Sinonasal inverted papilloma (SIP) is a benign tumor
which originates from the sinonasal Schneiderian mucosa and
accounts for 0.5 to 4% of all nasal and sinus neoplasm (1). Pathologically, SIP epithelium inverts
into submucosal stroma, which is distinguished from other types of
nasal papilloma. Unlike other benign tumors, SIP exhibits
remarkable aggressive behaviors, including invasiveness, recurrence
and malignant transformation (~10%) (2). Therefore, SIP can spread into the
sparanasal sinus, orbit, and cranial base, which can lead to poor
prognosis for SIP patients (2).
To date, the treatment for SIP includes surgery and
surgery combined with radiotherapy for SIP-associated squamous cell
carcinoma (SIP/SCC). Currently, the majority of surgeons prefer
endoscopic methods to traditional external approaches, due to
similar success rates, less trauma and no facial scars. However,
the common view is that SIP recurrence is due to inadequate removal
during the first surgery (2–4). Therefore, preoperative evaluation as
well as postoperative follow up is very important.
Understanding clinical risk factors is critical for
preventing the recurrence of SIP. Similar to other head and neck
tumors, smoking has been identified as a risk factor of SIP
recurrence in two previous studies (containing 132 and 162 SIP
patients, respectively) (5,6). Outdoor and industrial occupations may be
another potential environmental risk factor, particularly exposure
to organic solvents, including diethylnitrosamine (7–9). These
factors include smoking history, smoking amount, and occupation
environment (5–9). Recently, Nomura et al (10) found that the SIP-affected area was
significantly associated with the concave side of the septal
deviation. Considering that the high wall shear stress of
high-velocity airflow in this location, the study may suggest a
causative role of human papilloma virus (HPV) and chemicals in the
occurrence of sinonasal papilloma due to the traumatic effects
caused by airflow (10). However,
whether nasal septal construction should be performed following SIP
surgery remains to be determined.
In the majority of head and neck tumors, the
clinical stage is associated with recurrence and poor prognosis
(11). The clinical stage of SIP has
been defined using the Krouse staging system (12), the Furuta staging system (13), the Cannady staging system (14) and the Han staging system (15). The Krouse staging system is currently
the most widely used (16). While
certain authors have emphasized the role of SIP stage system on SIP
recurrence (17,18), it is not clear whether clinical stage
is associated with SIP recurrence. An association between Krouse
stage system and recurrence of SIP was not identified in a recent
study involving 156 SIP patients (19). In a multicenter study involving 578
SIP patients, three stage systems (the Krouse staging system, the
Furuta staging system and the Cannady staging system) did not
associate with SIP recurrence rates (2). This study also suggested that patients
with advanced stage of SIP who underwent single endoscopic surgery
presented a higher recurrence rate. Furthermore, the study also
found that SIP involving the frontal sinus or maxillary sinus was
associated with higher recurrence rates (2). Consistently, in 57 patients with SIP
based within the sphenoid sinus, a multi-institutional
retrospective study revealed that the attachment site of SIP over
the optic nerve and carotid artery correlated with a 14.6% rate of
recurrence (20). Our previous study
conducted by the present authors did not show the correlation
between Han staging systems and recurrence rates of SIP in 89 SIP
cases, but indicated that there is a statistically higher
recurrence rate (27.3%) in patients who underwent secondary surgery
(21). Consistent with this study, a
recent study reported a 50% SIP recurrence rate following secondary
surgery compared with a 12% rate following primary resection
(18). However, this study did not
classify the SIP as benign or SIP/SCC. Recently, two studies,
involving 87 and 32 SIP/SCC patients, suggested that advanced
American Joint Committee on Cancer (AJCC) stages and therapeutic
methods may be risk factors for poor prognosis (22,23). To
the best of our knowledge, these are the largest series of SIP/SCC
cases reported to date. Collectively, these studies proposed that
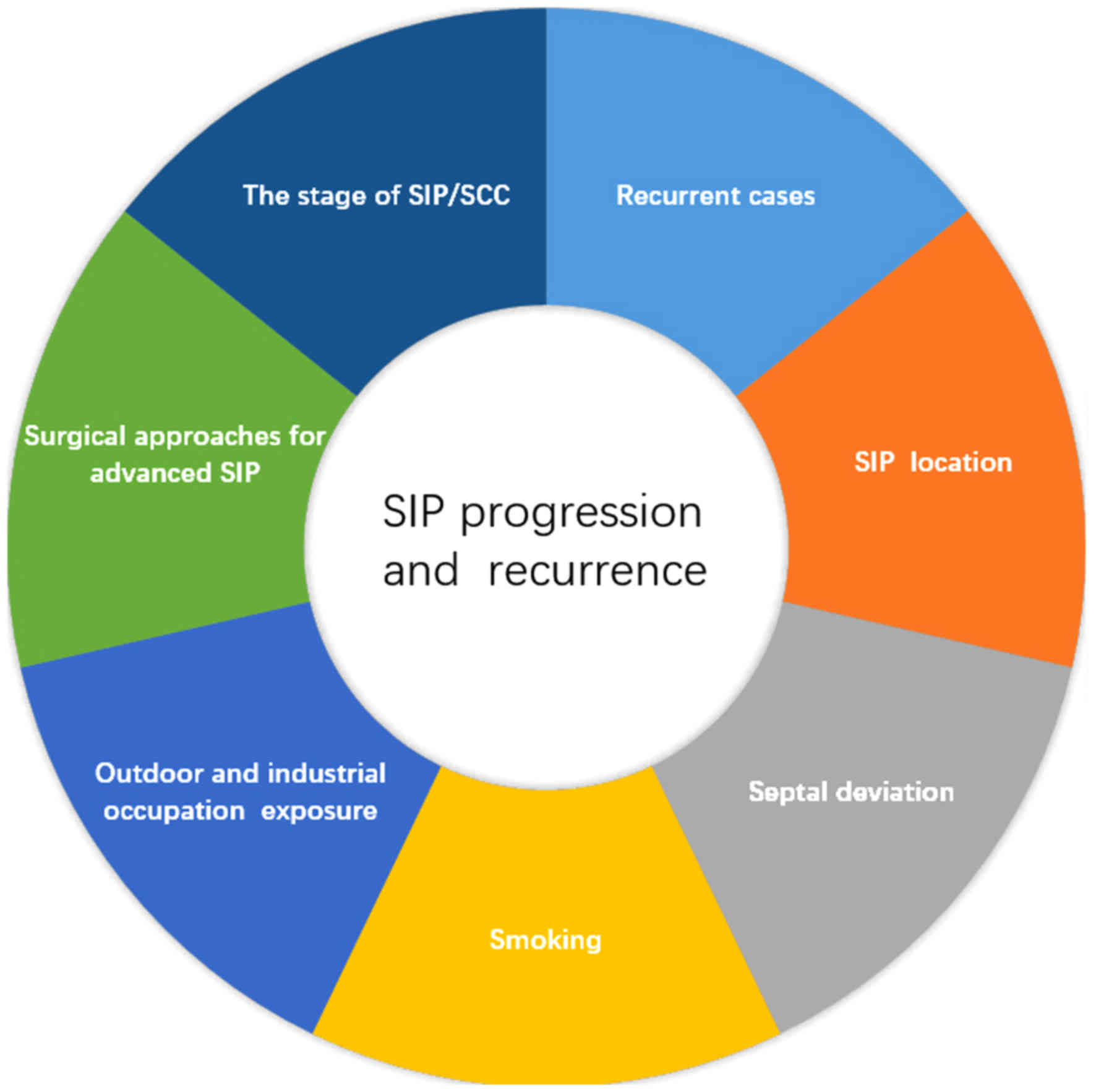
smoking, chemical exposure, septal deviation, SIP location,
secondary surgery and AJCC stage of SIP/SCC may be clinical risk
