Introduction
Colorectal cancer (CRC) is the third leading cause
of cancer-associated mortality in Japan (1), and the clinical outcomes of patients
with unresectable metastatic CRC are particularly poor. Although
novel anti-cancer agents, molecularly targeted drugs and surgical
procedures have improved the prognosis of unresectable metastatic
CRC, the clinical outcomes associated with unresectable metastatic
CRC remain unfavorable, with a median survival time of only ~30
months (2,3).
Adhesion molecules are involved in cell-to-cell
adhesion and cell-to-extracellular matrix (ECM) adhesion (4,5). The loss
of adhesion molecules in CRC is reported to have an important role
in the metastasis and invasion of tumors (4,6) and to be
associated with a poor clinical outcome (5–8). In
addition, the loss of adhesion molecules is reported to be
associated with resistance to chemotherapy (9).
E-cadherin serves a pivotal role in cell-to-cell
adhesion (10,11), and the loss of E-cadherin is
associated with tumor de-differentiation and metastasis, and,
therefore, poor clinical outcome (6).
Additionally, the loss of E-cadherin is associated with
chemotherapy resistance via numerous pathways, including the
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway and
the Wingless type (Wnt)/β-catenin pathway (12–15).
Cluster of differentiation (CD) 44, a type 1
transmembrane glycoprotein, is a receptor for hyaluronan (HA) and
has pathological and physiological roles in the homing of
lymphocytes, cell-to-ECM adhesion, tumor growth, angiogenesis, and
inflammation (5,16–21). The
decrease of cell-to-ECM adhesion caused by the loss of CD44 induces
tumor cell detachment from the basal membrane and the invasion of
cancer cells (8). Furthermore, loss
of CD44 expression is reported to be associated with chemotherapy
resistance and with a poor clinical outcome (6).
The focus of the present study was to evaluate the
significance of E-cadherin and CD44 expression, which have separate
roles in cellular adhesion, in unresectable metastatic CRC.
Materials and methods
Patient characteristics and
therapy
The characteristics of 49 patients with unresectable
metastatic CRC, who underwent surgery for the primary tumor at the
Department of Surgical Oncology, Osaka City University (Osaka,
Japan) between April 2005 and December 2013, were retrospectively
reviewed. The median follow-up period was 26.7 months (range,
5.8–63.2 months). All of the patients underwent first-line
combination chemotherapy with oxaliplatin (OX) or irinotecan (IRI)
+ 5-fluorouracil (5-FU)/leucovorin (LV), or a prodrug of 5-FU,
which is converted to 5-fluorouracil (5-FU) in vivo to exert
antitumor activity, such as S-1 and capecitabine. The chemotherapy
regimens that were administered were as follows: 5-FU/LV+OX
(FOLFOX; n=30), 5-FU/LV+IRI (FOLFIRI; n=6), capecitabine+OX
(CapeOX; n=12), and S-1+OX (SOX; n=1). In total, 21 patients
underwent chemotherapy combined with a molecularly targeted agent.
Written informed consent was obtained from the patients for
participation, and the Ethics Committee of Osaka City University
approved the current study protocol. The investigation was
conducted according to the principles expressed in the Declaration
of Helsinki. All patients were followed up until May 2015 or until
the date of their mortality.
Antibodies
Commercially available monoclonal antibodies were
selected. Mouse anti-human E-cadherin (catalog no., M106; 2 µg/ml)
was purchased from Takara Bio, Inc. (Otsu, Japan), and mouse
anti-human CD44 (catalog no., M708201-2; dilution, 1:50) was
purchased from Dako (Agilent Technologies, Inc., Santa Clara, CA,
USA).
Immunohistochemistry
All tissue specimens were fixed in 10% buffered
formalin and embedded in paraffin. Immunohistochemical staining for
E-cadherin and CD44 was performed on 4-µm-thick sections of each of
the CRC tissue samples. The slides were deparaffinized in xylene
and rehydrated in decreasing concentrations of ethanol.
Immunohistochemical staining was performed as previously described
(22). Briefly, the sections were
subjected to endogenous peroxidase blocking in 1%
H2O2 solution in methanol for 15 min. Antigen
retrieval was performed by autoclaving the sections at 105°C for 15
min in Dako Target Retrieval solution (Dako; Agilent Technologies,
Inc. Santa Clara, CA, USA). Serum blocking was performed with 10%
normal rabbit serum for 10 min. Following
H2O2 and serum blocking, the slides were
incubated with the primary antibody at 4°C overnight. The secondary
antibody was a biotin-labeled rabbit anti-mouse IgG + IgA + IgM
(Nichirei Biosciences, Inc., Tokyo, Japan; dilution, 1:500). Normal
rabbit serum, a biotin-labeled rabbit anti-mouse antibody and
peroxidase-labeled streptavidin, were used, which are included in
the Histfine SAB-PO(M) kit (catalog no., 424032; Nichirei
Biosciences, Inc., Tokyo, Japan.) according to the manufacturer's
protocol. Detection was performed with a 3,3′-diaminobenzidine
tetrahydrochloride kit (Histofine Simple Stain kit; catalog no.,
415174; Nichirei Biosciences, Inc., Tokyo, Japan). The sections
were counterstained with hematoxylin, dehydrated, cleared and
mounted on glass coverslips. Sections in which the primary
antibodies were absent were used as negative controls.
