Introduction
Lung cancer, particularly non-small-cell lung cancer
(NSCLC), remains the leading cause of global cancer-associated
mortality, with a 5-year survival rate of <20% (1). Despite years of research into novel
chemotherapy combinations, few treatment options are available for
the majority of patients with advanced or metastatic disease
(2). Adenocarcinoma patients with
epithelial growth factor receptor tyrosine kinase inhibitor
(EGFR-TKI)-sensitive mutations generally exhibit improved
progression-free and overall survival times following TKI treatment
(3). However, these patients
inevitably encounter resistance to first-generation EGFR-TKIs,
usually after 6–8 months of treatment (4). There are several mechanisms involved in
the generation of EGFR-TKI resistance in non-small cell lung cancer
(NSCLC) (5). These include the T790M
EGFR mutation, which induces secondary resistance in >50% of
patients (6); the compensatory
contribution of other receptor tyrosine kinases, including c-MET
amplification (7) and activating
mutations in human epidermal growth factor receptor 2 (HER-2)
(8); the activation of compensatory
signaling pathways, including the phosphoinositide-3
kinase/AKT/mammalian target of rapamycin signaling pathway
(9) and the TOPK-c-Jun pathway
(10); and histological
transformation, including EMT phenotypic transforming (11) and SCLC transformation (12). The transformation of lung
adenocarcinoma to SCLC, as described by the present case report, is
a relatively rare TKI resistance mechanism.
Case report
A 74-year-old male with a 50-pack-year smoking
history presented to the Department of Respiratory Medicine
(Huashan Hospital, Shanghai, China) with a 3-year history of
coughing, sputum production and shortness of breath in March 2014.
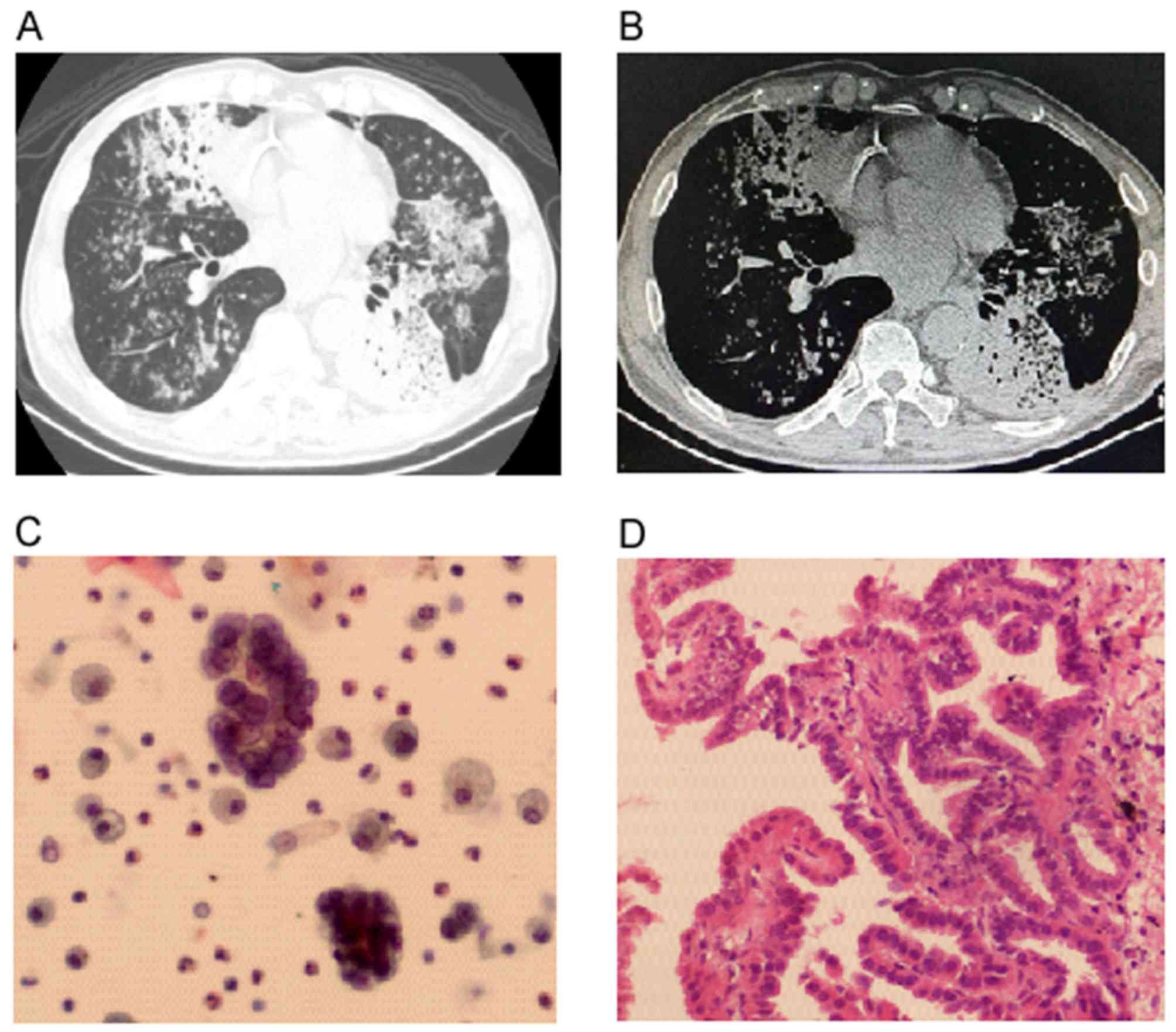
A computed tomography (CT) scan revealed multiple patchy and
nodular high-density shadows, and bilateral lung bronchiectasis
with consolidation in the left lower lobe (Fig. 1A). The structure of the mediastinum