factors for progression and recurrence of SIP. Notably, the AJCC
stage of SIP/SCC may contribute to treatment selection (Fig. 1) (22,23).
The choice of surgical methods may be another
potential risk factor for SIP recurrence. Although endoscopic sinus
surgery (ESS) has been considered the treatment of choice for the
majority of SIP cases, surgical decisions should take into account
the extent, volume, and lesion location (24). A study of 212 SIP patients
demonstrated that SIP lesions with an extensive involvement of the
frontal sinus and/or supraorbital cell may require a combined
approach (25). In addition, the
Korean multicenter study suggested that surgeons should consider
combined approaches to reduce recurrence for advanced SIP [Krouse
staging system: T3 stage (12);
Furuta staging system: T3-A stage (13); Cannady staging system: group B
(14)], particularly for novice
surgeons (2). Although certain
authors propose that SIP involving attachment sites within the
maxillary sinus require a endoscopic-external combined technique
(1,26), emerging evidence suggests that novel
tailored ESS techniques (endoscopic modified medial maxillectomy,
and transnasal endoscopic anterior and medial maxillectomy) allow
enhanced visualization and preserve important structures, including
the inferior turbinate and nasolacrimal duct (27–29).
However, other authors proposed that the endoscopic-external
combined approach remains essential for recurrent maxillary SIP
(30). Therefore, a multicenter study
or large meta-analysis is required to determine the most
significant factors affecting progression and recurrence of
SIP.
In summary, the location of SIP, secondary surgery,
AJCC stage of SIP/SCC and the choice of surgical method for
advanced SIP directly contribute to incomplete or inadequate
removal of tumors. Therefore, incomplete or inadequate removal is a
direct cause of recurrence. SIP location, secondary surgery, AJCC
stage of SIP/SCC, and surgical approaches for advanced SIP are the
direct risk factors of SIP recurrence.
The management of risk factors of SIP recurrence
involves precise identification of the SIP attachment site,
anatomical anomalies in sinonasal regions, careful planning of
surgical procedures and a well-planned postoperative follow-up
(19). The use of computed tomography
(CT) and magnetic resonance imaging (MRI) is critical for
preoperative prediction of SIP attachment sites and differentiation
(31,32). Radical ablation of SIP attachment
sites is crucial for the first surgical resection. Therefore,
imaging is important in preoperative prediction of SIP attachment
sites.
Since the majority of recurrence is localized to the
same site as the primary tumor, the accurate preoperative
prediction of SIP attachment sites is crucial for the first
surgical resection (3). Notably, SIPs
with an origin in close proximity to vital structures, including
the optic nerve and carotid artery, may be associated with higher
rates of recurrence (20), which may
be a factor for consideration when choosing the surgical
approach.
Prior reports have suggested that focal osteitis
within the SIP tissue may be a predictor of SIP origin (31). While the mechanisms underlying the
origin of SIP-induced osteitis remains to be determined, certain
studies report that the SIP attachment site provides blood supply
to the large bulky tumor volume, which leads to
hypervascularization of the attachment site (31). Hypervascularization within the origin
site may cause bone growth (31),
driven by bone morphogenetic protein 4 expressed by SIP cells
(33) or cytokines released due to
inflammation (34). Bhalla et
al (34) found that the
predictive value of osteitis was 95% via CT scan, and similar
results were also reported by Yousuf et al (35). Lee et al (31) evaluated 55 lesions associated with
focal hyperostosis using CT images and revealed that the location
of hyperostosis coincided with the actual tumor attachment sites in
49 (89.1%) of all the lesions. Notably, Lee et al (31) suggested that areas of cone-shaped
hyperostosis matched with the SIP origin rather than plaque-like
hyperostosis.
Accurate tumor mapping is likely to be challenging
due to inadequate differentiation of the tumor from pathological
inflammation (36) and squamous cell
carcinoma (32). MRI may have an
advantage in differentiating soft tissue. MRI is able to identify
inflammation more clearly and is also able to identify tumor
margin, tumor extent (32) and
convoluted cerebriform pattern (CCP), which is considered a
valuable SIP characteristic (37).
