Introduction
Complete surgical cure of pancreatic cancer is
possible. However, Japanese patients with early-stage disease have
few symptoms, and only 10% of pancreatic cancer cases are diagnosed
at a resectable stage (1). Most
patients present with unresectable advanced disease at the time of
diagnosis, and the recurrence rate is high even when aggressive
resection is performed (2). In Japan,
the number of deaths from pancreatic cancer per year has steadily
risen, to approximately 30,000 at present, making it the fourth
(7.4%) leading cause of cancer deaths (3). In recent years, patients with borderline
resectable pancreatic cancer have been treated with surgery
following preoperative chemoradiotherapy or intensive multi-agent
chemotherapy. However, quality of life is often impaired by cancer
pain and biliary tract infections in patients with locally advanced
unresectable pancreatic cancer, which has a poor prognosis.
Therefore, multimodality therapy and selection of optimal treatment
methods are essential for achieving prolonged survival in these
patients. Chemoradiotherapy with fluorouracil (5-FU) was formerly
the standard therapy for advanced pancreatic cancer. However,
Burris et al (4). Reported
that chemotherapy with gemcitabine hydrochloride (GEM) produced
significant benefits in terms of the symptom-alleviating effect and
the survival rate. Thereafter, GEM replaced 5-FU as the standard
chemotherapy for pancreatic cancer. Furthermore, a randomized
comparative study of chemoradiotherapy with 5-FU vs. GEM, conducted
by Li et al (5), showed
significantly prolonged survival in the GEM group. Although these
therapies are effective, the survival rate in patients with
pancreatic cancer is significantly lower than that in patients with
other carcinomas. Thus, we investigated the significance of using
hyperthermia concurrently with multimodality therapy for improving
the outcomes of patients with locally advanced unresectable
pancreatic cancer.
Materials and methods
Patient selection
We retrospectively studied 13 patients who received
concurrent hyperthermia and chemoradiotherapy (HCR) or
chemoradiotherapy (CR) alone during the period from 2002 to 2013
(Table I). Documentation of informed
consent for treatment was signed by each patient and placed in the
patient's medical record. These patients were diagnosed as having
locally advanced unresectable pancreatic cancer based on
histopathological and imaging findings. Among the 13 patients, five
received concurrent HCR and eight were given CR. The drugs used for
chemotherapy were 5-FU in five patients and GEM in the other eight.
GEM was used in all 5 patients who received concurrent HCR
(Table II). Patients who consented
to undergo hyperthermia treatment were given GEM CR. As for the
selection of chemotherapeutic agents, 5-FU was used at our hospital
until the approval of GEM in Japan.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | n=13 |
|---|
| Follow-up
(months) |
|
Median | 12 |
|
Range | 4–8 |
| Sex |
| Male | 4 |
|
Female | 9 |
| Age |
|
Median | 78 |
|
Range | 48–80 |
| Pathology |
|
Adenoca | 4 (31) |
| Unknown
(CT and MRI) | 9 (69) |
| PS |
| 1 | 11 (85) |
| 2 | 2 (15) |
| Location |
|
Pancreatic head | 7 (54) |
|
Pancreatic body | 8 (46) |
| Tumor size (cm) |
|
Median | 5.5 |
|
Range | 3–9 |
| Tumor marker (CA 19-9
U/ml) |
|
Median | 5.5 |
|
Range | 0.1–13,850 |
| Stage (UICC 7th) |
| cT4 cN0
cM0 stage III | 9 (69) |
| cT4 cN1
cM0 stage III | 3 (23) |
| cT2 cN1
cM0 stage III | 1 (8) |
 | Table II.Five patients received concurrent
hyperthermia and chemoradiotherapy. |
Table II.