Evaluation
First, to determine the tumor area, the entire
section was surveyed with a low-magnification objective lens.
Subsequently, three locations within the selected tumor area were
evaluated with a ×200 lens with BX43 (Olympus Coporation, Tokyo,
Japan); the three microscopic fields were randomly selected to
calculate the mean number of positively stained cells. The
membranous staining was focused on to assess the expression levels
of E-cadherin and CD44. With regard to E-cadherin expression,
tissues in which <25% of the cells were stained or in which
there was an absence of staining were assigned to the low
expression group, whilst tissues in which ≥25% of the cells were
stained were assigned to the high expression group (Fig. 1) (6).
With regard to CD44 expression, tissues in which
<10% of the cells were stained or in which there was an absence
of staining were assigned to the low expression group, whilst
tissues in which ≥10% of the cells were assigned to the expression
group (Fig. 2) (23).
The staining intensity was disregarded. Two
pathologists who were blinded to the clinicopathological or
survival data of the patients at the time of the analysis,
evaluated the data. If the observers reported different results,
they reviewed the slides by microscope until a consensus was
reached.
Statistical analysis
The statistical differences between the groups were
analyzed using the χ2 test, Fisher's exact test and
Student's t-test. The duration of survival was calculated using the
Kaplan-Meier method. Differences in the survival curves were
assessed using the log-rank test. A multivariate analysis of the
associations between clinicopathological characteristics and
survival was performed using a Cox proportional hazards model. JMP
software version 12 (SAS Institute, Inc., Cary, NC, USA) was used
for all of the statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical characteristics
The clinicopathological characteristics of the
patients are presented in Table I.
The study population consisted of 26 male and 23 female patients
with a median age of 63 years (range, 40–80 years). In total, 32
patients had colon cancer and 17 patients had rectal cancer. There
were 44 patients with low-grade tumors (including
well-differentiated or moderately differentiated adenocarcinomas),
and the 5 remaining patients had high-grade tumors (including
poorly-differentiated or mucinous adenocarcinomas). With regard to
metastases, 38 patients had liver metastases, 14 had lung
metastases, 14 had peritoneal disseminations and 14 had distant
lymph node metastases. The site of metastasis was a single organ in
28 patients, two organs in 16 patients and three organs in 5
patients.
 | Table I.Clinicopathological characteristics
of the patients. |
Table I.
Clinicopathological characteristics
of the patients.
| Clinicopathological
characteristics | n=49 |
|---|
| Sex,
male:female | 26:23 |
| Age, years, median
(range) | 63 (40–80) |
| Location,
colon:rectum | 32:17 |
| Differentiation,
well + moderately: mucinous + poorly | 44:5 |
| Tumor depth,
T1-3:T4 | 24:25 |
| Lymph node
metastasis, negative:positive | 6:43 |
| Lymph vessel
invasion, negative: positive: unknown | 4:41:4 |
| Venous invasion,
negative:positive:unknown | 23:22:4 |
| Liver metastasis,
negative:positive | 11:38 |
| Lung metastasis,
negative:positive | 35:14 |
| Peritoneal
dissemination, negative:positive | 35:14 |
| No. of organs
affected by metastasis, 1:≥2 | 28:21 |
| CD44,
negative:positive | 14:35 |
| E-cadherin,
negative:positive | 8:41 |
| Chemotherapy,
FOLFOX+CapeOX+ | 43:6 |
| SOX:FOLFIRI |
| Molecular targeted
agent, negative:positive | 18:31 |
Associations between the expression of
E-cadherin/CD44 and clinicopathological characteristics
The expression of E-cadherin alone was not
significantly associated with any of the clinicopathological
factors (Table II). The expression
of CD44 was only significantly associated with sex (P=0.0202;
Table II).
 | Table II.Associations between the adhesion
molecules and the clinical backgrounds of the patients. |
Table II.
