally, clinical trials are also required to test the efficacy of
iguratimod in patients with bone cancer pain.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81372852). The authors would
like to thank Dr Dai Shi and Dr Xuehai Guan (Department of
Anesthesiology, Tongji Hospital, Wuhan, China) for their surgical
and mechanical allodynia test suggestions. The authors would also
like to thank Dr Qiaochu Fu (Department of Anesthesiology, Tongji
Hospital) for her provision of the Walker 256 cells.
References
|
1
|
Roodman GD: Genes associate with abnormal
bone cell activity in bone metastasis. Cancer Metastasis Rev.
31:569–578. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holland PM, Miller R, Jones J, Douangpanya
H, Piasecki J, Roudier M and Dougall WC: Combined therapy with the
RANKL inhibitor RANK-Fc and rhApo2L/TRAIL/dulanermin reduces bone
lesions and skeletal tumor burden in a model of breast cancer
skeletal metastasis. Cancer Biol Ther. 9:539–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zinonos I, Luo KW, Labrinidis A, Liapis V,
Hay S, Panagopoulos V, Denichilo M, Ko CH, Yue GG, Lau CB, et al:
Pharmacologic inhibition of bone resorption prevents cancer-induced
osteolysis but enhances soft tissue metastasis in a mouse model of
osteolytic breast cancer. Int J Oncol. 45:532–540. 2014.PubMed/NCBI
|
|
4
|
Gui Q, Xu C, Zhuang L, Xia S, Chen Y, Peng
P and Yu S: A new rat model of bone cancer pain produced by rat
breast cancer cells implantation of the shaft of femur at the third
trochanter level. Cancer Biol Ther. 14:193–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kane CM, Hoskin P and Bennett MI: Cancer
induced bone pain. BMJ. 350:h3152015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vahtsevanos K, Kyrgidis A, Verrou E,
Katodritou E, Triaridis S, Andreadis CG, Boukovinas I, Koloutsos
GE, Teleioudis Z, Kitikidou K, et al: Longitudinal cohort study of
risk factors in cancer patients of bisphosphonate-related
osteonecrosis of the jaw. J Clin Oncol. 27:5356–5362. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stopeck AT, Lipton A, Body JJ, Steger GG,
Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA,
Viniegra M, et al: Denosumab compared with zoledronic acid for the
treatment of bone metastases in patients with advanced breast
cancer: A randomized, double-blind study. J Clin Oncol.
28:5132–5139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koo HJ, Yoon WJ, Sohn EH, Ham YM, Jang SA,
Kwon JE, Jeong YJ, Kwak JH, Sohn E, Park SY, et al: The analgesic
and anti-inflammatory effects of Litsea japonica fruit are mediated
via suppression of NF-κB and JNK/p38 MAPK activation. Int
Immunopharmacol. 22:84–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin JJ, Pollock CB and Kelly K: Mechanisms
of cancer metastasis to the bone. Cell Res. 15:57–62. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bussard KM, Venzon DJ and Mastro AM:
Osteoblasts are a major source of inflammatory cytokines in the
tumor microenvironment of bone metastatic breast cancer. J Cell
Biochem. 111:1138–1148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takiguchi S, Korenaga N, Inoue K, Sugi E,
Kataoka Y, Matsusue K, Futagami K, Li YJ, Kukita T, Teramoto N and
Iguchi H: Involvement of CXCL14 in osteolytic bone metastasis from
lung cancer. Int J Oncol. 44:1316–1324. 2014.PubMed/NCBI
|
|
12
|
Luo Q, Sun Y, Liu W, Qian C, Jin B, Tao F,
Gu Y, Wu X, Shen Y and Xu Q: A novel disease-modifying
antirheumatic drug, iguratimod, ameliorates murine arthritis by
blocking IL-17 signaling, distinct from methotrexate and
leflunomide. J Immunol. 191:4969–4978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okamura K, Yonemoto Y, Okura C, Kobayashi
T and Takagishi K: Efficacy of the clinical use of iguratimod
therapy in patients with rheumatoid arthritis. Mod Rheumatol.
25:235–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guan XH, Fu QC, Shi D, Bu HL, Song ZP,
Xiong BR, Shu B, Xiang HB, Xu B, Manyande A, et al: Activation of
spinal chemokine receptor CXCR3 mediates bone cancer pain through
an Akt-ERK crosstalk pathway in rats. Exp Neurol. 263:39–49. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mao-Ying QL, Zhao J, Dong ZQ, Wang J, Yu
J, Yan MF, Zhang YQ, Wu GC and Wang YQ: A rat model of bone cancer
pain induced by intra-tibia inoculation of Walker 256 mammary gland
carcinoma cells. Biochem Biophys Res Commun. 345:1292–1298. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du F, Lü LJ, Fu Q, Dai M, Teng JL, Fan W,
Chen SL, Ye P, Shen N, Huang XF, et al: T-614, a novel
immunomodulator, attenuates joint inflammation and articular damage
in collagen-induced arthritis. Arthritis Res Ther. 10:R1362008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu B, Guan XH, Yu JX, Lv J, Zhang HX, Fu
QC, Xiang HB, Bu HL, Shi D, Shu B, et al: Activation of spinal
phosphatidylinositol 3-kinase/protein kinase B mediates pain
behavior induced by plantar incision in mice. Exp Neurol.
255:71–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pogatzki EM and Raja SN: A mouse model of
incisional pain. Anesthesiology. 99:1023–1027. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bloom AP, Jimenez-Andrade JM, Taylor RN,
Castañeda-Corral G, Kaczmarska MJ, Freeman KT, Coughlin KA,
Ghilardi JR, Kuskowski MA and Mantyh PW: Breast cancer-induced bone
remodeling, skeletal pain, and sprouting of sensory nerve fibers. J
Pain. 12:698–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Medhurst SJ, Walker K, Bowes M, Kidd BL,
Glatt M, Muller M, Hattenberger M, Vaxelaire J, O'Reilly T,
Wotherspoon G, et al: A rat model of bone cancer pain. Pain.