Wang et al (38) have
demonstrated that there were significant differences between SIP
and malignancy in T2 homogeneity, CCP and other MRI parameters. The
authors concluded that non-enhanced and static combined with
dynamic contrast-enhanced MRI facilitates the identification of SIP
and malignant tumors (38). Another
study demonstrated the diagnostic value of tumor blood flow
obtained by pseudocontinuous arterial spin labeling is able to
effectively differentiate between SCC, non-aggressive SIP and
aggressive SIP using a 3.0-T MRI (39). Nakamaru et al (40) analyzed 10 consecutive patients with
SIP and diagnosis in these patients was confirmed by histological
assessment. The study indicated that MRI indicated greater
specificity compared with CT scan and suggested that a combination
of preoperative CT and MRI may able to provide more useful
information compared with using either CT or MRI alone (40).
Currently, there are no distinct clinical signs and
symptoms that differentiate SIP and malignant transformation of SIP
(2,32). The diagnosis of SIP malignant
transformation is based on the observation of synchronous
transformation, which appears at the same time as papilloma, and
metachronous transformation, which appears at the site of a
previous papilloma (41).
Furthermore, preoperative histological examination
is difficult and ineffective to differentiate SIP and malignant
transformation of SIP (22). Several
investigators have suggested that CT/MRI may be ineffective in
distinguishing SIP from SCC (42),
while others have reported that bone invasion may be a
differentiating feature of synchronous malignant transformation of
SIP on CT scan (43). Therefore,
recent studies introduced fluorine-18-fluorodeoxyglucose (18F-FDG)
positron emission tomography (PET)/CT for the identification of
SIP, which depends on the extent of FDG uptake by different tissues
though glycolysis (44). Allegra
et al (42) analyzed 12
patients (7 with primary diagnosis of SIP and 5 with suspected
recurrence of SIP) using 18FDG-PET/CT for the diagnosis of SIP with
a sensitivity and specificity rate of 100% (42). Similarly, a 2015 study containing 27
patients demonstrated that 18FDG-PET/CT is able to distinguish
polyposis, SIP and SCC by distinct standardized uptake value
(SUVmax) values (45).
Shojaku et al (46) confirmed
that higher SIP SUVmax values may indicate the
probability of an associated malignancy, even when preoperative
biopsy indicates a benign papilloma. By contrast, another study
containing 8 patients reported a wide discrepancy between MRI and
PET/CT findings (47). Taken
together, these studies suggest that preoperative SIP imaging
should involve a combination of CT, MRI and PET/CT.
Histologically, the most common malignancy
associated with SIP is SCC. However, growing evidence has
demonstrated a pathologic collision exists between SIP and other
tumors. Karam et al (48)
reported a case with a pathologic collision of SIP with
esthesioneuroblastoma. The tumor was resected; however the
postoperative surgical margin was positive, and neck lymph nodes
were metastatic. Therefore the patient was treated with adjuvant
concomitant chemoradiation, and evidence of tumor recurrence was
not detected in the 42-month follow-up (48). In another study, a patient with nasal
type natural killer/T-cell lymphoma and SIP, received surgery with
postoperative chemotherapy. Tumor recurrence was not observed in
the subsequent 10-month follow-up (49). Shahrjerdi et al (50) reported a case of co-existing
unilateral SIP and angiofibroma. The nasal mass was treated by
radical surgical resection, and the 3-month follow-up indicated
that the patient was asymptomatic with no signs of cancer
recurrence (50). Additionally, a
patient with SIP and accompanying monophasic fibrous synovial
sarcoma in the sphenoid sinus was also reported (49). The treatment for this case involved
surgery, postoperative adjuvant radiotherapy and subsequent
chemotherapy. There were no signs of recurrence following the
50-month follow-up (51).
Furthermore, SIP with fungal ball in the maxillary sinus has also
been reported (52). SIP accompanied
with a malignancy of a different pathology is extremely rare, which
may lead to pretherapeutic misdiagnosis (51) and an increased risk of recurrence
potentially due to a lack of therapeutic regimen such as surgical
margin, dose and cycles of radiotherapy, selection of
chemotherapeutic agent, and lack of evidence-based analysis of in
large well-controlled studies (Table
I).
Apart from histological variations of SIP,
exceptional clinical cases should also be emphasized. It has been
reported that SIP may spread to the middle ear and temporal bone.
The spread of SIP may be mediated either due to migration via the
eustachian tube or due to embryological migration of the
Schneiderian mucosa into the middle ear (53,54).
Garcia et al (55) reported
that SIP/SCC in the maxillary sinus may extend to the mouth as an
early symptom. Furthermore, as a common unilateral nasal
occurrence, a case with a bilateral SIP involving both sides of
frontal sinus was reported in Keskin et al (56). Sharma et al (57) reported a patient with a history of
multiple locations, who presented with recurrent SIP with a
pathologically benign large mass on the left side of the upper
neck. Additionally, another study reported that SIP/SCC is
associated with neck metastasis (58). These cases demonstrate that SIP may
origin from multiple sites and therefore should not be ignored.
Preoperative examinations should include a complete head and neck
assessment.
A number of studies suggest that the length
follow-up for SIP was usually >3 years (2,16).
Unfortunately, it is difficult to distinguish inflammation from SIP
recurrence using nasal endoscopy (19,59). MRI
and PET/CT may be recommended for post-surgery follow-up. In
addition to imaging techniques, the serum level of squamous cell
carcinoma antigen may also be used as a molecular marker for the
recurrence of SIP (59,60).