Associations between the adhesion
molecules and the clinical backgrounds of the patients.
|
| CD44
expression | E-cadherin
expression |
|---|
|
|
|
|
|---|
|
Characteristics | High n=35 | Low n=14 | P-value | High n=41 | Low n=8 | P-value |
|---|
| Sex |
|
| 0.0202 |
|
| 0.5587 |
|
Male | 20 | 3 |
| 21 | 5 |
|
|
Female | 15 | 11 |
| 20 | 3 |
|
| Age, years, median
(range) | 63.0 (40–80) | 64.5 (48–80) | 0.3105 | 64.0 (40–80) | 61.0 (53–72) | 0.8939 |
| Location |
|
| 0.2055 |
|
| 0.1494 |
|
Colon | 21 | 11 |
| 25 | 7 |
|
|
Rectum | 14 | 3 |
| 16 | 1 |
|
| Tumor invasion |
|
| 0.0707 |
|
| 0.4777 |
|
T1-3 | 20 | 4 |
| 21 | 3 |
|
| T4 | 15 | 10 |
| 20 | 5 |
|
| Histology |
|
| 0.5505 |
|
| 0.8146 |
| Well +
moderately | 32 | 12 |
| 37 | 7 |
|
| Poorly
+ mucinous | 3 | 2 |
| 4 | 1 |
|
| Lymphatic vessel
invasion |
|
| 0.1817 |
|
| 0.3299 |
|
Negative | 4 | 0 |
| 4 | 0 |
|
|
Positive | 28 | 13 |
| 33 | 8 |
|
| Blood vessel
invasion |
|
| 0.2793 |
|
| 0.3957 |
|
Negative | 18 | 5 |
| 20 | 3 |
|
|
Positive | 14 | 8 |
| 17 | 5 |
|
| Lymph node
metastasis |
|
| 0.7127 |
|
| 0.2416 |
|
Negative | 4 | 2 |
| 4 | 2 |
|
|
Positive | 31 | 11 |
| 36 | 6 |
|
| Liver
metastasis |
|
| 0.9135 |
|
| 0.8501 |
|
Negative | 8 | 3 |
| 9 | 2 |
|
Positive | 27 | 11 |
| 32 | 6 |
|
| Lung
metastasis |
|
| 0.4839 |
|
| 0.5411 |
|
Negative | 24 | 11 |
| 30 | 5 |
|
|
Positive | 11 | 3 |
| 11 | 3 |
|
| Peritoneal
dissemination |
|
| 0.1615 |
|
| 0.2713 |
|
Negative | 27 | 8 |
| 28 | 7 |
|
|
Positive | 8 | 6 |
| 13 | 1 |
|
| Number of organs
affected |
|
| 1.0000 |
|
| 0.7378 |
| by metastasis |
| 1 | 20 | 8 |
| 23 | 5 |
|
| ≥2 | 15 | 6 |
| 18 | 3 |
|
Associations between the expression of
E-cadherin/CD44 and the efficacy of chemotherapy
The low expression of E-cadherin was significantly
associated with a lower objective response rate (ORR; P=0.0491),
but it did not correlate with a lower disease control rate (DCR) to
a significant extent (P=0.3438; Table
III). CD44 expression did not correlate with the efficacy of
chemotherapy (Table III). The
patients were categorized into four groups according to combination
of E-cadherin and CD44 expression: Group 1, high expression of
E-cadherin and CD44 (n=29); Group 2, low expression of E-cadherin
and high expression of CD44 (n=5); Group 3, high expression of
E-cadherin and low expression of CD44 (n=12); and Group 4, low
expression of E-cadherin and CD44 (n=3). Patients were further
categorized into two groups: Group A consisted of all of the
patients in Groups 1, 2 and 3, and Group B consisted of the
patients in Group 4. The ORRs of Groups A and B were 71.7 and 0%,
respectively. Additionally, the DCRs of Groups A and B were 89.1
and 33.3%, respectively (Table
III). The ORRs and DCRs of the patients in Group A were
significantly higher compared with those in Group B (P=0.0076 and
P=0.0294, respectively; Table
III).
 | Table III.Effects of chemotherapy and CD44 and
E-cadherin expression levels. |
Table III.
Effects of chemotherapy and CD44 and
E-cadherin expression levels.
|
| CD44
expression | E-cadherin
expression | Combination of
E-cadherin and CD44 expression |
|---|
|
|
|
|
|
|---|
| Response | High n=35 | Low n=14 | P-value | High n=41 | Low n=8 | P-value | Other n=46 |
E-cadherin(−)/CD44(−) n=3 | P-value |
|---|
| Complete response,
n | 2 | 0 |
| 2 | 0 |
| 2 | 0 |
|
| Partial response,
n | 23 | 8 |
| 28 | 3 |
| 31 | 0 |
|
| Stable disease,
n | 6 | 3 |
| 6 | 3 |
| 8 | 1 |
|
| Progressive
disease, n | 4 | 3 |
| 5 | 2 |
| 5 | 2 |
|
| ORR, % | 71.4 | 57.1 | 0.3412 | 73.2 | 37.5 | 0.0491 | 71.7 | 0.0 | 0.0076 |
| DCR, % | 88.6 | 78.6 | 0.3813 | 87.8 | 75.0 | 0.3438 | 89.1 | 33.3 | 0.0294 |
It has been previously demonstrated that molecularly
targeted agents may improve the survival of these patients
(2,3).