Five patients received concurrent
hyperthermia and chemoradiotherapy.
| No. | Age (years)/sex | Pathology | Location | CA19.9 (U/ml) | Tumor size (cm) | Stage | Hyperthermia | Gemcitabine dose | Radiation dose
(Gy) | Adverse event | Survival time
(months) |
|---|
| 1 | 64/M | Adenoca | Pancreatic body | 511 | 5 | cT4cN0 | 6 times | 500 mg/m2
(3 times/month) | 60 | Grade 3 Gastric
ulcer | 18 |
| 2 | 62/F | No | Pancreatic body | 85.3 | 6 | cT4cN0 | 5 times | 600 mg/m2
(3 times/month) | 50 | Grade 2 Gastritis and
enterocolitis | 17 |
| 3 | 49/F | No | Pancreatic body | 173.2 | 7 | cT4cN0 | 5 times | 1000 mg/m2
(1 time/month) | 50 | Grade 2 Gastritis and
enterocolitis | 15 |
| 4 | 62/F | Adenoca | Pancreatic head | 68 | 6 | cT4cN1 | 6 times | 600 mg/m2
(3 times/month) | 50 | Grade 2 Gastritis and
enterocolitis | 12 |
| 5 | 73/M | No | Pancreatic body | 140.8 | 5 | cT4cN1 | 5 times | 500 mg/m2
(3 times/month) | 50 | Grade 3
neutropenia | 18 |
Radiotherapy
The device employed for radiotherapy was the SIEMENS
PRIMUS KD2 (SIEMENS Oncology Care Systems, Concord, CA, USA). For
treatment planning, the XiO (version 4.4.0–4.6.0; Elekta, Hamburg,
Germany) was employed and the isocenter dose was calculated by
Clarkson's method. Irradiation fields were determined as follows:
First, a 1.5-cm margin was added to the clinical target volume
(CTV), which was the planning target volume (PTV), and then a 5-mm
margin was added to the PTV, which was the irradiation field.
Irradiation was performed using 10MV X-rays; irradiation with
opposed anterior and posterior beams was performed on two patients
and 4- or more field irradiation was performed on the others.
Hyperfractionation was applied in three cases and 2 Gy/fraction in
the others (including all 5 patients who received concurrent HCR).
Total doses were 60 Gy in two patients and approximately 50 Gy in
the others. In the two patients given 60 Gy in total, localized
irradiation of tumor sites was performed as boost radiation after
the dose had exceeded 50 Gy (Table
III).
 | Table III.Summary of RT approaches. |
Table III.
Summary of RT approaches.
| Approach | Details |
|---|
| Chemoradiation | 13 cases |
| Radiation dose |
| 1.4 Gy × 2
fractions/day, total 60.8 Gy | 1 case |
| 1.6 Gy × 2
fractions/day, total 51.6 Gy | 2 cases |
| 2 Gy/fraction,
total 50 Gy | 9 cases |
| 2 Gy/fraction,
total 60 Gy | 1 case |
| Radiation
field |
| CTV | Pancreatic tumor
only or pancreatic tumor with LN meta + 10–15 mm |
| PTV | CTV + 5–8 mm |
| 50 Gy over | CTV + less than 5
mm on the small bowel surface |
Chemotherapy
Treatment regimens were as follows: 5-FU, 300
mg/m2 3 times/month (3 weeks of treatment followed by a
1-week treatment-free period); GEM, 1,000 mg/m2
once/month or 500 to 600 mg/m2 3 times/month (3 weeks of
treatment followed by a 1-week treatment-free period).
Hyperthermia
In combination with radiotherapy, hyperthermia was
administered for 50 min/session once or twice a week, 5 to 6 times
in total, employing an 8-MHz RF-capacitive heating device
(Thermotron-RF8; Yamamoto Vinita Co., Ltd., Osaka, Japan). The 25-
or 30-cm electrodes of the Thermotron-RF8, with output settings
ranging from 800 to 1,200 W, were applied to lesions. The lesions
were heated to 41°C or higher and then evaluated using images
obtained with the Thermotron-RF8 thermo-simulator.