The mechanisms leading to the occurrence, recurrence
and malignant transformation of SIP remain a matter of debate. To
date, many studies have aimed to resolve this issue (Tables II and III). Putative aberrant mediators that may
participate in the recurrence of SIP include palate, lung, and
nasal epithelium clone protein (65),
keratin, type I cytoskeletal 14 (66), Ki-67 (66), survivin (67), B-cell lymphoma 2 (67), osteopontin (OPN) (68), vascular endothelial growth factor
(68), fascin (69), mean vessel density (69,70), CCAAT
enhancer binding proteins (71),
cyclooxygenase-2 (COX-2) (72),
angiomotin (73),
phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and
dual-specificity protein phosphatase (PTEN) (74), hypoxia-inducible factor 1-α (HIF-1α)
(74), HPV infection and stathmin
(75).
Malignant transformation of SIP may be associated
with the following factors: HPV 16/18 infection (76), epidermal growth factor receptor (EGFR)
1 (77,78); cell cycle proteins [p21 (79); p16 (79,80); p53
(80,81); p63 (79,81); p27;
cyclin D1 (82); proliferating cell
nuclear antigen reverse transcription (65,83); Ki-67
(65,84); metallothionein-2-5A/G (reference
single nucleotide polymorphisms cluster ID, 28366003) (85); TFPI-2 (86); fascin (87); matrix metallopeptidase-2 (76); sex determining region Y-box 2
(88); topoisomerase II-α (84,89,90); OPN
(91); homeobox protein MSX-2
(89–91); desmoglein 3 (92); survivin (83,93);
cathepsin S (94); stefin A (94); E-cadherin (95); β-catenin (82,95); COX-2
(96,97); deleted in lung and esophageal cancer
protein 1 (98); IQ motif containing
GTPase activating protein 1 (99);
Smac (93); PTEN (74); HIF-1α (74); Dvl-1 (82); retinoblastoma protein (100); and regulatory T cells (101). However, there were several key
limitations in these studies. The materials and methods used were
simple (1). The majority of
literature analyzed primary resected SIP tissue samples and
performed immunohistochemistry as a common method. However,
accurate research on molecular biological mechanisms requires
comprehensive materials and methods. For instance, the
establishment of SIP cell lines and animal models is necessary for
research on SIP (2). The major
dependent factors of SIP. The basement of target therapy is that
tumor cells depended on a core factor for their progression.
Starska et al (85) emphasized
EGFR mutations as a regulator of SIP to SIP/SCC. Variations in the
EGFR gene have been identified as a key factor that are associated
with poor prognosis in head and neck squamous cell carcinoma
(102). It is likely that EGFR may
be a target for SIP treatment; however further studies are required
to confirm this hypothesis.
In summary, the clinical risk factors of SIP
progression and recurrence include smoking, outdoor and industrial
occupational exposure, septal deviation, SIP location, recurrent
cases, stage of SIP/SCC and choice of surgical method for advanced
SIP. The best preventative measure for SIP recurrence is the
complete removal of the tumor during the first surgery and a
comprehensive follow-up. Additionally, further studies are required
to elucidate the molecular mechanisms underlying the recurrence and
malignant transformation of SIP.
|
1
|
Wood JW and Casiano RR: Inverted
papillomas and benign nonneoplastic lesions of the nasal cavity. Am
J Rhinol Allergy. 26:157–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim DY, Hong SL, Lee CH, Jin HR, Kang JM,
Lee BJ, Moon IJ, Chung SK, Rha KS, Cho SH, et al: Inverted
papilloma of the nasal cavity and paranasal sinuses: A Korean
multicenter study. Laryngoscope. 122:487–494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saha SN, Ghosh A, Sen S, Chandra S and
Biswas D: Inverted papilloma: A clinico-pathological dilemma with
special reference to recurrence and malignant transformation.
Indian J Otolaryngol Head Neck Surg. 62:354–359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Busquets JM and Hwang PH: Endoscopic
resection of sinonasal inverted papilloma: A meta-analysis.
Otolaryngol Head Neck Surg. 134:476–482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moon IJ, Lee DY, Suh MW, Han DH, Kim ST,
Min YG, Lee CH and Rhee CS: Cigarette smoking increases risk of
recurrence for sinonasal inverted papilloma. Am J Rhinol Allergy.
24:325–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong SL, Kim BH, Lee JH, Cho KS and Roh
HJ: Smoking and malignancy in sinonasal inverted papilloma.
Laryngoscope. 123:1087–1091. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sham CL, Lee DL, van Hasselt CA and Tong
MC: A case-control study of the risk factors associated with
sinonasal inverted papilloma. Am J Rhinol Allergy. 24:e37–e40.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
d'Errico A, Zajacova J, Cacciatore A,
Baratti A, Zanelli R, Alfonzo S and Beatrice F: Occupational risk
factors for sinonasal inverted papilloma: A case-control study.
Occup Environ Med. 70:703–708. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herrold KM: Epithelial papillomas of the
nasal cavity; Experimental induction in syrian hamsters. Arch
Pathol. 78:189–195. 1964.PubMed/NCBI
|
|
10
|
Nomura K, Ogawa T, Sugawara M, Honkura Y,
Oshima H, Arakawa K, Oshima T and Katori Y: Association between
septal deviation and sinonasal papilloma. Tohoku J Exp Med.
231:315–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teymoortash A and Werner JA: Current
advances in diagnosis and surgical treatment of lymph node
metastasis in head and neck cancer. GMS Curr Top Otorhinolaryngol
Head Neck Surg. 11:Doc04. 2012.PubMed/NCBI
|
|
12
|
Krouse JH: Development of a staging system
for inverted papilloma. Laryngoscope. 110:965–968. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oikawa K, Furuta Y, Nakamaru Y, Oridate N
and Fukuda S: Preoperative staging and surgical approaches for
sinonasal inverted papilloma. Ann Otol Rhinol Laryngol.