was clear and the lymph nodes were not enlarged (Fig. 1B). The patient was administered
ceftazidime and levofloxacin to treat the suspected infection prior
to hospitalization. However, the patient did not respond to
treatment and the symptoms and CT scan images gradually
worsened.
Following admission, adenocarcinoma cells and a
Lophomonasblattarum infection were detected by pathological
cytology following a bronchoalveolar lavage (BAL) that was
performed in the right middle lobe of the lung (Fig. 1C). A subsequent transbronchial lung
biopsy was performed on the basal segment of the left lower lobe,
which detected well-differentiated adenocarcinoma cells (Fig. 1D). Next-generation sequencing (NGS,
Illumina Hiseq 4000; Illumina, Inc., San Diego, CA, USA) was
employed to check the 8 driver genes with targeted drugs and it was
found that exon 19 of EGFR was deleted (Table I). A positron emission tomography
(PET)/CT scan revealed intense uptake of 18F-fluorodeoxyglucose in
the left lower lobe, no hypermetabolic lymph nodes, and no
hypermetabolic lesions in the brain, abdomen or bone. The patient
was prescribed 500 mg metronidazole once a day for 3 months to cure
the L. blattarum infection and 250 mg gefitinib once a day
for lung cancer treatment, and was followed up every 2–3 months.
The patient's symptoms improved markedly, the serum
carcinoembryonic antigen level decreased from 5.52 ng/ml to within
the normal range (<5 ng/ml) and a CT scan revealed partial
remission (Fig. 2).
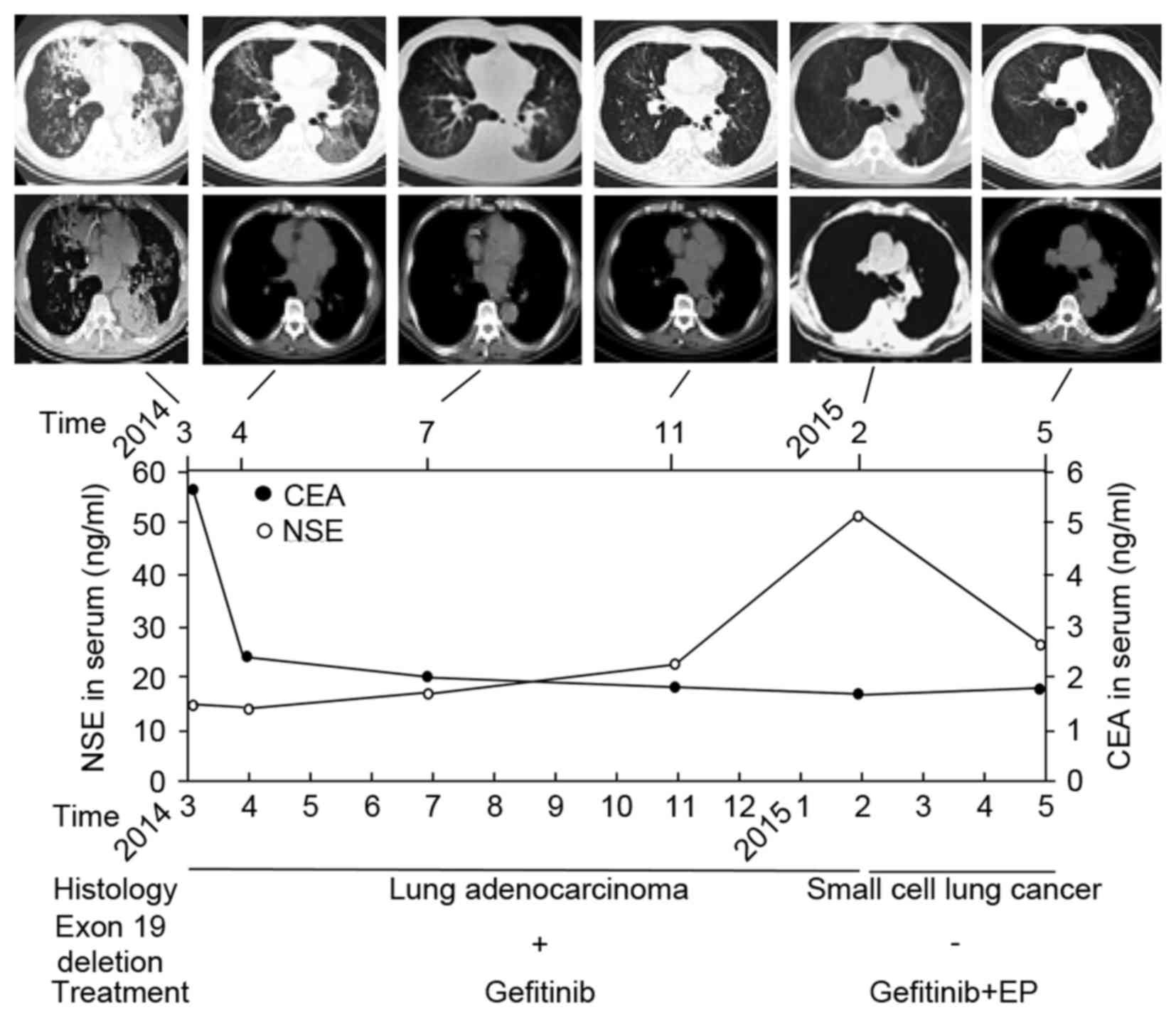 | Figure 2.Follow-up schematic diagram. The top
panel shows a series of CT scans taken between March 2014 and May
2015; the upper row of images depicts the pulmonary window and the
lower row of images depicts the mediastinal window. The middle
panel depicts follow-up examinations of serum CEA levels, which
decreased markedly after gefitinib treatment, and serum NSE levels,
which increased markedly after SCLC transformation. The bottom