96:129–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao YJ and Ji RR: c-Fos and pERK, which is
a better marker for neuronal activation and central sensitization
after noxious stimulation and tissue injury? Open Pain J. 2:11–17.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang LN, Yao M, Yang JP, Peng J, Peng Y,
Li CF, Zhang YB, Ji FH, Cheng H, Xu QN, et al: Cancer-induced bone
pain sequentially activates the ERK/MAPK pathway in different cell
types in the rat spinal cord. Mol Pain. 7:482011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang XW, Li TT, Zhao J, Mao-Ying QL, Zhang
H, Hu S, Li Q, Mi WL, Wu GC, Zhang YQ and Wang YQ: Extracellular
signal-regulated kinase activation in spinal astrocytes and
microglia contributes to cancer-induced bone pain in rats.
Neuroscience. 217:172–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Doré-Savard L, Otis V, Belleville K,
Lemire M, Archambault M, Tremblay L, Beaudoin JF, Beaudet N,
Lecomte R, Lepage M, et al: Behavioral, medical imaging and
histopathological features of a new rat model of bone cancer pain.
PLoS One. 5:e137742010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kingsley LA, Fournier PG, Chirgwin JM and
Guise TA: Molecular biology of bone metastasis. Mol Cancer Ther.
6:2609–2617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sterling JA, Edwards JR, Martin TJ and
Mundy GR: Advances in the biology of bone metastasis: How the
skeleton affects tumor behavior. Bone. 48:6–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Siclari VA, Guise TA and Chirgwin JM:
Molecular interactions between breast cancer cells and the bone
microenvironment drive skeletal metastases. Cancer Metastasis Rev.
25:621–633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mantyh P: Bone cancer pain: Causes,
consequences, and therapeutic opportunities. Pain. 154 Suppl
1:S54–S62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sosnoski DM, Krishnan V, Kraemer WJ,
Dunn-Lewis C and Mastro AM: Changes in cytokines of the bone
microenvironment during breast cancer metastasis. Int J Breast
Cancer. 2012:1602652012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jimenez-Andrade JM, Mantyh WG, Bloom AP,
Ferng AS, Geffre CP and Mantyh PW: Bone cancer pain. Ann N Y Acad
Sci. 1198:173–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoneda T, Hata K, Nakanishi M, Nagae M,
Nagayama T, Wakabayashi H, Nishisho T, Sakurai T and Hiraga T:
Involvement of acidic microenvironment in the pathophysiology of
cancer-associated bone pain. Bone. 48:100–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clark AK, Old EA and Malcangio M:
Neuropathic pain and cytokines: Current perspectives. J Pain Res.
6:803–814. 2013.PubMed/NCBI
|
|
33
|
Ellis A and Bennett DL: Neuroinflammation
and the generation of neuropathic pain. Br J Anaesth. 111:26–37.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Falk S and Dickenson AH: Pain and
nociception: Mechanisms of cancer-induced bone pain. J Clin Oncol.
32:1647–1654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du F, Lü LJ, Teng JL, Shen N, Ye P and Bao
CD: T-614 alters the production of matrix metalloproteinases (MMP-1
andMMP-3) and inhibits the migratory expansion of rheumatoid
synovial fibroblasts, in vitro. Int Immunopharmacol. 13:54–60.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
David Roodman G: Role of stromal-derived
cytokines and growth factors in bone metastasis. Cancer. 97 3
Suppl:S733–S738. 2003. View Article : Google Scholar
|
|
37
|
Ara T and Declerck YA: Interleukin-6 in
bone metastasis and cancer progression. Eur J Cancer. 46:1223–1231.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ara T, Song L, Shimada H, Keshelava N,
Russell HV, Metelitsa LS, Groshen SG, Seeger RC and DeClerck YA:
Interleukin-6 in the bone marrow microenvironment promotes the
growth and survival of neuroblastoma cells. Cancer Res. 69:329–337.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Paice JA and Ferrell B: The management of
cancer pain. CA Cancer J Clin. 61:157–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aikawa Y, Yamamoto M, Yamamoto T, Morimoto
K and Tanaka K: An anti-rheumatic agent T-614 inhibits NF-kappaB
activation in LPS- and TNF-alpha-stimulated THP-1 cells without
interfering with IkappaBalpha degradation. Inflamm Res. 51:188–194.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Okamura K, Yonemoto Y, Suto T, Okura C and
Takagishi K: Efficacy at 52 weeks of daily clinical use of
iguratimod in patients with rheumatoid arthritis. Mod Rheumatol.
25:534–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hara M, Abe T, Sugawara S, Mizushima Y,
Hoshi K, Irimajiri S, Hashimoto H, Yoshino S, Matsui N and Nobunaga
M: Long-term safety study of iguratimod in patients with rheumatoid
arthritis. Mod Rheumatol. 17:10–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hara M, Ishiguro N, Katayama K, Kondo M,
Sumida T, Mimori T, Soen S, Nagai K, Yamaguchi T and Yamamoto K;
Iguratimod-Clinical Study Group, : Safety and efficacy of
combination therapy of iguratimod with methotrexate for patients
with active rheumatoid arthritis with an inadequate response to
methotrexate: An open-label extension of a randomized,
double-blind, placebo-controlled trial. Mod Rheumatol. 24:410–418.
2014. View Article : Google Scholar : PubMed/NCBI
|