The number of patients who underwent chemotherapy combined with a
molecularly targeted agent in Group A was 20 (43.5%), while in
Group B it was 1 (33.3%). However, this difference was not
statistically significant (P=0.7277).
Survival analysis according to the
expression of E-cadherin and CD44
The expression of E-cadherin was not significantly
associated with progression-free survival (PFS; P=0.2825; Fig. 3A), or overall survival (OS; P=0.6617;
Fig. 3B). The expression of CD44 was
not significantly associated with PFS (P=0.4365; Fig. 4A), however, it tended
(non-significantly) to correlate with OS (P=0.0699; Fig. 4B).
Survival analysis according to the
combination of E-cadherin and CD44 expression
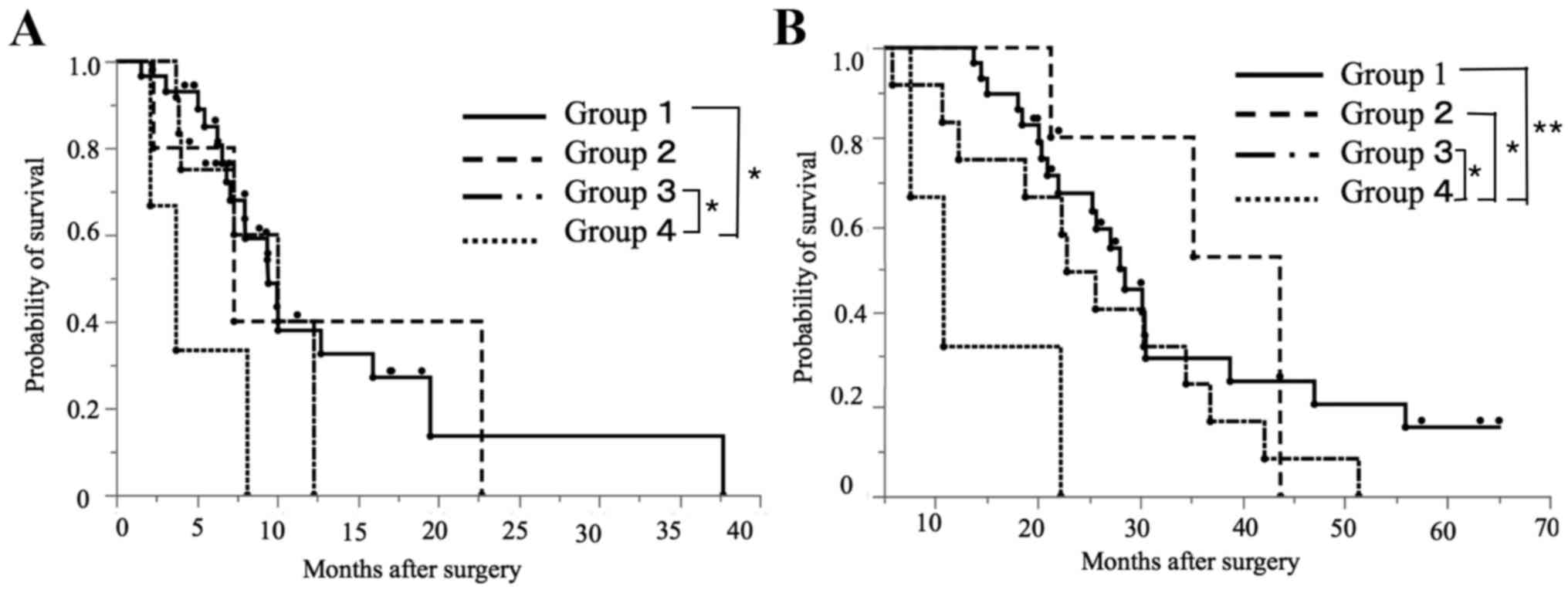
Group 4 was associated with decreased PFS in
comparison with Group 1 (P=0.0126) and Group 3 (P=0.0317; Fig. 5A). Group 4 was associated with
significantly reduced OS compared with Groups 1 (P=0.0011), 2
(P=0.0279) and 3 (P=0.0352; Fig. 5B).
The PFS and OS rates of the patients in Group B were significantly
reduced compared with those of the patients in Group A (P=0.0101
and P=0.0009, respectively; Fig.
6).
The correlations between the clinicopathological
characteristics and prognosis were then examined. In the univariate
analysis, sex (P=0.0333) and the combination of E-cadherin and CD44
expression (P=0.0474) were identified to be significantly
associated with PFS (Table IV). When
multivariate analysis was performed, the peritoneal dissemination
and the number of organs affected by metastasis, which are
established prognostic factors, were added as covariates (24,25). A
multivariate analysis demonstrated that the combination of
E-cadherin and CD44 expression was the only independent risk factor
for PFS [hazard ratio (HR), 8.276, 95% confidence interval (CI),
1.383–43.311; P=0.0227; Table
IV].
 | Table IV.Results of the univariate and
multivariate analyses of prognostic factors for progression-free
survival. |
Table IV.