panel is the pathological diagnosis corresponding to EGFR exon 19
deletion status and the treatment regimen. CT, computed tomography;
CEA, carcinoembryonic antigen; NSE, neuron-specific enolase; SCLC,
small cell lung cancer; EGFR, epithelial growth factor receptor,
EP, etoposide and cisplatin chemotherapy. |
 | Table I.Driver gene profile of primary lung
adenocarcinoma and secondary small cell lung cancer. |
Table I.
Driver gene profile of primary lung
adenocarcinoma and secondary small cell lung cancer.
| Driver gene | Primary lung
adenocarcinoma | Secondary small cell
lung cancer |
|---|
| EGFR exon 19
deletion | + | − |
| ALK
rearrangement | − | − |
| HER2 mutations | − | − |
| BRAF V600E
mutation | − | − |
| High-level MET
amplification or MET exon 14 skipping mutation | − | − |
| RET
rearrangements | − | − |
| ROS1
rearrangements | − | − |
| KRAS mutation | − | − |
However, after 1 year of gefitinib treatment, in
February 2015, serum levels of neuron-specific enolase (NSE)
markedly increased from normal range (<15 ng/ml) to 50.6 ng/ml,
with a CT scan revealing a slightly enlarged left lung hilum
(Fig. 2). After a further 3 months of
gefitinib treatment, the patient again presented with coughing and
shortness of breath. A CT scan revealed a substantially enlarged
left lung hilum with left lower lobe bronchial patency (Fig. 2). Another endobronchial ultrasound
transbronchial needle aspiration biopsy of the left lower lobe and
a set of 4-μm-thick paraffin sections were cut and mounted on
silanized slides. Sections were incubated for 60 min at room
temperature with primary antibodies from Abcam (Cambridge, UK):
Anti-transcription termination factor 1 (cat no. ab204411;
dilution, 1:500), anti-vimentin (cat no. ab92547; dilution, 1:200),
anti-anaplastic lymphoma kinase (cat no. ab16770; dilution, 1:100),
anti-synaptophysin (cat no. ab32127; dilution, 1:400),
anti-leukocyte common antigen (cat no. ab40763; dilution, 1:250),
anti-NSE (cat no. ab53025; dilution, 1:100), anti-S100 (cat no.