116:674–680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cannady SB, Batra PS, Sautter NB, Roh HJ
and Citardi MJ: New staging system for sinonasal inverted papilloma
in the endoscopic era. Laryngoscope. 117:1283–1287. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han JK, Smith TL, Loehrl T, Toohill RJ and
Smith MM: An evolution in the management of sinonasal inverting
papilloma. Laryngoscope. 111:1395–1400. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin GC, Akkina S, Chinn S, Prince ME,
McHugh JB, Carey T and Zacharek MA: Sinonasal inverted papilloma:
Prognostic factors with emphasis on resection margins. J Neurol
Surg B Skull Base. 75:140–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gras-Cabrerizo JR, Montserrat-Gili JR,
Massegur-Solench H, León-Vintró X, De Juan J and Fabra-Llopis JM:
Management of sinonasal inverted papillomas and comparison of
classification staging systems. Am J Rhinol Allergy Allergy Rhinol
Allergy. 24:66–69. 2010. View Article : Google Scholar
|
|
18
|
Tomazic PV, Hubmann F and Stammberger H:
The problem of high recurrence rate in endoscopic revision surgery
for inverted papilloma. Laryngorhinootologie. 94:447–450. 2015.(In
German). PubMed/NCBI
|
|
19
|
Xiao-Ting W, Peng L, Xiu-Qing W, Hai-Bo W,
Wen-Hui P, Bing L, Er-Peng Z and Guang-Gang S: Factors affecting
recurrence of sinonasal inverted papilloma. Eur Arch
Otorhinolaryngol. 270:1349–1353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suh JD, Ramakrishnan VR, Thompson CF,
Woodworth BA, Adappa ND, Nayak J, Lee JM, Lee JT, Chiu AG and
Palmer JN: Inverted papilloma of the sphenoid sinus: Risk factors
for disease recurrence. Laryngoscope. 125:544–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang XD, Dong Z, Li GY, Gao G and Zhu DD:
Endoscopic surgery for 89 cases of nasal inverted papilloma.
Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 45:186–189. 2010.(In
Chinese). PubMed/NCBI
|
|
22
|
Yu HX and Liu G: Malignant transformation
of sinonasal inverted papilloma: A retrospective analysis of 32
cases. Oncol Lett. 8:2637–2641. 2014.PubMed/NCBI
|
|
23
|
Liang QZ, Li DZ, Wang XL, Huang H, Xu ZG
and Wu YH: Survival outcome of squamous cell carcinoma arising from
sinonasal inverted papilloma. Chin Med J (Engl). 128:2457–2461.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Osuch-Wójcikiewicz E, Wojas O, Nyckowska
J, Checiński P, Sielska-Badurek E, Bruzgielewicz A, Szwedowicz P
and Niemczyk K: Management of recurrent sinonasal inverted
papilloma in the experience of ENT department medical university of
warsaw. Otolaryngol Pol. 64:73–76. 2010.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lombardi D, Tomenzoli D, Buttà L, Bizzoni
A, Farina D, Sberze F, Karligkiotis A, Castelnuovo P and Nicolai P:
Limitations and complications of endoscopic surgery for treatment
for sinonasal inverted papilloma: A reassessment after 212 cases.
Head Neck. 33:1154–1161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lawson W, Kaufman MR and Biller HF:
Treatment outcomes in the management of inverted papilloma: An
analysis of 160 cases. Laryngoscope. 113:1548–1556. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Q, Yu H, Minovi A, Wei W, Wang D,
Zheng C, Li F and Zhang Z: Management of maxillary sinus inverted
papilloma via transnasal endoscopic anterior and medial
maxillectomy. ORL J Otorhinolaryngol Relat Spec. 72:247–251. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wada K, Ishigaki T, Ida Y, Yamada Y,
Hosono S and Edamatsu H: Endoscopic modified medial maxillectomy
for resection of an inverted papilloma originating from the entire
circumference of the maxillary sinus. Case Rep Otolaryngol.
2015:9529232015.PubMed/NCBI
|
|
29
|
Erbek SS, Koycu A and Buyuklu F:
Endoscopic modified medial maxillectomy for treatment of inverted
papilloma originating from the maxillary sinus. J Craniofac Surg.
26:e244–e246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lian F and Juan H: Different endoscopic
strategies in the management of recurrent sinonasal inverted
papilloma. J Craniofac Surg. 23:e44–e48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee DK, Chung SK, Dhong HJ, Kim HY, Kim HJ
and Bok KH: Focal hyperostosis on CT of sinonasal inverted
papilloma as a predictor of tumor origin. AJNR Am J Neuroradiol.
28:618–621. 2007.PubMed/NCBI
|
|
32
|
Gomaa MA, Hammad MS, Abdelmoghny A,
Elsherif AM and Tawfik HM: Magnetic resonance imaging versus
computed tomography and different imaging modalities in evaluation
of sinonasal neoplasms diagnosed by histopathology. Clin Med
Insights Ear Nose Throat. 6:9–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okamoto T, Kodama S, Nomi N, Umemoto S and
Suzuki M: Expression of bone morphogenic protein in sinonasal
inverted papilloma with new bone formation. Allergy Rhinol
(Providence). 2:16–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhalla RK and Wright ED: Predicting the
site of attachment of sinonasal inverted papilloma. Rhinology.
47:345–348. 2009.PubMed/NCBI
|
|
35
|
Yousuf K and Wright ED: Site of attachment
of inverted papilloma predicted by CT findings of osteitis. Am J
Rhinol. 21:32–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sham CL, King AD, van Hasselt A and Tong
MC: The roles and limitations of computed tomography in the
preoperative assessment of sinonasal inverted papillomas. Am J
Rhinol. 22:144–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeon TY, Kim HJ, Chung SK, Dhong HJ, Kim
HY, Yim YJ, Kim ST, Jeon P and Kim KH: Sinonasal inverted
papilloma: Value of convoluted cerebriform pattern on MR imaging.