Statistical analysis
Survival was calculated by the Kaplan-Meier method,
and differences were expressed at a 5% significance level with a
two-tailed log-rank test. All calculations and survival displays
were conducted using the SPSS 21.0J statistical software package
(SSPS Inc., Chicago, IL, USA). Acute and late complications were
graded according to the National Cancer Institute-Common
Terminology Criteria (NCI-CTC), version 4.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcae_4_with_lay_terms.pdf).
Results
Overall survival
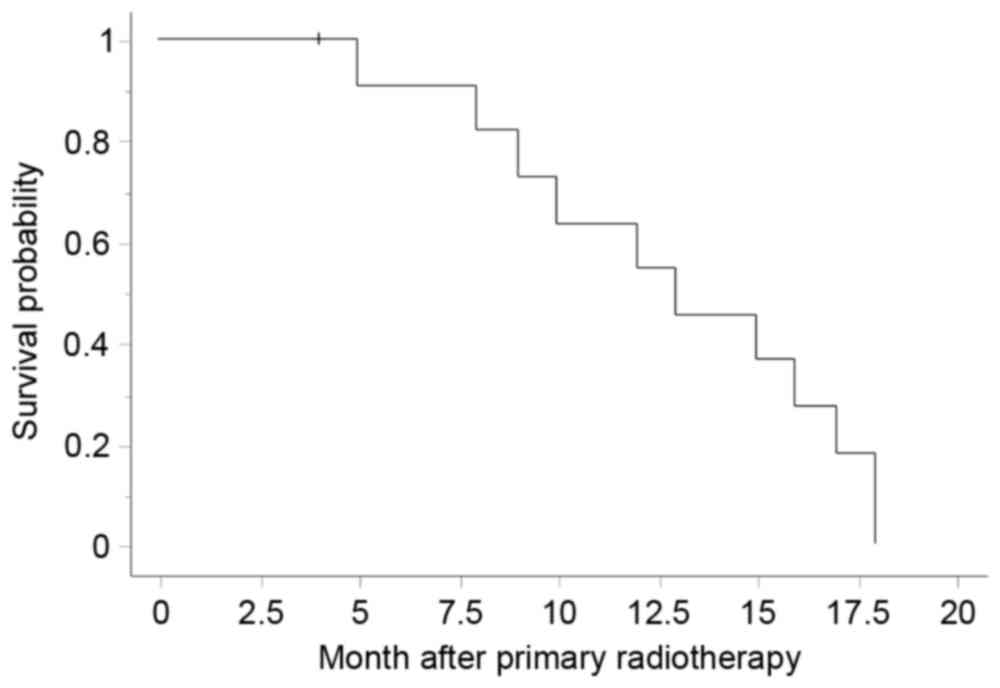
