Introduction
Pseudomyxoma peritonei (PMP) is a rare disease
caused by primary mucinous tumors that arise from different sites,
usually from the appendix or ovary (1). It is estimated to occur in 1 to 2
individuals per million (2). PMP is a
fatal clinical syndrome that may be associated with pelvic/ovarian
masses and abundant mucinous ascites, caused by the rupture or
leakage of a mucinous neoplasm within the abdomen (3). If untreated, patients frequently succumb
to malnutrition due to the compression and blockage of the
digestive tract by accumulating intraperitoneal mucin; however,
significant invasive surgical procedures to treat PMP are
associated with numerous complications and morbidities (3). Usually a diagnosis is produced with
ultrasonography, computed tomography (CT), magnetic resonance
imaging (MRI) and immunohistochemical markers.
The coexistence of mucinous appendiceal and ovarian
tumors is commonly exhibited in PMP; however, the origin of these
tumors in such patients is the subject of considerable debate; a
mucinous ovarian tumor, in the presence of PMP, is likely to be of
appendiceal origin (4). The
identification of two truly independent primary mucinous tumors
involving the appendix and ovary associated with PMP is uncommon.
Sites of origin may be determined based on clinical, pathological
and immunohistochemical features. The current study presents a rare
case of PMP caused by synchronous primary mucinous tumors of the
ovary and appendix in a 73-year-old female.
Case report
A 73-year-old female was referred to the Department
of Gynecology of the Pusan National University Hospital on 28 July
2015 for the evaluation of a pelvic mass, and presented with
progressively worsening indigestion and abdominal distension.
Ultrasound examination of the pelvis and abdomen revealed a large
multiseptated cystic mass on the right ovary with a large amount of
peritoneal fluid in the upper abdomen. Expression levels of the
tumor markers cancer antigen (CA) 125 and human epididymis protein
4 were elevated to 51.79 U/ml and 98.07 ng/ml, respectively, and
the risk of ovarian malignancy algorithm was ~39.38%, suggestive of
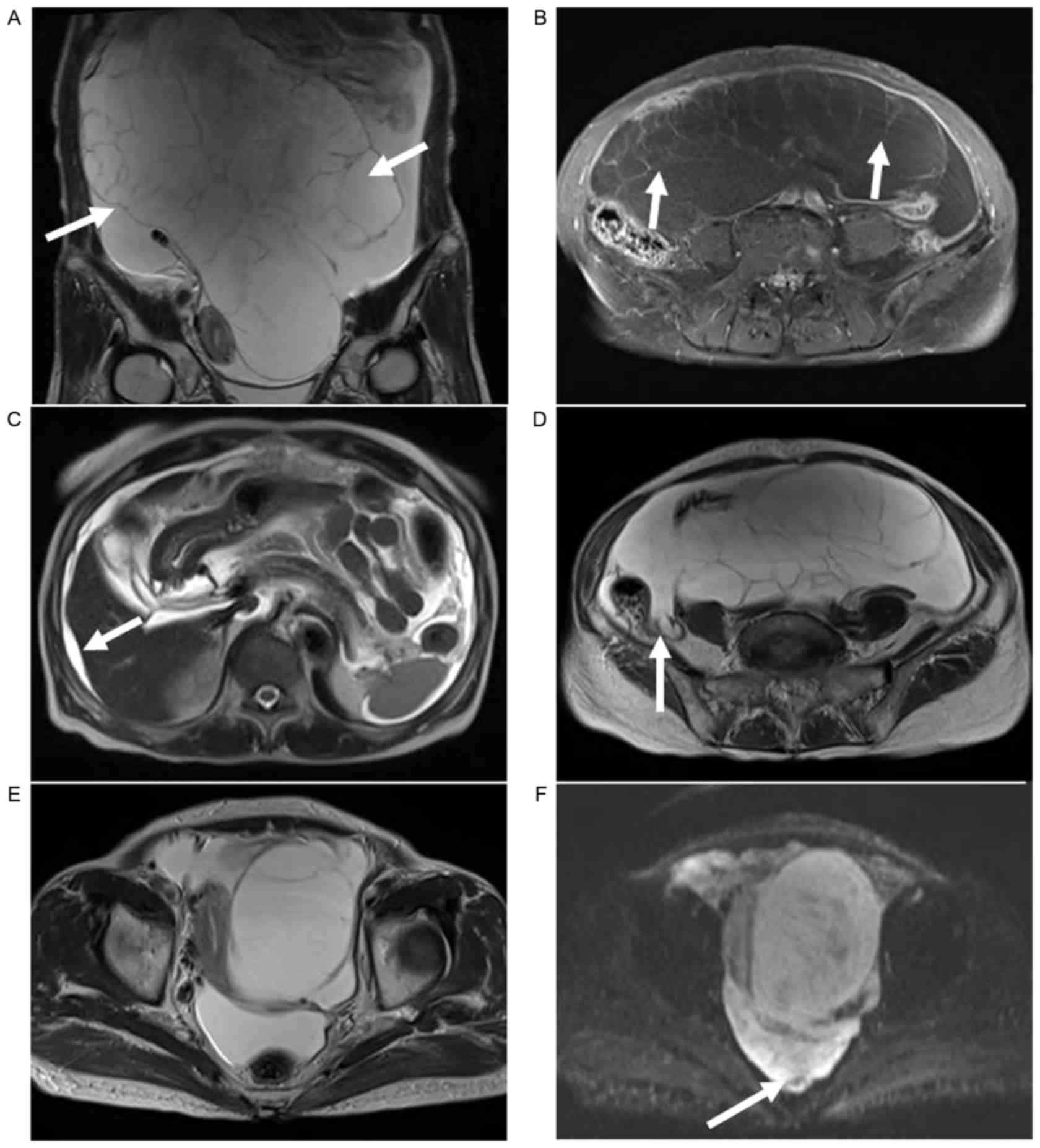