ab868; dilution, 1:100), anti-pan cytokeratin (AE1/AE3; cat no.
ab86734; dilution, 1:50), and control staining was performed using
isotype rabbit IgG (cat no. 3900) or isotype mouse IgG, (cat no.
5415) (Cell Signaling Technology, Inc., Danvers, MA, USA). Control
antibodies diluted to the same concentrationas the primary antibody
used for analysis in each case. After washing, sections were
incubated for 60 min at room temperature with horseradish
peroxidase polymer (pre-diluted; cat no. ab214879 or ab214880;
Abcam) and developed with DAB. Immunohistochemical staining
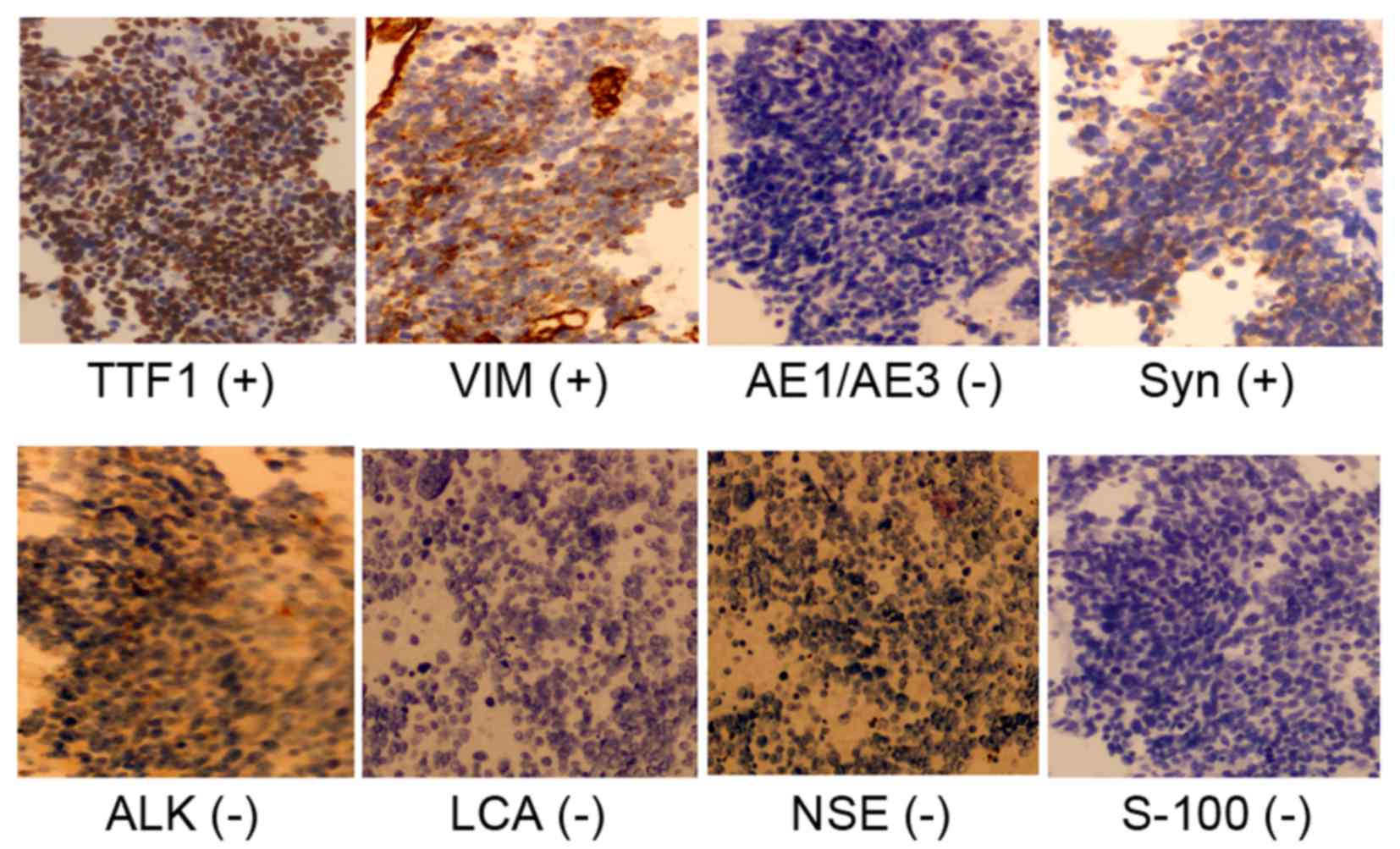
confirmed a histological transformation of the adenocarcinoma to
SCLC, and positivity for the expression of transcription
termination factor 1, synaptophysin and vimentin (Fig. 3). However, the deletion of EGFR exon
19 could no longer be detected by NGS (Table I). A chemotherapy regimen was added to
the gefitinib treatment: Etoposide (100 mg/m2, days 1–3)
plus cisplatin (30 mg/m2, days 1–3) (EP). After two
cycles of chemotherapy and a 30-Gy curative dose of radiotherapy,
the patient showed no improvement, and a CT scan revealed that the
left lower lobe was almost totally obstructed by an invasive tumor.
As the patient could not tolerate any further chemotherapy,
high-frequency electrocoagulation was employed to remove the tumor
from the left lower lobe of the lung and a stent was implanted to
keep the bronchial lumen patent. The patient was then prescribed
the best supportive care and succumbed to the disease 18 months
after the initial diagnosis.
Written informed consent was obtained for this case
report to be published.
Discussion
Transformation from lung adenocarcinoma to SCLC
following EGFR-TKI treatment is a relatively rare mechanism of
resistance to EGFR-TKIs (13). SCLC
transformation is more frequent in lung adenocarcinomas that have
EGFR-activating mutations than in EGFR wild-type tumors (14). One previous study reported an
incidence of SCLC transformation of 3.5% (4/115) (15), while other studies found that the