The median overall survival period of all patients
was 12 months and the 1-year survival rate was 55% (Fig. 1); the corresponding values were 10
months and 50% in the 5-FU CR group, 12 months and 57% in the GEM
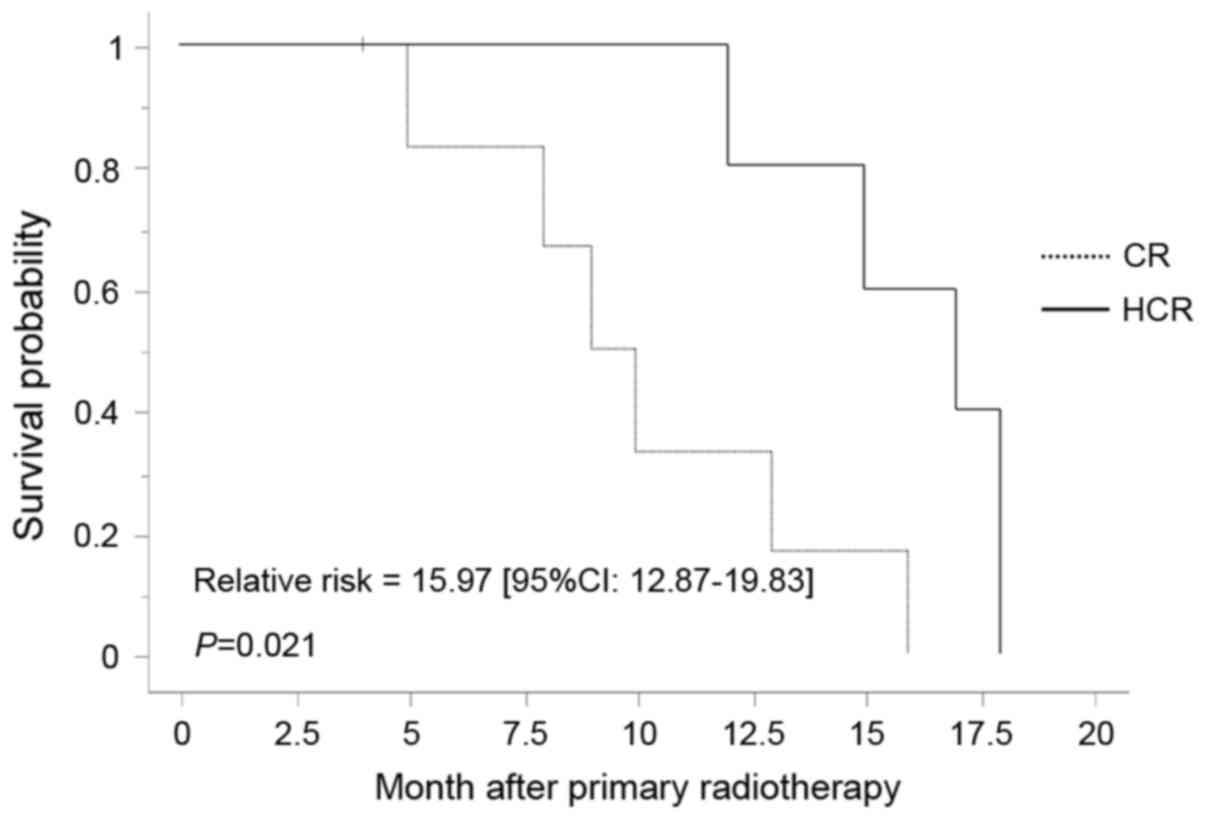
CR group, and 15 months and 80% in the HCR group. Univariate
analysis for factors associated with outcomes revealed a
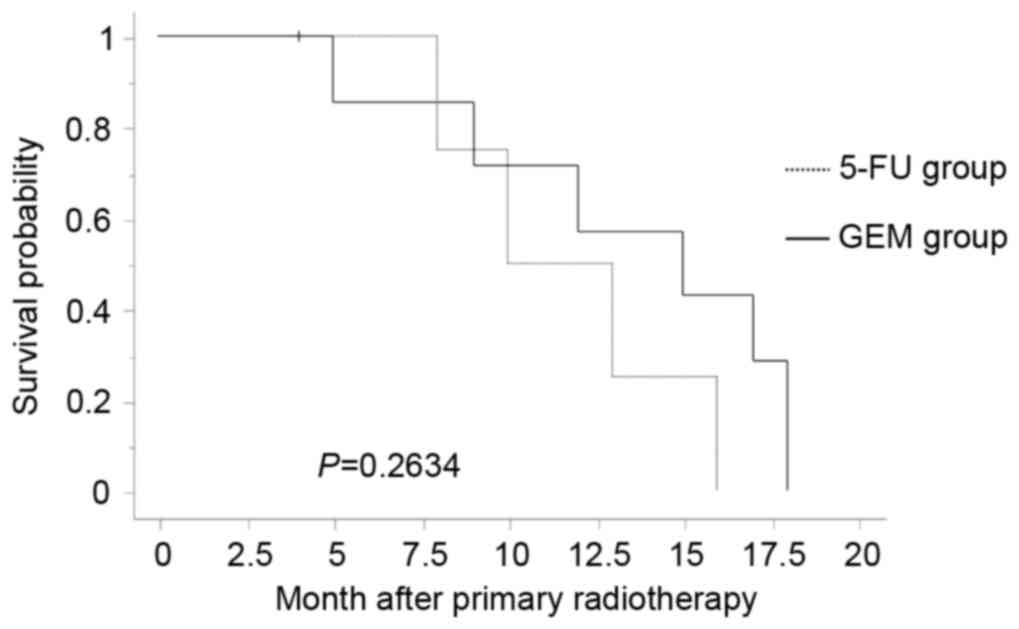
significant difference favoring the HCR group (P=0.021) (Fig. 2) and no significant difference in the
GEM or the 5-FU CR group (P=0.263) (Fig.
