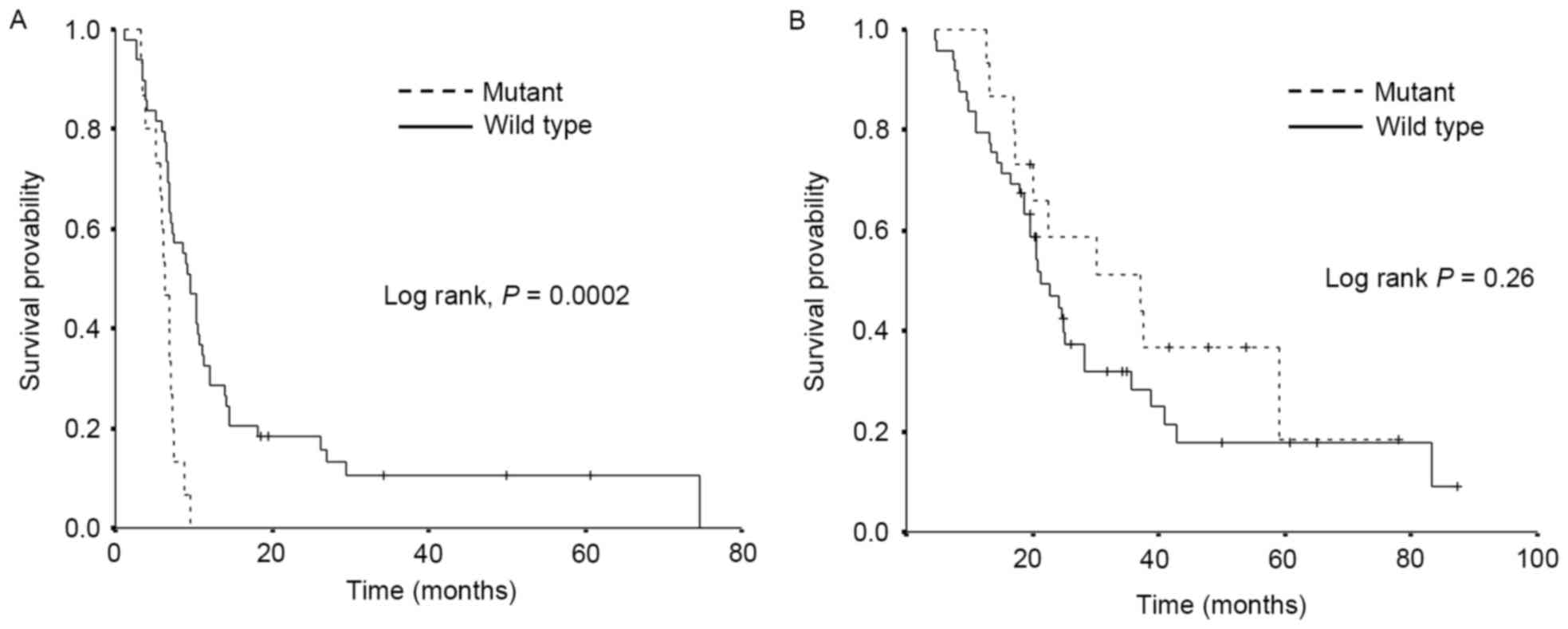
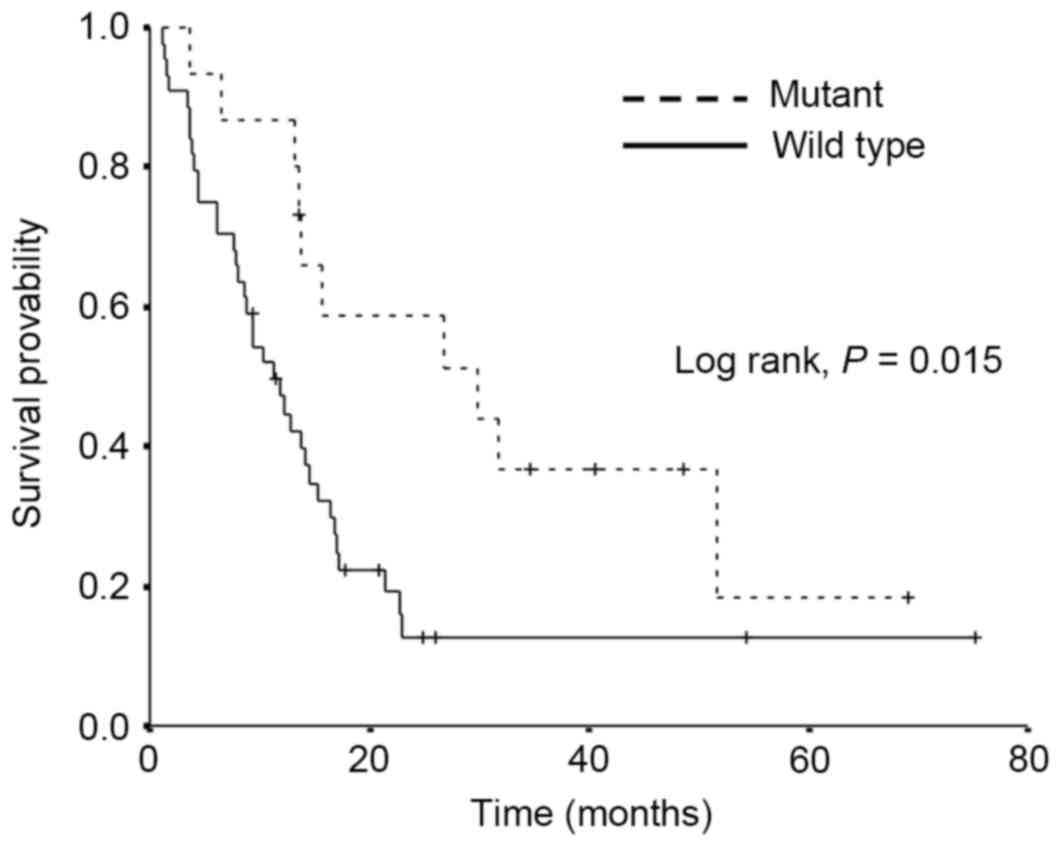
|
1
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pritchard RS and Anthony SP: Chemotherapy
plus radiotherapy compared with radiotherapy alone in the treatment
of locally advanced, unresectable, non-small-cell lung cancer. A
meta-analysis. Ann Intern Med. 125:723–729. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Furuse K, Fukuoka M, Kawahara M, Nishikawa
H, Takada Y, Kudoh S, Katagami N and Ariyoshi Y: Phase III study of
concurrent versus sequential thoracic radiotherapy in combination
with mitomycin, vindesine, and cisplatin in unresectable stage III
non-small-cell lung cancer. J Clin Oncol. 17:2692–2699. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Segawa Y, Kiura K, Takigawa N, Kamei H,
Harita S, Hiraki S, Watanabe Y, Sugimoto K, Shibayama T, Yonei T,
et al: Phase III trial comparing docetaxel and cisplatin
combination chemotherapy with mitomycin, vindesine, and cisplatin
combination chemotherapy with concurrent thoracic radiotherapy in
locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin
Oncol. 28:3299–3306. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto N, Nakagawa K, Nishimura Y,
Tsujino K, Satouchi M, Kudo S, Hida T, Kawahara M, Takeda K,
Katakami N, et al: Phase III study comparing second- and
third-generation regimens with concurrent thoracic radiotherapy in
patients with unresectable stage III non-small-cell lung cancer:
West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol.
28:3739–3745. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagai Y, Miyazawa H, Huqun, Tanaka T,