T790M mutation is the major acquired resistance mechanism (in
40–55% of cases) after first-generation EGFR-TKI treatment
(5,16). A further study found that SCLC
transformation occurred more frequently in lung adenocarcinomas
with a deletion in EGFR exon 19 [in 50.0% (9/18) of cases examined]
than in those with exon 21 L858R mutation (27.8%, 5/18) (12). The majority of the SCLC
transformations retained the same gene mutation as the primary lung
adenocarcinoma (83.3%, 15/18), with only a small proportion (16.7%,
3/18) losing the original gene mutation or gaining another type of
gene mutation (12). This phenomenon
may therefore be associated with a different mechanism of SCLC
transformation.
Heterogeneity in lung cancer (including driver gene
diversity and histological heterogeneity) occurs spatially and
temporally during the evolution of cancer cells. The regionally
separated, temporally altered driver mutations, coupled with genome
instability, markedly complicates the treatment of NSCLC (17,18) and is
associated with an increased likelihood of postoperative relapse in
patients with localized lung adenocarcinomas (19). The most common histological
heterogeneity is that of adenocarcinoma mixed with squamous cell
carcinoma, termed adenosquamous carcinoma (20). Combined SCLC and NSCLC histology has
been reported by two large studies. The first study analyzed 176
SCLC tumors and found that 17 (9.7%) tumors had an NSCLC component
(21). The second study examined the
histology of 429 SCLC tumors, finding that 9 (2.1%) tumors
contained NSCLC cells (6 adenocarcinoma and 3 squamous cell
carcinoma) (22). According to these
data, re-biopsy when tumors do not respond as initially expected or
when the responses among two or more different lesions are
discordant in advanced lung cancer may confirm a dominant histology
that was not identified at the initial diagnosis. Histological
transformation from NSCLC to SCLC following treatment with an
EGFR-TKI has been previously reported as a rare type of
histological heterogeneity (23).