malignancy. MRI was performed to characterize the pelvic mass, and
a T2-weighted MRI revealed a large multilocular cystic mass,
measuring 21.8 cm. No mural nodules or enhancing solid components
were observed within the mass; however, ascites and mild scalloping
of the liver surface were evident. Diffusion-weighted images (DWI)
with a b-value of 400 s/mm2 demonstrated hypointense
septa in the fluid collection at the cul-de-sac (Fig. 1). The presumed preoperative diagnosis
was PMP associated with an ovarian mucinous malignancy.
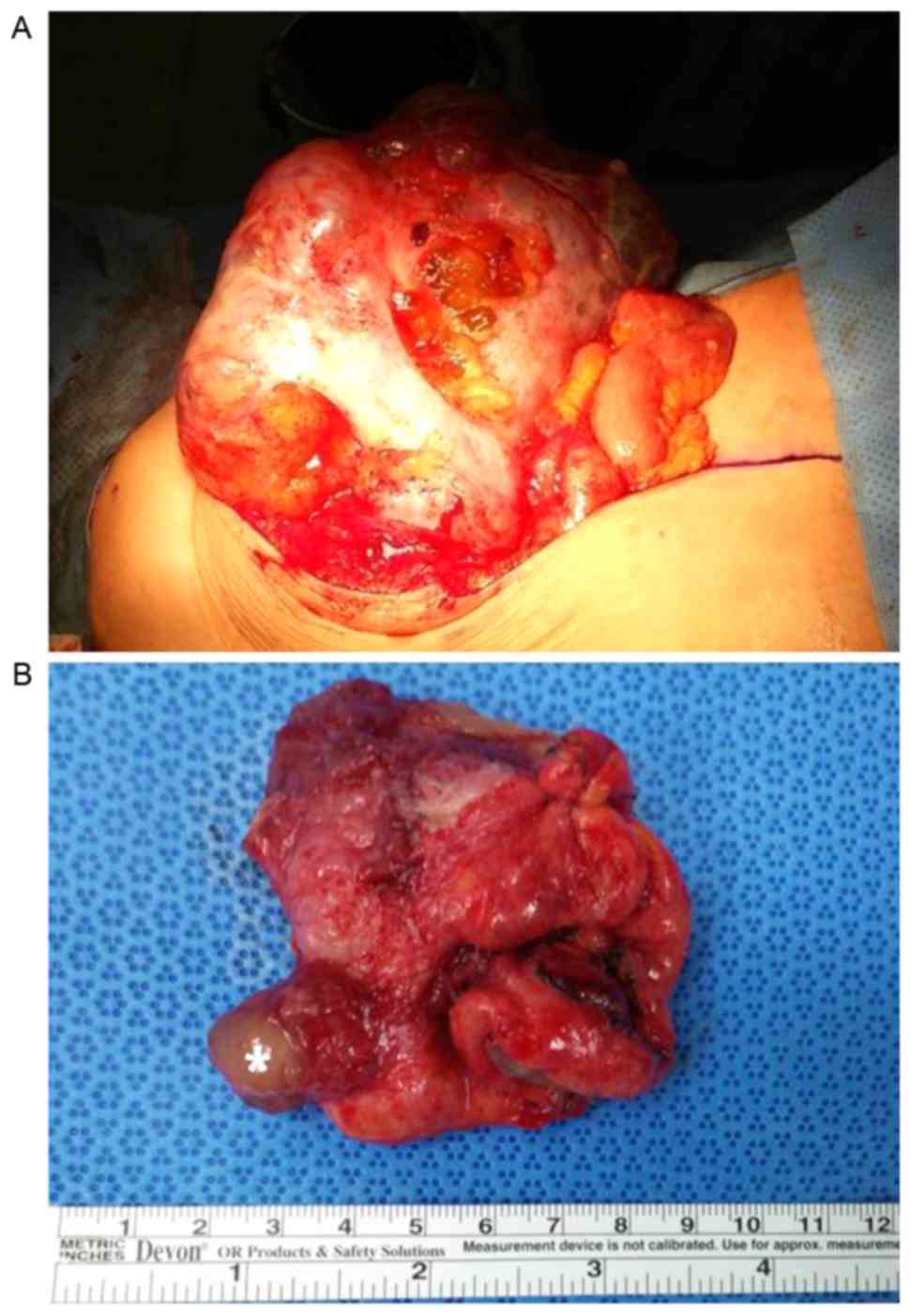
Laparotomy results demonstrated the presence of a
large, well-circumscribed and ruptured cyst arising from the right
ovary, a normal uterus and left ovary and considerable gelatinous
mucinous peritoneal effusion occupying the whole pelvis and
abdomen. The appendix was identified to be partially amputed
following a previous rupture, distended and filled with mucin
(Fig. 2). Bilateral
salpingo-oophorectomy, hysterectomy, ileocecectomy, omentectomy,
excisions of multifocal peritoneal mucinous implants and peritoneal
lavage were performed on 4 August 2015.