AJNR Am J Neuroradiol. 29:1556–1560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang X, Zhang Z, Chen X, Li J and Xian J:
Value of magnetic resonance imaging including dynamic
contrast-enhanced magnetic resonance imaging in differentiation
between inverted papilloma and malignant tumors in the nasal
cavity. Chin Med J (Engl). 127:1696–1701. 2014.PubMed/NCBI
|
|
39
|
Fujima N, Nakamaru Y, Sakashita T, Homma
A, Tsukahara A, Kudo K and Shirato H: Differentiation of squamous
cell carcinoma and inverted papilloma using non-invasive MR
perfusion imaging. Dentomaxillofac Radiol. 44:201500742015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakamaru Y, Fujima N, Takagi D, Tsukahara
A, Yoshida D and Fukuda S: Prediction of the attachment site of
sinonasal inverted papillomas by preoperative imaging. Ann Otol
Rhinol Laryngol. 123:468–474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mirza S, Bradley PJ, Acharya A, Stacey M
and Jones NS: Sinonasal inverted papillomas: Recurrence, and
synchronous and metachronous malignancy. J Laryngol Otol.
121:857–864. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Allegra E, Cristofaro MG, Cascini LG,
Lombardo N, Tamburrini O and Garozzo A: 18FDG uptake in sinonasal
inverted papilloma detected by positron emission
tomography/computed tomography. ScientificWorldJournal.
2012:9434122012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Myers EN, Fernau JL, Johnson JT, Tabet JC
and Barnes EL: Management of inverted papilloma. Laryngoscope.
100:481–490. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vansteenkiste JF, Stroobants SG, Dupont
PJ, de Leyn PR, Verbeken EK, Deneffe GJ, Mortelmans LA and Demedts
MG: Prognostic importance of the standardized uptake value on
(18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in
non-small-cell lung cancer: An analysis of 125 cases. Leuven Lung
Cancer Group. J Clin Oncol. 17:3201–3206. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yılmaz I, Reyhan M, Canpolat T, Yılmazer
C, Erkan AN, Yaşar M, Akdoğan V and Özlüoğlu LN: Positron emission
tomography evaluation of sinonasal inverted papilloma and related
conditions: A prospective clinical study. Kulak Burun Bogaz Ihtis
Derg. 25:9–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shojaku H, Fujisaka M, Yasumura S, Ishida
M, Tsubota M, Nishida H, Watanabe Y, Kawano M, Shimizu M and
Fukuoka J: Positron emission tomography for predicting malignancy
of sinonasal inverted papilloma. Clin Nucl Med. 32:275–278. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Allegra E, Lombardo N, Cascini G, La Boria
A, Garozzo A and Tamburrini O: Possible role of 18FDG-PET/CT for
the surveillance of sinonasal inverted papilloma. Clin Otolaryngol.
35:249–251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Karam SD, Jay AK, Anyanwu C, Steehler MK,
Davidson B, Debrito P and Harter KW: Pathologic collision of
inverted papilloma with esthesioneuroblastoma. Front Oncol.
4:442014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Roy AD, Tuli IP and Joshi D: NK/T cell
lymphoma with inverted papilloma: A rare coexistence. Australas Med
J. 7:318–322. 2014.PubMed/NCBI
|
|
50
|
Shahrjerdi B, Angoyaroko A and Abdullah B:
Co-existing of sinonasal inverted papilloma and angiofibroma: Case
report and review of the literature. Acta Inform Med. 20:261–263.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jiang X, Huang Q, Tang J and Hoffman MR:
Monophasic epithelial synovial sarcoma accompanied by an inverted
papilloma in the sphenoid sinus. Case Rep Med.
2012:3797202012.PubMed/NCBI
|
|
52
|
Hsin LJ and Yang SW: Concomitant inverted
papilloma and fungus ball in unilateral maxillary sinus. B-ENT.
9:71–75. 2013.PubMed/NCBI
|
|
53
|
Barbosa JL, Pinheiro SD, Freitas MR, Nunes
AA and Leite EB: Sinonasal inverted papilloma involving the middle
ear and the mastoid. Braz J Otorhinolaryngol. 78:1222012.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu ZW, Walden A and Lee CA: Sinonasal
inverted papilloma involving the temporal bone via the eustachian
tube: Case report. J Laryngol Otol. 127:318–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Garcia AS, Bravo-Calderón DM, Ferreira MP
and Oliveira DT: Squamous cell carcinoma arising from inverted
Schneiderian papilloma: A case report with oral involvement. Case
Rep Otolaryngol. 2014:4780922014.PubMed/NCBI
|
|
56
|
Keskin IG, Topdağ M, Ila K, Topdağ DÖ and
Öztürk M: Bilateral inverted papilloma originating from the frontal
sinus. Kulak Burun Bogaz Ihtis Derg. 24:349–353. 2014.(In Turkish).
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sharma J, Goldenberg D, Crist H and McGinn
J: Multifocal inverted papillomas in the head and neck. Ear Nose
Throat J. 94:E20–E23. 2015.PubMed/NCBI
|
|
58
|
Mathew P and Idiculla JJ: Malignant
sinonasal papilloma with neck metastasis: A rare report and
literature review. Int J Oral Maxillofac Surg. 41:368–370. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Matoušek P, Zelenik K, Safarčík K,
Cábalová L and Kominek P: Squamous cell carcinoma antigen as a
marker of sinonasal inverted papilloma. Eur Arch Otorhinolaryngol.
271:535–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Suzuki M, Deng Z, Hasegawa M, Uehara T,
Kiyuna A and Maeda H: Squamous cell carcinoma antigen production in
nasal inverted papilloma. Am J Rhinol Allergy. 26:365–370. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Healy DY Jr, Chhabra N, Metson R, Holbrook
EH and Gray ST: Surgical risk factors for recurrence of inverted
papilloma. Laryngoscope. 126:796–801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Adriaensen GF, van der Hout MW, Reinartz
SM, Georgalas C and Fokkens WJ: Endoscopic treatment of inverted
papilloma attached in the frontal sinus/recess. Rhinology.