Udagawa K, Kato M, Fukuyama S, Yokote A, Kobayashi K, Kanazawa M
and Hagiwara K: Genetic heterogeneity of the epidermal growth
factor receptor in non-small cell lung cancer cell lines revealed
by a rapid and sensitive detection system, the peptide nucleic
acid-locked nucleic acid PCR clamp. Cancer Res. 65:7276–7282. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yatabe Y, Hida T, Horio Y, Kosaka T,
Takahashi T and Mitsudomi T: A rapid, sensitive assay to detect
EGFR mutation in small biopsy specimens from lung cancer. J Mol
Diagn. 8:335–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akamatsu H, Kaira K, Murakami H, Serizawa
M, Koh Y, Ono A, Shukuya T, Tsuya A, Nakamura Y, Kenmotsu H, et al:
The impact of clinical outcomes according to EGFR mutation status
in patients with locally advanced lung adenocarcinoma who recieved
concurrent chemoradiotherapy. Am J Clin Oncol. 37:144–147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yagishita S, Horinouchi H, Taniyama T
Katsui, Nakamichi S, Kitazono S, Mizugaki H, Kanda S, Fujiwara Y,
Nokihara H, Yamamoto N, et al: Epidermal growth factor receptor
mutation is associated with longer local control after definitive
chemoradiotherapy in patients with stage III nonsquamous
non-small-cell lung cancer. Int J Radiat Oncol Biol Phys.
91:140–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayashi H, Okamoto I, Kimura H, Sakai K,
Nishimura Y, Nishio K and Nakagawa K: Clinical outcomes of thoracic
radiotherapy for locally advanced NSCLC with EGFR mutations or
EML4-ALK rearrangement. Anticancer Res. 32:4533–4537.