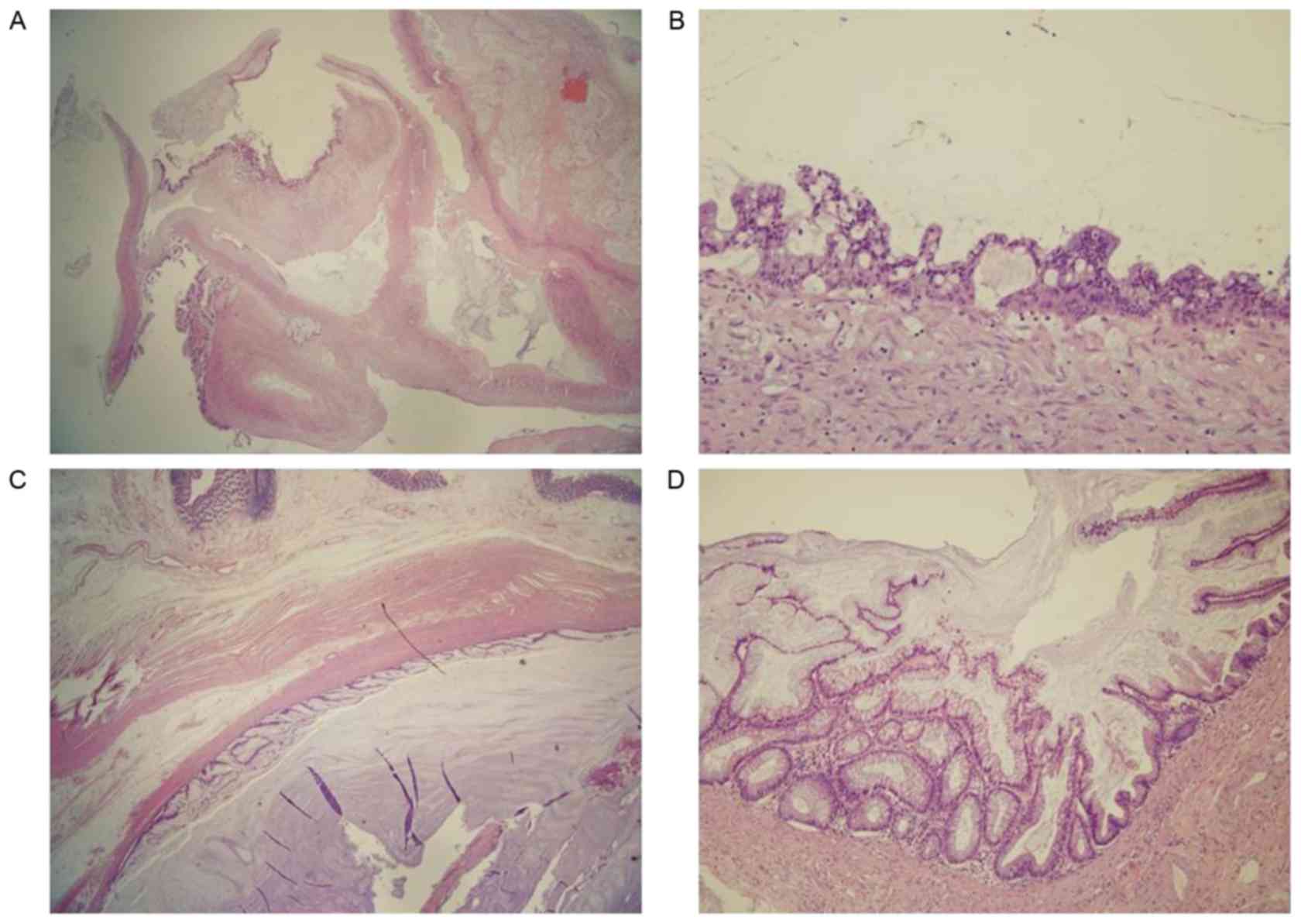
Microscopically, the ovarian tumor was a multicystic
lesion with mucinous epithelium and mucin pools in the wall. The
lining of the mucinous epithelium exhibited nuclear enlargement,
stratification and a complex architecture. The appendiceal mucinous
tumor contained a large mucin pool and the lining epithelium
exhibited low-grade dysplasia, but did not demonstrate infiltrative
growth. The final pathological diagnosis was a borderline mucinous
tumor of the right ovary and a low-grade appendiceal mucinous
neoplasm associated with PMP (Fig.
3).