53:317–324. 2015.PubMed/NCBI
|
|
63
|
Akkari M, Lassave J, Mura T, Gascou G,
Pierre G, Cartier C, Garrel R and Crampette L: Atypical
presentations of sinonasal inverted papilloma: Surgical management
and influence on the recurrence rate. Am J Rhinol Allergy.
30:149–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Karligkiotis A, Lepera D, Volpi L,
Turri-Zanoni M, Battaglia P, Lombardi D, Accorona R, Bignami M,
Nicolai P and Castelnuovo P: Survival outcomes after endoscopic
resection for sinonasal squamous cell carcinoma arising on inverted
papilloma. Head Neck. 38:1604–1614. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tsou YA, Huang HJ, Wang TC, Tai CJ, Chen
CM and Chen CY: Evaluation of correlation of cell cycle proteins
and Ki-67 interaction in paranasal sinus inverted papilloma
prognosis and squamous cell carcinoma transformation. Biomed Res
Int. 2014:6349452014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gunia S, Liebe D and Koch S: Loss of basal
cell keratin 14 reflects increased risk of recurrence in surgically
resected sinonasal inverted papilloma. J Clin Pathol. 61:707–712.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liang J, Gao S, Zhang J, Ao H, Wei X and
Luo H: Expression of survivin and Bcl-2 in sinonasal inverted
papilloma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
23:933–935. 2009.(In Chinese). PubMed/NCBI
|
|
68
|
Liu W, Li Z, Luo Q, Lai Y, Zhang J, Chen
F, Shi J, Li H, Xiong G, Xu G and Wang H: The elevated expression
of osteopontin and vascular endothelial growth factor in sinonasal
inverted papilloma and its relationship with clinical severity. Am
J Rhinol Allergy. 25:313–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cai Y and Zhang J: Expression of fascin
and correlation with MVD in sinonasal inverted papilloma. Lin Chung
Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 26:629–632. 2012.(In
Chinese). PubMed/NCBI
|
|
70
|
Pajor AM, Danilewicz M,
Stasikowska-Kanicka O and Józefowicz-Korczyńska M: The
immunoexpression of CD34, Bcl-2, and Ki-67 antigens in sinonasal
inverted papillomas. Am J Rhinol Allergy. 28:e31–e34. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shabana EH, Depondt J, Hourseau M, Walker
F and Berdal A: Production and significance of CCAAT enhancer
binding proteins alpha and beta in sinonasal inverted papilloma.
Histol Histopathol. 28:53–60. 2013.PubMed/NCBI
|
|
72
|
Suh JD, Palma-Diaz F, Bhuta S and Wang MB:
COX-(2) overexpression in sinonasal inverted papilloma. Int Forum
Allergy Rhinol. 3:997–1000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Byun JY, Lee SH, Shin JM, Baek BJ and Lee
JY: Overexpression of angiomotin in sinonasal inverted papilloma.
Int Forum Allergy Rhinol. 4:512–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang W, Wen S, Zhang T, Wang B, Gao W and
Li L: Expression and significance of PTEN and HIF-1α proteins in
sinonasal inverted papilloma. Zhonghua Er Bi Yan Hou Tou Jing Wai
Ke Za Zhi. 49:399–403. 2014.(In Chinese). PubMed/NCBI
|
|
75
|
Lin H, Lin D and Xiong XS: Roles of human
papillomavirus infection and stathmin in the pathogenesis of
sinonasal inverted papilloma. Head Neck. 38:220–224. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lee HJ and Kim JW: Immunohistochemical
study on the expression of matrix metalloproteinase 2 and high-risk
human papilloma virus in the malignant progression of papillomas. J
Korean Assoc Oral Maxillofac Surg. 39:224–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Udager AM, Rolland DC, McHugh JB, Betz BL,
Murga-Zamalloa C, Carey TE, Marentette LJ, Hermsen MA, DuRoss KE,
Lim MS, et al: High-frequency targetable EGFR mutations in
sinonasal squamous cell carcinomas arising from inverted sinonasal
papilloma. Cancer Res. 75:2600–2606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Scheel A, Lin GC, McHugh JB, Komarck CM,
Walline HM, Prince ME, Zacharek MA and Carey TE: Human
papillomavirus infection and biomarkers in sinonasal inverted
papillomas: Clinical significance and molecular mechanisms. Int
Forum Allergy Rhinol. 5:701–707. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kim SG, Lee OY, Choi JW, Park YH, Kim YM,
Yeo MK, Kim JM and Rha KS: Pattern of expression of cell
cycle-related proteins in malignant transformation of sinonasal
inverted papilloma. Am J Rhinol Allergy. 25:75–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lin GC, Scheel A, Akkina S, Chinn S,
Graham M, Komarck C, Walline H, McHugh JB, Prince ME, Carey TE and
Zacharek MA: Epidermal growth factor receptor, p16, cyclin D1, and
p53 staining patterns for inverted papilloma. Int Forum Allergy
Rhinol. 3:885–889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Oncel S, Cosgul T, Calli A, Calli C and
Pinar E: Evaluation of p53, p63, p21, p27, ki-67 in paranasal sinus
squamous cell carcinoma and inverted papilloma. Indian J
Otolaryngol Head Neck Surg. 63:172–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jung YG, Lee HW, Kim MG, Dhong HJ, Cho KS
and Roh HJ: Role of Wnt signaling pathway in progression of
sinonasal inverted papilloma to squamous cell carcinoma. Am J
Rhinol Allergy. 29:e81–e86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Peng L, Shan C, Feng Z and Yang L:
Expression and significance of survivin and PCNA in sinonasal
inverted papilloma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
27:264–266. 2013.(In Chinese). PubMed/NCBI
|
|
84
|
Hadar T, Shvero J, Yaniv E, Shvili I,
Leabu M and Koren R: Human topoisomerase II-alpha is highly
expressed in sinonasal-inverted papilloma, but not in inflammatory
polyp. J Cell Mol Med. 12:1551–1558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Starska K, Bryś M, Forma E, Olszewski J,
Pietkiewicz P, Lewy-Trenda I, Stasikowska-Kanicka O, Danilewicz M
and Krześlak A: Metallothionein 2A core promoter region genetic
polymorphism and its impact on the risk, tumor behavior, and
recurrences of sinonasal inverted papilloma (Schneiderian
papilloma). Tumour Biol. 36:8559–8571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yu H, Liu Q, Wang H, Wang D, Hu L, Sun X
and Liu J: The role of tissue factor pathway inhibitor-2 in
malignant transformation of sinonasal inverted papilloma. Eur Arch
Otorhinolaryngol. 271:2191–2196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wu HH, Zafar S, Huan Y, Yee H, Chiriboga L
and Wang BY: Fascin over expression is associated with dysplastic
changes in sinonasal inverted papillomas: A study of 47 cases. Head
Neck Pathol. 3:212–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Schröck A, Göke F, Wagner P, Bode M,
Franzen A, Braun M, Huss S, Agaimy A, Ihrler S, Menon R, et al: Sex
determining region Y-box 2 (SOX2) amplification is an independent
indicator of disease recurrence in sinonasal cancer. PLoS One.