The fact that the majority of transformed SCLCs
retained an original EGFR-activating mutation in the study by Jiang
et al (12) means that SCLC
may have been transformed from primary adenocarcinoma or that the
SCLC and adenocarcinoma cells originated from the same cancer stem
cells as a mechanism of resistance to EGFR-TKI treatment. A small
proportion of transformed SCLC cells did not have the same EGFR
mutations present in the original adenocarcinomas, as in the
current case report (12). These
patients may have tumors with a combined adenocarcinoma and SCLC
histology, which was not apparent at the time of the initial
diagnosis. When the adenocarcinoma was successfully treated with an
EGFR-TKI, the SCLC component became dominant as it was resistant to
EGFR-TKI treatment. A prior study reported that retinoblastoma
protein (Rb) expression was lost in cases where the histological
phenotype had changed from NSCLC to SCLC following EGFR-TKI
therapy, whereas Rb was rarely lost in those that retained the
NSCLC phenotype. This is considered one of the molecular mechanisms
of SCLC transformation (24).
Neuroendocrine differentiation can occur during the transformation
of SCLC and may lead to increased chemosensitivity (25). It can therefore be concluded that SCLC
cells may trans-differentiate from primary adenocarcinoma cells,
originate from the minor pre-existent SCLC under the selection
pressure of EGFR-TKIs, or arise from multi-potent cancer stem
cells. This phenomenon emphasizes the importance of re-biopsy in
the clinical design of a treatment regimen.
A rapid increase in serum NSE and a poor response to
EGFR-TKIs is usually an indication of transformation from
adenocarcinoma to SCLC (26). In the
present case, the markedly increased serum levels of NSE
highlighted the necessity of repeat biopsies. The present case
indicates that patients could benefit from the routine testing of
serum NSE levels to monitor SCLC transformation. The majority of
cases of SCLC transformation have exhibited neuroendocrine
differentiation with synaptophysin positive expression and
responded well to initial EP chemotherapy (12,21,27). The
SCLC tissue in the present case was positive for synaptophysin
expression, but was resistant to first-line EP chemotherapy.
Immunohistochemical analysis of SCLC tissue reveals vimentin
expression (28). Vimentin is a major
constituent of the intermediate filament family of proteins and a
biomarker of mesenchymal tissue that is overexpressed in various
epithelial cancer types, including in lung cancer cells (29). Vimentin expression in lung cancer
cells is an independent prognostic predictor of poor survival in
primary NSCLC (30), and its
expression is significantly lower in squamous cell carcinoma than
in adenocarcinoma (31). Vimentin is
recognized as a canonical marker for the epithelial-mesenchymal
transition (32), and is associated
with tumor growth and metastasis (33), serving as a potential molecular target
for cancer therapy (34). Withaferin
A, a small-molecule antagonist of vimentin, can elicit apoptosis,
decrease angiogenesis and induce vimentin cleavage in
vimentin-expressing tumor cells (35). Vimentin is also a predictive biomarker
of a worse outcome from erlotinib therapy (36). In the present case, vimentin
expression in SCLC was the molecular mechanism behind the
resistance to chemotherapy.
In conclusions, the present study reported the case
of a 74-year-old man who was initially diagnosed with lung
adenocarcinoma with a deletion in exon 19 of EGFR. The patient was
treated with gefitinib and relapsed after 1 year. Transformation to
EGFR-exon 19 deletion-negative SCLC was the reason for gefitinib
resistance. The patient was refractory to EP chemotherapy owing to
vimentin expression. Transformation from adenocarcinoma to SCLC may
originate from a minor pre-existent SCLC cell population under the
selective pressure of EGFR-TKI treatment. NSE serum level may be
useful for detecting early histological transformation, while
re-biopsy is also important to EGFR-TKI-resistant patients, as it
allows for genetic and histological re-evaluation of the
disease.
Acknowledgements
This study was supported by the National Natural
Science Foundation (grant nos. 81272586 and 81272646). The authors
would like to thank Dr Fred Bogott for providing assistance with
English language editing.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wakelee H and Belani CP: Optimizing
first-line treatment options for patients with advanced NSCLC.
Oncologist. 10 Suppl 3:S1–S10. 2005. View Article : Google Scholar
|
|
3
|
Wu JY, Wu SG, Yang CH, Chang YL, Chang YC,
Hsu YC, Shih JY and Yang PC: Comparison of gefitinib and erlotinib
in advanced NSCLC and the effect of EGFR mutations. Lung Cancer.