Surgical specimens were submitted for
immunohistochemical examinations to distinguish the origin of the
tumor. Immunohistochemistry was performed on serial 4-µm thick
paraffin sections. The paraffin sections were deparaffinized in
xylene and rehydrated with a descending ethanol series. Bond
Epitope Retrieval Solution 1 (pH 6; Leica Microsystems GmbH,
Wetzlar, Germany) was used for antigen retrieval for 20 min at
100°C. Monoclonal CK7 (dilution, 1:100; cat. no., NCL-L-CK7-OVTL),
CK20 (dilution, 1:100; cat. no., NCL-L-CK20-561; both from
Novocastra; Leica Microsystems GmbH) and CDX2 antibodies (dilution
1:250; cat. no., 235R-15; Sigma Alrich; Merck KGaA) were applied on
the slides. Immunohistochemical staining was performed with a Leica
Bond-MAX™ autostainer and a Peroxidase/DAB
Bond™ Polymer Refine Detection system was used for
visualization (both Leica Microsystems GmbH). Slides were incubated
with a peroxide block for 5 min, the primary antibody for 15 min, a
post-primary reagent for 8 min and the polymer for 8 min, at room
temperature. Cells were considered positive for CK7 and CK20 when
cytoplasm was stained, and positive for CDX2 when nuclear staining
was observed, using a light microscope (BX53, Olympus Corporation,
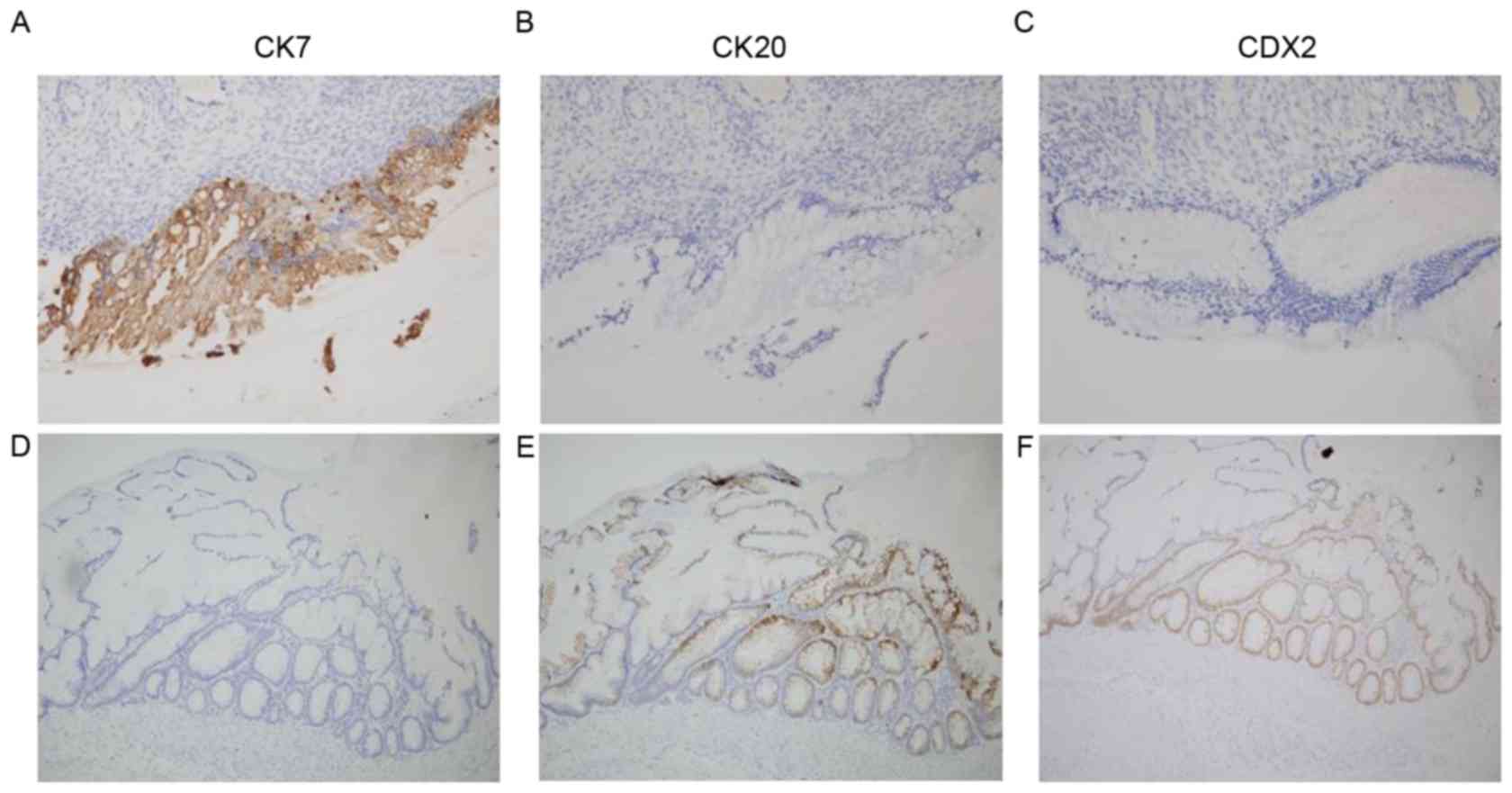
Tokyo, Japan). The ovarian tissue sample stained strongly positive
for cytokeratin (CK)-7 and negative for CK-20 and homeobox protein
CDX-2 (CDX2), whereas the appendiceal tumor stained negative for
CK-7 and positive for CK-20 and CDX2 (Fig. 4).
The patient's postoperative course was uneventful
and she was discharged in a stable condition. Follow up was
performed using CT imaging and serial measurement of serum
carcinoembryonic antigen (CEA) and CA19-9 tumor markers. The CEA
and CA19-9 levels were determined using radioimmunoassay kits
manufactured from Abbott Laboratories (Chicago, IL, USA). The CEA
and CA19-9 levels of the patient remained within normal levels and
no recurrence was observed during the first 14 months subsequent to
surgery.
Discussion
Pseudomyxoma peritonei is almost always associated
with a mucinous neoplasm of the appendix (5), and is also commonly associated with an