3).
Adverse events
Ten patients (10/13, 77%) developed gastroenteritis
(acute adverse events ≥ Grade 2) including five (5/13, 38%) with
Grade 3 gastroenteritis. Of these five patients, three developed
enteritis, necessitating hospitalization for medical therapy, and
two developed duodenal ulcers (corresponding to Grade 2 severity)
requiring medical therapy. Thus, there were no occurrences of
adverse events ≥ Grade 4. In the HCR group, two patients (2/5, 40%)
developed Grade 3 gastroenteritis; one each with enteritis and
duodenal ulcers.
Hematotoxicity ≥ Grade 2 occurred in nine patients
(9/13, 69%) including three with Grade 3 and one (in the 5-FU CR
group) with Grade 4 hematotoxicity. All five patients in the HCR
group developed hematotoxicity ≥ Grade 2: Grade 2 in three patients
and Grade 3 in two. No serious late adverse events were noted.
Discussion
Surgery is the only treatment by which complete cure
of pancreatic cancer can be achieved. However, according to data
from the pancreatic cancer registry in Japan, early-stage
resectable pancreatic cancer accounts for only 10% of all cases
(1). As for lymphadenectomy, there is
thought to be no significant difference in outcomes between radical
and standard lymphadenectomy (6,7). Since
surgery is the only radical therapy for pancreatic cancer,
increasing the percentage of pancreatic cancer cases diagnosed at a
stage when tumors are still resectable is essential. In the NCCN
guidelines (http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#pancreatic),
pancreatic cancer is classified into 3 types: Resectable,
unresectable, and borderline resectable. Surgical treatment of
borderline resectable pancreatic cancer after neoadjuvant
chemotherapy is a promising approach.
As for chemotherapy, marked advances in
postoperative adjuvant chemotherapy have improved the outcomes of
cancer patients (8), and
postoperative treatment strategies are an area of ongoing research
with promising new developments. However, for patients with
unresectable pancreatic cancer, satisfactory prognostic improvement
has not yet been achieved, despite advances obtained with the use
of GEM, which was approved in Japan in 2001. In recent years,
therapies with FOLFIRINOX (9) and
with the combination of GEM plus Abraxane have improved the
outcomes of these patients (10).
However, in Japan, these therapies appear to be associated with
higher incidences of adverse events (11,12).
It has long been accepted that radiotherapy in
combination with 5-FU chemotherapy (5-FU CR) is the standard
treatment regimen for unresectable pancreatic cancer since greater
improvement of outcomes was noted in patients given CR as compared
to those receiving radiotherapy alone (13). A comparative study of GEM CR vs. GEM
chemotherapy (14) showed that the
former had significantly better outcomes, though the incidence of
adverse events was high. In contrast, a clinical study comparing
concurrent FP (5-FU plus CDDP) CR vs. GEM chemotherapy (15) found that the latter showed a
significant difference in outcomes with a low incidence of adverse
events. Another study demonstrated concurrent TS-1 (tegafur,
gimeracil, and oteracil potassium) CR to be useful (16). Incidences of adverse events are
relatively high with CR and there was no significant difference in
outcomes between chemotherapy alone and that with concurrent CR.