72:205–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu M, Xie Y, Ni S and Liu H: The latest
therapeutic strategies after resistance to first generation
epidermal growth factor receptor tyrosine kinase inhibitors (EGFR
TKIs) in patients with non-small cell lung cancer (NSCLC). Ann
Transl Med. 3:962015.PubMed/NCBI
|
|
5
|
Camidge DR, Pao W and Sequist LV: Acquired
resistance to TKIs in solid tumours: Learning from lung cancer. Nat
Rev Clin Oncol. 11:473–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W, Ren S, Li J, Li A, Fan L, Li X, Zhao
C, He Y, Gao G, Chen X, et al: T790M mutation is associated with
better efficacy of treatment beyond progression with EGFR-TKI in
advanced NSCLC patients. Lung Cancer. 84:295–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brugger W and Thomas M: EGFR-TKI resistant
non-small cell lung cancer (NSCLC): New developments and
implications for future treatment. Lung Cancer. 77:2–8. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cretella D, Saccani F, Quaini F, Frati C,
Lagrasta C, Bonelli M, Caffarra C, Cavazzoni A, Fumarola C, Galetti
M, et al: Trastuzumab emtansine is active on HER-2 overexpressing
NSCLC cell lines and overcomes gefitinib resistance. Mol Cancer.
13:1432014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin Y, Wang X and Jin H: EGFR-TKI
resistance in NSCLC patients: Mechanisms and strategies. Am J
Cancer Res. 4:411–435. 2014.PubMed/NCBI
|
|
10
|
Li Y, Yang Z, Li W, Xu S, Wang T, Wang T,
Niu M, Zhang S, Jia L and Li S: TOPK promotes lung cancer
resistance to EGFR tyrosine kinase inhibitors by phosphorylating
and activating c-Jun. Oncotarget. 7:6748–6764. 2016.PubMed/NCBI
|
|
11
|
Rudisch A, Dewhurst MR, Horga LG, Kramer
N, Harrer N, Dong M, van der Kuip H, Wernitznig A, Bernthaler A,
Dolznig H and Sommergruber W: High EMT signature score of invasive
non-small cell lung cancer (NSCLC) cells correlates with NFκB
driven colony-stimulating factor 2 (CSF2/GM-CSF) secretion by
neighboring stromal fibroblasts. PLoS One. 10:e01242832015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang SY, Zhao J, Wang MZ, Huo Z, Zhang J,
Zhong W and Xu Y: Small-cell lung cancer transformation in patients
with pulmonary adenocarcinoma: A case report and review of
literature. Medicine (Baltimore). 95:e27522016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe S, Sone T, Matsui T, Yamamura K,
Tani M, Okazaki A, Kurokawa K, Tambo Y, Takato H, Ohkura N, et al:
Transformation to small-cell lung cancer following treatment with
EGFR tyrosine kinase inhibitors in a patient with lung
adenocarcinoma. Lung Cancer. 82:370–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oser MG, Niederst MJ, Sequist LV and
Engelman JA: Transformation from non-small-cell lung cancer to
small-cell lung cancer: Molecular drivers and cells of origin.
Lancet Oncol. 16:e165–e172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumor specimens at the time of acquired resistance to
EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu SG, Liu YN, Tsai MF, Chang YL, Yu CJ,
Yang PC, Yang JC, Wen YF and Shih JY: The mechanism of acquired
resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib
in lung adenocarcinoma patients. Oncotarget. 7:12404–12413.
2016.PubMed/NCBI
|
|
17
|
de Bruin EC, McGranahan N, Mitter R, Salm
M, Wedge DC, Yates L, Jamal-Hanjani M, Shafi S, Murugaesu N, Rowan
AJ, et al: Spatial and temporal diversity in genomic instability
processes defines lung cancer evolution. Science. 346:251–256.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McGranahan N, Favero F, De Bruin EC,
Birkbak NJ, Szallasi Z and Swanton C: Clonal status of actionable
driver events and the timing of mutational processes in cancer
evolution. Sci Transl Med. 7:283ra542015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Fujimoto J, Zhang J, Wedge DC,
Song X, Zhang J, Seth S, Chow CW, Cao Y, Gumbs C, et al: Intratumor
heterogeneity in localized lung adenocarcinomas delineated by
multiregion sequencing. Science. 346:256–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uramoto H, Yamada S and Hanagiri T:
Clinicopathological characteristics of resected adenosquamous cell
carcinoma of the lung: Risk of coexistent double cancer. J
Cardiothorac Surg. 5:922010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adelstein DJ, Tomashefski JF Jr, Snow NJ,
Horrigan TP and Hines JD: Mixed small cell and non-small cell lung
cancer. Chest. 89:699–704. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mangum MD, Greco FA, Hainsworth JD, Hande
KR and Johnson DH: Combined small-cell and non-small-cell lung
cancer. J Clin Oncol. 7:607–612. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim WJ, Kim S, Choi H, Chang J, Shin HJ,
Park CK, Oh IJ, Kim KS, Kim YC and Choi YD: Histological
transformation from non-small cell to small cell lung carcinoma
after treatment with epidermal growth factor receptor-tyrosine
kinase inhibitor. Thorac Cancer. 6:800–804. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Niederst MJ, Sequist LV, Poirier JT,
Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN,
Moran T, et al: RB loss in resistant EGFR mutant lung
adenocarcinomas that transform to small-cell lung cancer. Nat
Commun. 6:63772015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang Y, Kim SY, Choi YJ, So KS, Rho JK,
Kim WS, Lee JC, Chung JH and Choi CM: Neuroendocrine
differentiation in acquired resistance to epidermal growth factor
receptor tyrosine kinase inhibitor. Tuberc Respir Dis (Seoul).