ovarian mucinous tumor. PMP exhibits well-established clinical
characteristics, including a protracted clinical course, multiple
recurrences and progressive fibrous adhesions leading to fatal
intestinal obstruction. Previously, aggressive cytoreductive
surgery (CRS) and hyperthermic intra-peritoneal chemotherapy
(HIPEC) have been suggested to improve clinical outcomes and
survival (6).
Considerable controversy surrounds the origin of
this disease; the coexistence of mucinous tumors of the ovary and
appendix associated with PMP is commonly encountered (4). Globally, ~1/3 to 1/2 of women with PMP
exhibit concurrent ovarian and appendiceal mucinous tumors
(7). Guo et al reported that
concurrent ovarian and appendiceal lesions occurred in 20/35 cases
(7). However, the origin of the tumor
in cases of synchronicity is widely debated (8). Clinicopathological and
immunohistochemical data suggest that synchronous mucinous tumors
of the ovary and appendix in women with PMP are derived from the
appendix (9–11). Patients with ovarian and appendiceal
tumors of independent origins have been identified (4). However, the presence of two truly
independent primary mucinous tumors involving the appendix and
ovary associated with PMP is uncommon.
It is now understood that primary mucinous tumors
are much less common than previously considered, and that
metastatic mucinous tumors from other organs are common (12). Several criteria help to distinguish
primary ovarian mucinous tumors from metastatic mucinous tumors: A
large size (>10 cm), unilaterality, the presence of benign or
borderline areas, an expansile pattern of invasion, a smooth
surface and the absence of extra-ovarian disease, a low grade and
low stage at presentation and less aggressive behavior all indicate
a primary ovarian neoplasm, whereas bilateral ovarian involvement,
smaller size, ovarian surface involvement, multiple nodules and an
infiltrative pattern of stromal invasion favor an extra-ovarian
origin (11–14). In the case of the present study, the
ovarian tumor was a large, unilateral and low-grade tumor, which is
consistent with a primary mucinous tumor of the ovary.