Results of the univariate and
multivariate analyses of prognostic factors for progression-free
survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex, male vs.
female | 2.336 | 1.069–5.265 | 0.0333 | 1.952 | 0.817–4.715 | 0.1309 |
| Age, ≥65 vs. <65
years | 0.826 | 0.382–1.730 | 0.6139 |
|
|
|
| Location, rectum
vs. colon | 0.739 | 0.306–1.620 | 0.4626 |
|
|
|
| Tumor invasion, T4
vs. T1-3 | 1.464 | 0.704–3.195 | 0.3111 |
|
|
|
| Histology, mucinous
+ poorly vs. well + moderately | 0.670 | 0.156–1.966 | 0.5010 |
|
|
|
| Lymphatic vessel
invasion, positive vs. negative | 3.094 | 0.603–57.148 | 0.2082 |
|
|
|
| Blood vessel
invasion, positive vs. negative | 0.677 | 0.677–1.508 | 0.3368 |
|
|
|
| Lymph node
metastasis, ≥N2 vs. N0+1 | 0.755 | 0.366–1.560 | 0.4446 |
|
|
|
| Liver metastasis,
positive vs. negative | 1.228 | 0.531–3.341 | 0.6492 |
|
|
|
| Lung metastasis,
positive vs. negative | 1.210 | 0.540–2.542 | 0.6285 |
|
|
|
| Peritoneal
dissemination, positive vs. negative | 0.990 | 0.438–2.099 | 0.9800 | 1.507 | 0.540–4.283 | 0.4329 |
| CD44/E-cadherin
expression, Group 4 vs. Groups 1–3 | 4.405 | 1.020–13.324 | 0.0474 | 8.276 | 1.383–43.311 | 0.0227 |
| No. of organs
affected by metastasis, ≥2 vs. 1 | 1.074 | 0.497–2.313 | 0.8542 | 0.929 | 0.326–2.500 | 0.8860 |
According to a univariate analysis, the combination
of E-cadherin and CD44 expression was significantly associated with
the OS (P=0.0177; Table V). When
multivariate analysis was performed, the peritoneal dissemination
and the number of organs affected by metastases, which are
established prognostic factors, were added as covariates. The
multivariate analysis demonstrated that the combination of
E-cadherin and CD44 expression was an independent risk factor for
OS (HR, 15.118; 95% CI, 2.645–77.490; P=0.0039; Table V).
 | Table V.Results of the univariate and
multivariate analyses of prognostic factors affecting the overall
survival. |
Table V.
Results of the univariate and
multivariate analyses of prognostic factors affecting the overall
survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex, male vs.
female | 1.621 | 0.327–1.159 | 0.1325 |
|
|
|
| Age, ≥65 vs. <65
years | 1.120 | 0.622–0.253 | 0.5787 |
|
|
|
| Location, rectum
vs. colon | 0.887 | 0.446–1.691 | 0.7210 |
|
|
|
| Tumor invasion, T4
vs. T1-3 | 1.794 | 0.947–3.463 | 0.0730 |
|
|
|
| Histology, mucinous
+ poorly vs. well + moderately | 0.742 | 0.178–2.070 | 0.6065 |
|
|
|
| Lymphatic vessel
invasion, positive vs. negative | 2.414 | 0.729–14.946 | 0.2118 |
|
|
|
| Blood vessel
invasion, positive vs. negative | 0.968 | 0.506–1.840 | 0.9210 |
|
|
|
| Lymph node
metastasis, ≥N2 vs. N0+1 | 1.376 | 0.732–2.625 | 0.3215 |
|
|
|
| Liver metastasis,
positive vs. negative | 1.142 | 0.548–2.684 | 0.7365 |
|
|
|
| Lung metastasis,
positive vs. negative | 1.220 | 0.582–2.377 | 0.0581 |
|
|
|
| Peritoneal
dissemination, positive vs. negative | 0.939 | 0.458–1.811 | 0.8546 | 1.055 | 0.469–2.265 | 0.8939 |
| CD44/E-cadherin
expression, Group 4 vs. Groups 1–3 | 6.423 | 1.468–19.953 | 0.0177 | 15.118 | 2.645–77.490 | 0.0039 |
| No. of organs
affected by metastasis, ≥2 vs. 1 | 1.281 | 0.677–2.410 | 0.4422 | 1.367 | 0.659–2.805 | 0.3971 |
Discussion
The current study has demonstrated that the combined
loss of E-cadherin and CD44 expression is associated with the
reduced efficacy of chemotherapy and also decreased survival rate.