75:95–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Li XY, Tang Y, Xu Y, Guo WH, Li
YC, Liu XK, Huang CY, Wang YS and Wei YQ: Rapid increase of serum
neuron specific enolase level and tachyphylaxis of EGFR-tyrosine
kinase inhibitor indicate small cell lung cancer transformation
from EGFR positive lung adenocarcinoma? Lung Cancer. 81:302–305.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hwang KE, Jung JW, Oh SJ, Park MJ, Shon
YJ, Choi KH, Jeong ET and Kim HR: Transformation to small cell lung
cancer as an acquired resistance mechanism in EGFR-mutant lung
adenocarcinoma: A case report of complete response to etoposide and
cisplatin. Tumori. 101:e96–e98. 2015.PubMed/NCBI
|
|
28
|
Molenaar WM, Oosterhuis JW, Oosterhuis AM
and Ramaekers FC: Mesenchymal and muscle-specific intermediate
filaments (vimentin and desmin) in relation to differentiation in
childhood rhabdomyosarcomas. Hum Pathol. 16:838–843. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kidd ME, Shumaker DK and Ridge KM: The
role of vimentin intermediate filaments in the progression of lung
cancer. Am J Respir Cell Mol Biol. 50:1–6. 2014.PubMed/NCBI
|
|
30
|
Al-Saad S, Al-Shibli K, Donnem T, Persson
M, Bremnes RM and Busund LT: The prognostic impact of NF-kappaB
p105, vimentin, E-cadherin and Par6 expression in epithelial and
stromal compartment in non-small-cell lung cancer. Br J Cancer.
99:1476–1483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tadokoro A, Kanaji N, Liu D, Yokomise H,
Haba R, Ishii T, Takagi T, Watanabe N, Kita N, Kadowaki N and
Bandoh S: Vimentin regulates invasiveness and is a poor prognostic
marker in non-small cell lung cancer. Anticancer Res. 36:1545–1551.
2016.PubMed/NCBI
|
|
32
|
Liu CY, Lin HH, Tang MJ and Wang YK:
Vimentin contributes to epithelial-mesenchymal transition cancer
cell mechanics by mediating cytoskeletal organization and focal
adhesion maturation. Oncotarget. 6:15966–15983. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan Y and Li X, Duan J, Yuan L, Fan S, Fan
J, Xiaokaiti Y, Yang H, Wang Y and Li X: Enoxaparin sensitizes
human non-small-cell lung carcinomas to gefitinib by inhibiting
DOCK1 expression, vimentin phosphorylation and Akt activation. Mol
Pharmacol. 87:378–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lahat G, Zhu QS, Huang KL, Wang S,
Bolshakov S, Liu J, Torres K, Langley RR, Lazar AJ, Hung MC and Lev
D: Vimentin is a novel anti-cancer therapeutic target; insights
from in vitro and in vivo mice xenograft studies. PLoS One.
5:e101052010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Richardson F, Young GD, Sennello R, Wolf
J, Argast GM, Mercado P, Davies A, Epstein DM and Wacker B: The
evaluation of E-Cadherin and vimentin as biomarkers of clinical
outcomes among patients with non-small cell lung cancer treated
with erlotinib as second- or third-line therapy. Anticancer Res.
32:537–552. 2012.PubMed/NCBI
|