8:e592012013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wu Q, Yang Y, Wu X, Zhao C, Cong L, Ruan B
and Zhang J: Expression and significance of Msx2 and topo II-alpha
in sinonasal inverted papilloma. Lin Chung Er Bi Yan Hou Tou Jing
Wai Ke Za Zhi. 26:343–346. 2012.(In Chinese). PubMed/NCBI
|
|
90
|
Zhang J, Yang Y, Tang Y, Wu X, Cong L and
Ruan B: The quantification and significance of muscle segment
homeobox gene Msx2, human topoisomerase II-α, HPV16 and VEGF in
sinonasal inverted papilloma. Lin Chung Er Bi Yan Hou Tou Jing Wai
Ke Za Zhi. 28:1819–1823. 2014.(In Chinese). PubMed/NCBI
|
|
91
|
Wu Y, Cui S, Wu Q, Ma Z and Yuan W:
Expression and significance of osteopontin and muscle segment
homeobox gene Msx2 in sinonasal inverted papilloma. Lin Chung Er Bi
Yan Hou Tou Jing Wai Ke Za Zhi. 27:1114–1117. 2013.(In Chinese).
PubMed/NCBI
|
|
92
|
Huang CC, Lee TJ, Chang PH, Lee YS, Chuang
CC, Jhang YJ, Chen YW, Chen CW and Tsai CN: Desmoglein 3 is
overexpressed in inverted papilloma and squamous cell carcinoma of
sinonasal cavity. Laryngoscope. 120:26–29. 2010.PubMed/NCBI
|
|
93
|
Yang L, Shan C, Huang H, Sun Y, Zhao Y,
Wang L and Jia W: The expression of Smac and survivin in sinonasal
inverted papilloma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
27:407–410. 2013.(In Chinese). PubMed/NCBI
|
|
94
|
Huang CC, Lee TJ, Chang PH, Lee YS, Chuang
CC, Jhang YJ, Chen YW, Chen CW, Fu CH and Tsai CN: Expression of
cathepsin S and its inhibitor stefin A in sinonasal inverted
papilloma. Rhinology. 48:352–357. 2010.PubMed/NCBI
|
|
95
|
Koo BS, Jung BJ, Kim SG, Liang ZL, Yeong
MK and Rha KS: Altered expression of E-cadherin and
β-catenin in malignant transformation of sinonasal
inverted papillomas. Rhinology. 49:479–485. 2011.PubMed/NCBI
|
|
96
|
Lee GH, Yoon YH, Kim YM, Yeo MK, Liang ZL,
Kim JM and Rha KS: Pattern of expression of cyclooxygenase-2 in
malignant transformation of sinonasal inverted papilloma. Am J
Otolaryngol. 33:585–589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yoon BN, Chon KM, Hong SL, Lee JH, Kim JY,
Cho KS and Roh HJ: Inflammation and apoptosis in malignant
transformation of sinonasal inverted papilloma: The role of the
bridge molecules, cyclooxygenase-2, and nuclear factor κB. Am J
Otolaryngol. 34:22–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chang PH, Huang CC, Lee TJ, Lee YS and
Tsai CN: Downregulation of DLEC1 in sinonasal inverted papilloma
and squamous cell carcinoma. J Otolaryngol Head Neck Surg.
41:94–101. 2012.PubMed/NCBI
|
|
99
|
Jin J, Lee JW, Rha KS, Kim DW and Kim YM:
Expression pattern of IQGAP1 in sinonasal inverted papillomas and
squamous cell carcinomas. Laryngoscope. 122:2640–2646. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yamashita Y, Hasegawa M, Deng Z, Maeda H,
Kondo S, Kyuna A, Matayoshi S, Agena S, Uehara T, Kouzaki H, et al:
Human papillomavirus infection and immunohistochemical expression
of cell cycle proteins pRb, p53, and p16(INK4a) in sinonasal
diseases. Infect Agent Cancer. 10:232015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lou H, Fang J, Li P, Zhou W, Wang Y, Fan
E, Li Y, Wang H, Liu Z, Xiao L, et al: Frequency, suppressive
capacity, recruitment and induction mechanisms of regulatory T
cells in sinonasal squamous cell carcinoma and nasal inverted
papilloma. PLoS One. 10:e01264632015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Sharafinski ME, Ferris RL, Ferrone S and
Grandis JR: Epidermal growth factor receptor targeted therapy of
squamous cell carcinoma of the head and neck. Head Neck.
32:1412–1421. 2010. View Article : Google Scholar : PubMed/NCBI
|