The cadherin family is one comprised of cell-to-cell adhesion
molecules, whilst CD44 is a cell-to-ECM adhesion molecule (21). The loss of cell adhesion molecules is
reported to be associated with the metastasis and invasion of CRC,
since the activation of cell motility and detachment from other
cells, the stroma and the ECM represent the biological basis of
metastasis and invasion (26–29). The downregulation of cell-to-cell
adhesion reduces the maintenance of cell shape and polarity
(30) and increases cellular motility
and migration (30). The loss of
cell-to-ECM adhesion induces cellular detachment from the basal
membrane, the ECM and connective tissue (8,5). This is
the hypothesis as to why the loss of the adhesion molecules is
associated with tumor progression (6,7).
Additionally, the loss of adhesion molecules is also reported to be
associated with resistance to chemotherapy (9).
E-cadherin is a transmembrane glycoprotein that is
required for calcium-dependent cell-to-cell adhesion in the
formation of adherens junctions (4,31,32). The hypothesized underlying mechanism
for the association between E-cadherin loss and chemotherapy
resistance is as follows: Cadherin switching [the alteration from
E-cadherin to neural (N)-cadherin] occurs in tumors; N-cadherin
subsequently activates the PI3K/Akt pathway (12); and the PI3K/Akt pathway induces
chemotherapy resistance by decreasing apoptosis and increasing
proliferation (13,14). In addition, the loss of E-cadherin
induces an increase in the levels of cytoplasmic β-catenin
(15), which is then relocated into
the nuclei, where it activates the Wnt/β-catenin pathway (15); the Wnt/β-catenin pathway is associated
with chemotherapy resistance through the maintenance and
proliferation of cancer stem cells (15).
As the loss of E-cadherin induces an increase in
cellular motility and loss of cell-to-cell adhesion, E-cadherin is
involved in tumor budding and facilitates invasion (33). Therefore, it is associated with a poor
clinical outcome (34). Consistently,
the present study identified that E-cadherin expression was
associated with the objective response to chemotherapy. Therefore,
although E-cadherin expression was not correlated with survival
(possibly due to the influence of numerous clinical factors on the
survival of patients with unresectable metastatic CRC), the current
study demonstrated that E-cadherin expression is associated with
the response to chemotherapy.
CD44, which is a class 1 transmembrane glycoprotein,
has important roles in lymphocyte homing, cell proliferation,
angiogenesis, inflammation and motility (5,16–21), and also serves an vital role in
cell-to-ECM adhesion in association with HA and glycosaminoglycans
(35,36). As cancer cells are connected with the
stroma or basal membrane by CD44, which is a receptor for HA, the
loss of CD44 induces detachment from the basal membrane and allows
tumor cell invasion (8). Since the
loss of CD44 leads to metastasis and invasion, it is associated
with decreased survival time (6). In
a previous study, the loss of CD44 in the tumor cells and clusters
at the invasive periphery of tumor tissue was associated with
chemotherapy resistance (9). The
current study failed to confirm the initial hypothesis that the
loss of CD44 expression may be associated with the efficacy of
chemotherapy. Thus, it is possible that the influence of CD44 on
chemotherapy resistance is minor, and that it contributes to the
decrease in OS via the aforementioned mechanism of promoting
invasion and metastasis.
In the present study, the individual losses of
E-cadherin and CD44 expression, which are reported to be poor
prognostic factors for patients who undergo curative surgery, were
not prognostic factors for patients with unresectable metastatic
CRC. However, there was a significant association between the
combined loss of E-cadherin and CD44 expression and a poor clinical
outcome. Although the mechanism remains to be elucidated, Ngan
et al (6) reported that
E-cadherin and CD44 may have an interdependent role in sustaining
the adhesive function, inhibiting invasion and metastasis, and
promoting chemotherapy resistance.
The current study has certain limitations as it was
retrospective in nature and included a small number of patients.
Furthermore, certain previous studies on E-cadherin and CD44
identified that neither E-cadherin nor CD44 correlated with
survival (37–40). The inconsistent results regarding the
importance of E-cadherin and CD44 expression as prognostic factors
may be the result of differing experimental methods, threshold
values for the expression of E-cadherin or CD44, or the evaluation
of immunoexpression. Additionally, the diversity in patient
backgrounds and the chemotherapies that are administered in
patients with unresectable metastatic CRC, may have led to results
that differed from the original hypothesis of the current
study.
Although these data are based on an analysis of 3
patients in whom the expression of E-cadherin and CD44 was low, the
prognosis of Group B was identified to be poorer compared with that
of Group A in the present study. As the present study included
patients who had distant metastasis and who underwent surgical
resection of their primary tumor, the number of eligible patients
was small. In addition to this, as the significance of the
E-cadherin and CD44 expression were evaluated with regard to OS and
also the efficacy of chemotherapy, only patients who underwent
combination chemotherapy as first-line chemotherapy were selected.