2012.PubMed/NCBI
|
|
18
|
Tanaka K, Hida T, Oya Y, Oguri T, Yoshida
T, Shimizu J, Horio Y, Hata A, Kaji R, Fujita II, et al: EGFR
mutationimpact on definitiveconcurrent chemoradiation therapy for
inoperable stage III adenocarcinoma. J Thorac Oncol. Sep
2–2015.(Epub ahead of print). View Article : Google Scholar
|
|
19
|
Das AK, Sato M, Story MD, Peyton M, Graves
R, Redpath S, Girard L, Gazdar AF, Shay JW, Minna JD and Nirodi CS:
Non-small-cell lung cancers with kinase domain mutations in the
epidermal growth factor receptor are sensitive to ionizing
radiation. Cancer Res. 66:9601–9608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Das AK, Chen BP, Story MD, Sato M, Minna
JD, Chen DJ and Nirodi CS: Somatic mutations in the tyrosine kinase
domain of epidermal growth factor receptor (EGFR) abrogate
EGFR-mediated radioprotection in non-small cell lung carcinoma.
Cancer Res. 67:5267–5274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
She Y, Lee F, Chen J, Haimovitz-Friedman
A, Miller VA, Rusch VR, Kris MG and Sirotnak FM: The epidermal
growth factor receptor tyrosine kinase inhibitor ZD1839 selectively
potentiates radiation response of human tumors in nude mice, with a
marked improvement in therapeutic index. Clin Cancer Res.
9:3773–3778. 2003.PubMed/NCBI
|
|
22
|
Chinnaiyan P, Huang S, Vallabhaneni G,
Armstrong E, Varambally S, Tomlins SA, Chinnaiyan AM and Harari PM:
Mechanisms of enhanced radiation response following epidermal
growth factor receptor signaling inhibition by erlotinib (Tarceva).
Cancer Res. 65:3328–3335. 2005.PubMed/NCBI
|
|
23
|
Raben D, Helfrich B, Chan DC, Ciardiello
F, Zhao L, Franklin W, Barón AE, Zeng C, Johnson TK and Bunn PA Jr:
The effects of cetuximab alone and in combination with radiation
and/or chemotherapy in lung cancer. Clin Cancer Res. 11:795–805.
2005.PubMed/NCBI
|
|
24
|
Koh PK, Faivre-Finn C, Blackhall FH and De
Ruysscher D: Targeted agents in non-small cell lung cancer (NSCLC):
Clinical developments and rationale for the combination with
thoracic radiotherapy. Cancer Treat Rev. 38:626–640. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okamoto I, Takahashi T, Okamoto H,
Nakagawa K, Watanabe K, Nakamatsu K, Nishimura Y, Fukuoka M and
Yamamoto N: Single-agent gefitinib with concurrent radiotherapy for
locally advanced non-small cell lung cancer harboring mutations of
the epidermal growth factor receptor. Lung Cancer. 72:199–204.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Center B, Petty WJ, Ayala D, Hinson WH,
Lovato J, Capellari J, Oaks T, Miller AA and Blackstock AW: A phase
I study of gefitinib with concurrent dose-escalated weekly
docetaxel and conformal three-dimensional thoracic radiation
followed by consolidative docetaxel and maintenance gefitinib for
patients with stage III non-small cell lung cancer. J Thorac Oncol.
5:69–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rothschild S, Bucher SE, Bernier J,
Aebersold DM, Zouhair A, Ries G, Lombrieser N, Lippuner T, Lütolf
UM, Glanzmann C and Ciernik IF: Gefitinib in combination with
irradiation with or without cisplatin in patients with inoperable
stage III non-small cell lung cancer: A phase I trial. Int J Radiat
Oncol Biol Phys. 80:126–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ready N, Jänne PA, Bogart J, Dipetrillo T,
Garst J, Graziano S, Gu L, Wang X, Green MR and Vokes EE: Cancer,
Leukemia Group B, Chicago, IL: Chemoradiotherapy and gefitinib in
stage III non-small cell lung cancer with epidermal growth factor
receptor and KRAS mutation analysis: Cancer and leukemia group B
(CALEB) 30106, a CALGB-stratified phase II trial. J Thorac Oncol.
5:1382–1390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choong NW, Mauer AM, Haraf DJ, Lester E,
Hoffman PC, Kozloff M, Lin S, Dancey JE, Szeto L, Grushko T, et al:
Phase I trial of erlotinib-based multimodality therapy for
inoperable stage III non-small cell lung cancer. J Thorac Oncol.
3:1003–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|