Previous evidence based on pathological, molecular
and immunohistochemical features suggest that an appendiceal origin
is the primary etiology of PMP in the majority of cases (15). Confirmation of a pathologically normal
appendix is essential for determining an extra-appendiceal origin,
and certain immunohistochemical markers, CK7, CK20 and CDX-2, may
assist in determining the origin of an ovarian tumor (16). CK7+/CK20-CDX2- and CK7-/CK20+CDX2+
patterns are typical of epithelial ovarian and intestinal tumors,
respectively (6). For the patient in
this case study, the presence of these immunohistochemical patterns
indicated malignant processes of independent origins.
Imaging studies, principally CT and MRI, aid the
diagnosis of PMP, which typically exhibits low or proteinaceous
attenuation ascites, scalloping of the liver, and splenic margins
and peritoneal implants that may cause extrinsic pressure on bowel
loops. Additionally, amorphous and curvilinear calcifications are
occasionally present in peritoneal implants (5). In particular, scalloping of the hepatic
and splenic margins is diagnostic (5). In the case of the present study, a 21-cm
large multiseptated cystic mass was encountered in the
pelvic/abdominal cavity with ascites and visceral scalloping of the
liver surface. In all cases of PMP, the appendix must be closely
observed for mucocele in cross-sectional images. The typical
imaging results of appendiceal mucocele are a cystic,
well-encapsulated mass, sometimes with mural calcification, in the
expected location (17). However, in
the present case study, appendiceal mucocele was missed when
interpreting preoperative MR images. In a number of cases, it may
be difficult to identify the originating appendiceal mucocele using
imaging, as the residual appendix may be small or fibrosed
subsequent to rupture (18). It must
also be understood that the ovaries require careful evaluation in
female patients with PMP.
In a recent study, it was suggested that DWI with
low b-values may aid the diagnosis of PMP by providing a clear
visualization of septa in the intra-abdominal fluid (19). In the present case, DWI with a b-value
of 400 s/mm2 facilitated the visualization of
hypointense septa in the pelvic fluid, whereas T2-weighted images
did not. This ability of DWI to visualize septa may be due to the
reduced signal intensity of highly mobile water molecules at low
b-values.
The diagnosis in the case of the present study was
predicted subsequent to the preoperative MRI examination and was
confirmed postoperatively, whereas the site of origin was
determined immunohistochemically.
CRS combined with HIPEC has previously resulted in
encouraging outcomes (6). The patient
in the present study was treated with CRS, which consisted of
bilateral salpingo-oophorectomy, hysterectomy, ileocecectomy,
omentectomy, excision of multifocal peritoneal mucinous implants
and peritoneal lavage. The site of origin does not affect the
natural history of PMP, as appendiceal and extra-appendiceal
neoplasms share prognoses and clinicopathological features
(20).
In conclusion, based on its clinicopathological and
immunohistochemical features, the case of the present study
represents a PMP resulting from two separate malignant processes of
primary mucinous tumors of the ovary and appendix. Therefore,
radiologists and clinicians must be aware of the rare occurrence of
synchronous mucinous tumors of the ovary and the appendix.
Acknowledgements
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no., 2013R1A1A4A01
010141).
References
|
1
|
Chira RI, Nistor-Ciurba CC, Mociran A and
Mircea PA: Appendicular mucinous adenocarcinoma associated with
pseudomyxoma peritonei, a rare and difficult imaging diagnosis. Med
Ultrason. 18:257–259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moran BJ and Cecil TD: The etiology,
clinical presentation, and management of pseudomyxoma peritonei.