As a consequence, the sample size was decreased. Further studies,
including prospective studies with a large study population, are
required to confirm whether the combined low expression of
E-cadherin and CD44 may be an independent predictor of prognosis in
patients with unresectable metastatic CRC, as indicated by the
results of the current study.
In conclusion, the present study demonstrated that
the combination of E-cadherin and CD44 expression may be an
effective prognostic factor for estimating the survival and
chemotherapeutic outcome of patients with unresectable metastatic
CRC. Further studies are required to confirm these results.
Acknowledgements
The authors would like to thank Brian Quinn who
provided medical writing services on behalf of JMC, Ltd.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
ECM
|
extra cellular matrix
|
|
HA
|
hyaluronan
|
|
OX
|
oxaliplatin
|
|
IRI
|
irinotecan
|
|
5-FU
|
5-fluorouracil
|
|
LV
|
leucovorin
|
|
FOLFOX
|
5-FU/LV+OX
|
|
FOLFIRI
|
5-FU/LV+IRI
|
|
CapeOX
|
capecitabine+OX
|
|
SOX
|
S-1+OX
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
HR
|
hazard ratio
|
|
95% CI
|
95% confidence interval
|
References
|
1
|
Matsuda A, Matsuda T, Shibata A, Katanoda
K, Sobue T and Nishimoto H; Japan Cancer Surveillance Research
Group, : Cancer incidence and incidence rates in Japan in 2008: A
study of 25 population-based cancer registries for the monitoring
of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol.
44:388–396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Loupakis F, Cremolini C, Masi G, Lonardi
S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi
R, et al: Initial therapy with FOLFOXIRI and bevacizumab for
metastatic colorectal cancer. N Engl J Med. 371:1609–1618. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beavon IR: The E-cadherin-catenin complex
in tumour metastasis: Structure, function and reglation. Eur J
Cancer. 36:1607–1620. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ponta H, Sherman L and Herrlich PA: CD44:
From adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ngan CY, Yamamoto H, Seshimo I, Ezumi K,
Terayama M, Hemmi H, Takemasa I, Ikeda M, Sekimoto M and Monden M:
A Multivariate analysis of adhesion molecules expression in
assessment of colorectal cancer. J Surg Oncol. 95:652–662. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lugli A, Lezzi G, Hostettler I, Murano MG,
Mele V, Tornillo L, Carafa V, Spagnoli G, Terracciano L and Zlobec
I: Prognostic impact of the expression of putative cancer stem cell
markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer.
Br J Cancer. 103:382–390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugino T, Gorham H, Yoshida K, Bolodeoku
J, Nargund V, Cranston D, Goodison S and Tarin D: Progressive loss
of CD44 gene expression in invasive bladder cancer. Am J Pathol.
149:873–882. 1996.PubMed/NCBI
|
|
9
|
Bhangu A, Wood G, Beown G, Darzi A, Tekkis
P and Goldin R: The role of epithelial mesenchymeal transition and
resistance to neoadjuvant therapy in localy advanced rectal cancer.
Colorectal Dis. 16:O133–O143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dorudi S, Sheffield JP, Pouulsom R,
Northover JM and Hart IR: E-cadherin expression in colorectal
cancer: An immunetochemical and in situ hybridization study. Am J
Pathol. 142:981–986. 1993.PubMed/NCBI
|
|
11
|
Ghadimi BM, Behrens J, Hoffman I, Haensch
W, Birchmeier W and Schlag PM: Immunohistological analysis of
E-cadherin, alpha-, beta- and gamma-catenin expression in
colorectal cancer: Implications for cell adhesion and signaling.
Eur J Cancer. 35:60–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wheelock MJ, Shintani Y, Maeda M, Fukumoto
Y and Johnson KR: Cadherin switching. J Cell Sci. 121:727–735.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shintani Y, Maeda M, Chaika N, Johnson KR
and Wheelock MJ: Collagen I promotes epithelial-to-mesenchymal
transition in lung cancer cells via transforming growth-factor
signaling. Am J Respir Cell Mol Biol. 38:95–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suyama K, Shapiro I, Guttman M and Hazan
RB: A signaling pathway leading to metastasis is controlled by
N-cadherin and the FGF receptor. Cancer Cell. 2:301–314. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanwar SS, Yu Y, Nautiyal J, Patel BB and
Majumdar AP: The Wnt/beta-catenin pathway regulates growth and
maintenance of colonospheres. Mol Cancer. 9:2122010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyake K, Underhill CB, Lesley J and
Kincade PW: Hyaluronate can function as a cell adhesion molecule
and CD44 participates in hyaluronate recognition. J Exp Ned.