Accordingly, at our hospital, we tend to use chemotherapy alone for
pancreatic cancer.
In a previous clinical study of 5-FU CR, the median
survival was 11 months and the 1-year survival rate was
approximately 30%. These results showed concurrent 5-FU CR to yield
better outcomes than radiotherapy alone, leading to the
establishment of 5-FU CR as a first-line treatment (11). After the advent of GEM, Crane et
al (17). Reported that GEM CR
achieved a median survival of 11 months and a 1-year survival rate
of 42%, while Gillen et al (2). Reported that GEM CR achieved a median
survival of 14.5 months.
At our institution, the median survival period is 15
months and the 1-year survival rate is 80% with concurrent GEM HCR;
these outcomes are better than those in previous studies of CR.
Comparison of concurrent GEM vs. 5-FU CR revealed no difference in
outcomes. Pancreatic cancer is generally a hypovascular tumor and
is thus more difficult to visualize with tumor staining than other
carcinomas. One of the reasons for prolonged survival in our
patients given concurrent HCR is considered to be the larger doses
of drugs delivered to tumor sites. This is because concurrent HCR
increases the blood supply to tumors. In GEM chemotherapy, nuclear
factor kappa B (NF-κB), a transcription factor, is activated
through immunological mechanisms, and this is regarded as one of
the causes of GEM resistance development. Heat shock protein
induced by concurrent HCR reportedly exerts inhibitory actions on
NF-κB activity (18,19), which is regarded as being a mechanism
underlying the antitumor effect of HCR. Accordingly, for patients
whose performance status is satisfactory, hyperthermia is
considered to contribute to prolonged survival regardless of
whether or not HCR, concurrent hyperthermia or maintenance GEM
chemotherapy is also administered.
HCR is also considered to be useful in patients who
have developed drug resistance after receiving chemotherapy alone.
However, patients who agree to receive HCR generally have a
positive attitude toward treatment, as well as a satisfactory
performance status, and this may bias outcomes. We plan to collect
more data on patients given HCR in future studies.
Although the sample size was small, there appeared
to be no difference in the incidence of adverse events between CR
and HCR. The safety of HCR in our study is thus considered to be
similar to that in previous studies (20).
However, there are several issues and challenges,
which must be addressed, before widespread adoption of hyperthermia
treatment. First, the procedure is time-consuming as it involves
application site pain despite cooling of the skin surface, and
there is a risk of heat transfer to stents placed in patients with
bile duct or pancreatic tumors. No specific methods for reducing
this discomfort have yet been devised. As for the latter problem,
the informed consent procedure for hyperthermia treatment is
carefully carried out at our hospital, with detailed explanations,
and patients thus undergo hyperthermia treatment safely. To date,
there have been no problems associated with the metallic stents.
Emaciation is widely recognized as occurring in patients with
pancreatic cancer; hyperthermia treatment is considered to be
suitable for emaciated patients.
Given the difficulty at present in achieving marked
improvement of outcomes in unresectable pancreatic cancer, measures
against the development of adverse events and drug resistance
during chemotherapy are considered to be essential. Such measures
can be applied with the use of hyperthermia; if the search for
application sites for hyperthermia is successful, this treatment
can provide benefits for cancer patients whose performance status
is satisfactory (21).
Concurrent HCR is reportedly effective for various
carcinomas as well as for pancreatic cancer. A significant
difference in outcomes was noted in patients with cervix carcinoma
who received hyperthermia treatment in addition to
Chemoradiotherapy (20). Another
study showed hyperthermia application to be useful for rectal
cancer as preoperative therapy (22).
These reports provide evidence of hyperthermia being a useful
treatment for various carcinomas.