Surg Oncol Clin N Am. 12:585–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pillai K, Akhter J, Mekkawy A, Chua TC and
Morris DL: Physical and chemical characteristics of mucin secreted
by pseudomyxoma peritonei (PMP). Int J Med Sci. 14:18–28. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chuaqui RF, Zhuang Z, Emmert-Buck MR,
Bryant BR, Nogales F, Tavassoli FA and Merino MJ: Genetic analysis
of synchronous mucinous tumors of the ovary and appendix. Hum
Pathol. 27:165–171. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zissin R, Gayer G, Fishman A, Edelstein E
and Shapiro-Feinberg M: Synchronous mucinous tumors of the ovary
and the appendix associated with pseudomyxoma peritonei: CT
findings. Abdom Imaging. 25:311–316. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baratti D, Kusamura S, Nonaka D, Cabras
AD, Laterza B and Deraco M: Pseudomyxoma peritonei: Biological
features are the dominant prognostic determinants after complete
cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann
Surg. 249:243–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo AT, Song X, Wei LX and Zhao P:
Histological origin of pseudomyxoma peritonei in Chinese women:
Clinicopathology and immunohistochemistry. World J Gastroenterol.
17:3531–3537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guerrieri C, Frånlund B, Fristedt S,
Gillooley JF and Boeryd B: Mucinous tumors of the vermiform
appendix and ovary, and pseudomyxoma peritonei: Histogenetic
implications of cytokeratin 7 expression. Hum Pathol. 28:1039–1045.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szych C, Staebler A, Connolly DC, Wu R,
Cho KR and Ronnett BM: Molecular genetic evidence supporting the
clonality and appendiceal origin of Pseudomyxoma peritonei in
women. Am J Pathol. 154:1849–1855. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Young RH, Gilks CB and Scully RE: Mucinous
tumors of the appendix associated with mucinous tumors of the ovary
and pseudomyxoma peritonei. A clinicopathological analysis of 22
cases supporting an origin in the appendix. Am J Surg Pathol.
15:415–429. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hart WR: Mucinous tumors of the ovary: A
review. Int J Gynecol Pathol. 24:4–25. 2005.PubMed/NCBI
|
|
12
|
Rouzbahman M and Chetty R: Mucinous
tumours of appendix and ovary: An overview and evaluation of
current practice. J Clin Pathol. 67:193–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yemelyanova AV, Vang R, Judson K, Wu LS
and Ronnett BM: Distinction of primary and metastatic mucinous
tumors involving the ovary: Analysis of size and laterality data by
primary site with reevaluation of an algorithm for tumor
classification. Am J Surg Pathol. 32:128–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zaino RJ, Brady MF, Lele SM, Michael H,
Greer B and Bookman MA: Advanced stage mucinous adenocarcinoma of
the ovary is both rare and highly lethal: A Gynecologic Oncology
Group study. Cancer. 117:554–562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nonaka D, Kusamura S, Baratti D, Casali P,
Younan R and Deraco M: CDX-2 expression in pseudomyxoma peritonei:
A clinicopathological study of 42 cases. Histopathology.
49:381–387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou F, Chen X, Li Y and Huang L: Two
independent primary mucinous tumors involving the appendix and
ovary accompanied with acellular pseudomyxoma peritonei. Int J Clin
Exp Pathol. 8:11831–11834. 2015.PubMed/NCBI
|
|
17
|
Levy AD, Shaw JC and Sobin LH: Secondary
tumors and tumorlike lesions of the peritoneal cavity: Imaging
features with pathologic correlation. Radiographics. 29:347–373.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sugarbaker PH, Ronnett BM, Archer A,
Averbach AM, Bland R, Chang D, Dalton RR, Ettinghausen SE, Jacquet
P, Jelinek J, et al: Pseudomyxoma peritonei syndrome. Adv Surg.
30:233–280. 1996.PubMed/NCBI
|
|
19
|
Himoto Y, Kido A, Fujimoto K, Kawada K,
Kitai T, Sakai Y and Togashi K: A case of pseudomyxoma peritonei:
Visualization of septa using diffusion-weighted images with low b
values. Abdom Radiol (NY). 41:1713–1717. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baratti D, Kusamura S, Milione M,
Pietrantonio F, Caporale M, Guaglio M and Deraco M: Pseudomyxoma
peritonei of extra-appendiceal origin: A comparative study. Ann
Surg Oncol. 23:4222–4230. 2016. View Article : Google Scholar : PubMed/NCBI
|