172:69–75. 1990. View Article : Google Scholar
|
|
17
|
Aruffo A, Stamenkovic I, Melnick M,
Underhill CB and Seed B: CD44 is the principal cell surface
receptor for hyaluronate. Cell. 61:1303–1313. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dougherty GJ, Lansdorp PM, Cooper DL and
Humphries RK: Molecular cloning of CD44R1 and CD44R2, two novel
isoforms of the human CD44 lymphocyte ‘homing’ receptor expressed
by hemopoietic cells. J Exp Med. 174:1–5. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldstein LA, Zhou DF, Picker LJ, Minty
CN, Bargatze RF, Ding JF and Butcher EC: A human lymphocyte homin
receptor, the hermes antigen, is related to cartel proteoglycan
core anf link proteins. Cell. 56:1063–1072. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stamenkovic I, Amiot M, Pesando JM and
Seed B: A lymphocyte molecule implicated in lymph node homing is a
member of the cartel link protein family. Cell. 56:1057–1062. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marhaba R and Zöller M: CD44 and in cancer
progression: Adhesion, migration and growth regulation. J Mol
Histol. 35:211–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sugano K, Maeda K, Ohtani H, Nagahara H,
Shibutani M and Hirakawa K: Expression of xCT as a predictor of
disease recurrence in patients with colorectal cancer. Anticancer
Res. 35:677–682. 2015.PubMed/NCBI
|
|
23
|
Al-Maghrabi J, Gomaa W, Buhmeida A,
Al-Qahtani M and Al-Ahwal M: Decreased immunoexpression of standard
form of CD44 is an independent favourable predictor of nodal
metastasis in colorectal carcinoma. Anticancer Res. 32:3455–3461.
2012.PubMed/NCBI
|
|
24
|
Jayne DG, Fook S, Loi C and Seow-Choen F:
Peritoneal carcinomatosis from colorectal cancer. Br J Surg.
89:1545–1550. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi H, Kotake K and Sugihara K:
Prognostic scoring system for stage IV colorectal cancer: Is the
AJCC sub-classification of stage IV colorectal cancer appropriate?
Int J Clin Oncol. 18:696–703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barker N and Clevers H: Tumor environment:
A potent driving force in colorectal cancer? Trends Mol Med.
7:535–537. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hur K, Toiyama Y, Takahashi M, Balaguer F,
Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR and Goel A:
MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT)
in human colorectal cancer metastasis. Gut. 62:1315–1326. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bates RC: Colorectal cancer progression:
Integrin alphavbeta6 and the epithelial-mesenchymal transition
(EMT). Cell Cycle. 4:1350–1352. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brabletz T, Hlubek F, Spaderna S,
Schmalhofer O, Hiendlmeyer E, Jung A and Kirchner T: Invasion and
metastasis in colorectal cancer: Epithelial-mesenchymal transition,
mesenchymal-epithelial transition, stem cells and beta-catenin.
Cells Tissues Organs. 179:56–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arias AM: Epithelial mesenchymal
interactions in cancer and development. Cell. 105:425–431. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal state: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wijnhoven BP, Dinjens WN and Pignatelli M:
E-cadherin-catenin cell-cell adhesion complex and human cancer. Br
J Surg. 87:992–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zlobec I, Lugli A, Baker K, Roth S, Minoo
P, Hayashi S, Terracciano L and Jass JR: Role of APAF-1, E-cadherin
and peritumoral lymphocytic infiltration in tumour budding in
colorectal cancer. J Pathol. 212:260–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He X, Chen Z, Jia M and Zhao X:
Downregulated E-cadherin expression indicates worse prognosis in
Asian patients with colorectal cancer: Evidence from meta-analysis.
PLoS One. 8:e708582013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sleeman J, Kondo K, Moll J, Ponta H and
Herrlich P: Variant exon v6 and v7 together expand the repertoire
of glycolsaminoglycans bound by CD44. J Biol Chem. 272:31837–31844.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang AD, Fan F, Camp ER, van Buren G, Liu
W, Somcio R, Gray MJ, Cheng H, Hoff PM and Ellis LM: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mulder JW, Kruyt PM, Sewnath M, Oosting J,
Seldenrijk CA, Weidema WF, Offerhaus GJ and Pals ST: Colorectal
cancer prognosis and expression of exon-v6-containing CD44
proteins. Lancet. 344:1470–1472. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morrin M and Delaney PV: CD44v6 is not
relevant in colorectal tumour progression. Int J Colorectal Dis.
17:30–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stanczak A, Stec R, Bodnar L, Olszewski W,
Cichowicz M, Kozlowski W, Szczylik C, Pietrucha T, Wieczorek M and
Lamparska-Przybysz M: Prognostic significance of Wnt-1, β-catenin
and E-cadherin expression in advanced colorectal carcinoma. Pathol
Oncol Res. 17:955–963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ilyas M, Novelli M, Wilkinson K, Tomlinson
IP, Abbasi AM, Forbes A and Talbot IC: Tumour recurrence is
associated with Jass grouping but not with differences in
E-cadherin expression in moderately differentiated Dukes' B
colorectal cancers. J Clin Pathol. 50:218–222. 1997. View Article : Google Scholar : PubMed/NCBI
|