Molecular targeted drugs are expected to achieve
improved outcomes in patients with locally advanced unresectable
pancreatic cancer whose performance status is satisfactory, while
modified or advanced hyperthermia techniques are also expected to
contribute to improving the outcomes of patients with various
carcinomas.
In conclusions, we studied multimodality therapy for
pancreatic cancer in a small patient sample, confirming the
efficacy and safety of concurrent hyperthermia and
chemoradiotherapy. Thus, hyperthermia treatment merits active
recommendation to pancreatic cancer patients who show a positive
attitude toward treatment and whose performance status is
satisfactory.
All clinical procedures were performed in accordance
with the ethical standards of the institutional and/or national
research committee and with the 1964 Helsinki declaration and its
later amendments or comparable ethical standards.
Acknowledgements
The authors thank Bierta Barfod for her contribution
to the language editing of this manuscript.
References
|
1
|
Egawa S, Toma H, Ohigashi H, Okusaka T,
Nakao A, Hatori T, Maguchi H, Yanagisawa A and Tanaka M: Japan
pancreatic cancer registry; 30th year anniversary: Japan pancreas
society. Pancreas. 41:985–992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gillen S, Schuster T, Zum Büschenfelde C
Meyer, Friess H and Kleeff J: Preoperative/neoadjuvant therapy in
pancreatic cancer: A systematic review and meta-analysis of
response and resection percentages. PLoS Med. 7:e10002672010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hori M, Matsuda T, Shibata A, Katanoda K,
Sobue T and Nishimoto H; Japan Cancer Surveillance Research Group,
: Cancer incidence and incidence rates in Japan in 2009: A study of
32 population-based cancer registries for the Monitoring of Cancer
Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 45:884–891.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li CP, Chao Y, Chi KH, Chan WK, Teng HC,
Lee RC, Chang FY, Lee SD and Yen SH: Concurrent chemoradiotherapy
treatment of locally advanced pancreatic cancer: Gemcitabine versus
5-fluorouracil, a randomized controlled study. Int J Radiat Oncol
Biol Phys. 57:98–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA,
Campbell KA, Sauter PK, Coleman J, Abrams RA and Hruban RH:
Pancreaticoduodenectomy with or without distal gastrectomy and
extended retroperitoneal lymphadenectomy for periampullary
adenocarcinoma, part 2: Randomized controlled trial evaluating
survival, morbidity, and mortality. Ann Surg. 236:355–668. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Riall TS, Cameron JL, Lillemoe KD,
Campbell KA, Sauter PK, Coleman J, Abrams RA, Laheru D, Hruban RH
and Yeo CJ: Pancreaticoduodenectomy with or without distal
gastrectomy and extended retroperitoneal lymphadenectomy for
periampullary adenocarcinoma-part 3: Update on 5-year survival. J
Gastrointest Surg. 9:1191–1206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maeda A, Boku N, Fukutomi A, Kondo S,
Kinoshita T, Nagino M and Uesaka K: Randomized phase III trial of
adjuvant chemotherapy with gemcitabine versus S-1 in patients with
resected pancreatic cancer: Japan adjuvant study group of
pancreatic cancer (JASPAC-01). Jpn J Clin Oncol. 38:227–229. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghorani E, Wong HH, Hewitt C, Calder J,
Corrie P and Basu B: Safety and efficacy of modified FOLFIRINOX for
advanced pancreatic adenocarcinoma: A UK Single-Centre experience.
Oncology. 89:281–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takeda Y, Katsura Y, Ohmura Y, Morimoto Y,
Ishida T, Motoyama Y, Ohneda Y, Sato Y, Kuwahara R, Murakami K, et
al: FOLFIRINOX combination chemotherapy in patients with metastatic
or recurrent pancreatic Cancer-A single institution experience. Gan
To Kagaku Ryoho. 42:2360–2363. 2015.(In Japanese). PubMed/NCBI
|
|
12
|
Ueno H, Ikeda M, Ueno M, Mizuno N, Ioka T,
Omuro Y, Nakajima TE and Furuse J: Phase I/II study of
nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese
patients with metastatic pancreatic cancer. Cancer Chemother
Pharmacol. 77:595–603. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moertel CG, Frytak S, Hahn RG, O'Connell
MJ, Reitemeier RJ, Rubin J, Schutt AJ, Weiland LH, Childs DS,
Holbrook MA, et al: Therapy of locally unresectable pancreatic
carcinoma: A randomized comparison of high dose (6000 rads)
radiation alone, moderate dose radiation (4000 rads +
5-fluorouracil), and high dose radiation + 5-fluorouracil: The
gastrointestinal tumor study group. Cancer. 48:1705–1710. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Loehrer PJ Sr, Feng Y, Cardenes H, Wagner
L, Brell JM, Cella D, Flynn P, Ramanathan RK, Crane CH, Alberts SR
and Benson AB III: Gemcitabine alone versus gemcitabine plus
radiotherapy in patients with locally advanced pancreatic cancer:
An eastern cooperative oncology group trial. J Clin Oncol.
29:4105–4112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chauffert B, Mornex F, Bonnetain F,
Rougier P, Mariette C, Bouché O, Bosset JF, Aparicio T, Mineur L,
Azzedine A, et al: Phase III trial comparing intensive induction
chemoradiotherapy (60 Gy, infusional 5-FU and intermittent
cisplatin) followed by maintenance gemcitabine with gemcitabine
alone for locally advanced unresectable pancreatic cancer.
Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol.
19:1592–1599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ueno H, Ioka T, Ikeda M, Ohkawa S,
Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et
al: Randomized phase III study of gemcitabine plus S-1, S-1 alone,
or gemcitabine alone in patients with locally advanced and
metastatic pancreatic cancer in Japan and Taiwan: GEST study. J
Clin Oncol. 31:1640–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crane CH, Abbruzzese JL, Evans DB, Wolff
RA, Ballo MT, Delclos M, Milas L, Mason K, Charnsangavej C, Pisters
PW, et al: Is the therapeutic index better with gemcitabine-based
chemoradiation than with 5-fluorouracil-based chemoradiation in
locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys.
52:1293–1302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adachi S, Kokura S, Okayama T, Ishikawa T,
Takagi T, Handa O, Naito Y and Yoshikawa T: Effect of hyperthermia
combined with gemcitabine on apoptotic cell death in cultured human
pancreatic cancer cell lines. Int J Hyperthermia. 25:210–219. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishikawa T, Kokura S, Sakamoto N, Ando T,
Imamoto E, Hattori T, Oyamada H, Yoshinami N, Sakamoto M, Kitagawa
K, et al: Phase II trial of combined regional hyperthermia and
gemcitabine for locally advanced or metastatic pancreatic cancer.
Int J Hyperthermia. 28:597–604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lutgens LC, Koper PC, Jobsen JJ, van der
Steen-Banasik EM, Creutzberg CL, van den Berg HA, Ottevanger PB,
van Rhoon GC, van Doorn HC, Houben R and van der Zee J: Radiation
therapy combined with hyperthermia versus cisplatin for locally
advanced cervical cancer: Results of the randomized RADCHOC trial.
Radiother Oncol. 120:378–382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tschoep-Lechner KE, Milani V, Berger F,
Dieterle N, Abdel-Rahman S, Salat C and Issels RD: Gemcitabine and
cisplatin combined with regional hyperthermia as second-line
treatment in patients with gemcitabine-refractory advanced
pancreatic cancer. Int J Hyperthermia. 29:8–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gani C, Schroeder C, Heinrich V, Spillner
P, Lamprecht U, Berger B and Zips D: Long-term local control and
survival after preoperative radiochemotherapy in combination with
deep regional hyperthermia in locally advanced rectal cancer. Int J
Hyperthermia. 32:187–192. 2016. View Article : Google Scholar : PubMed/NCBI
|