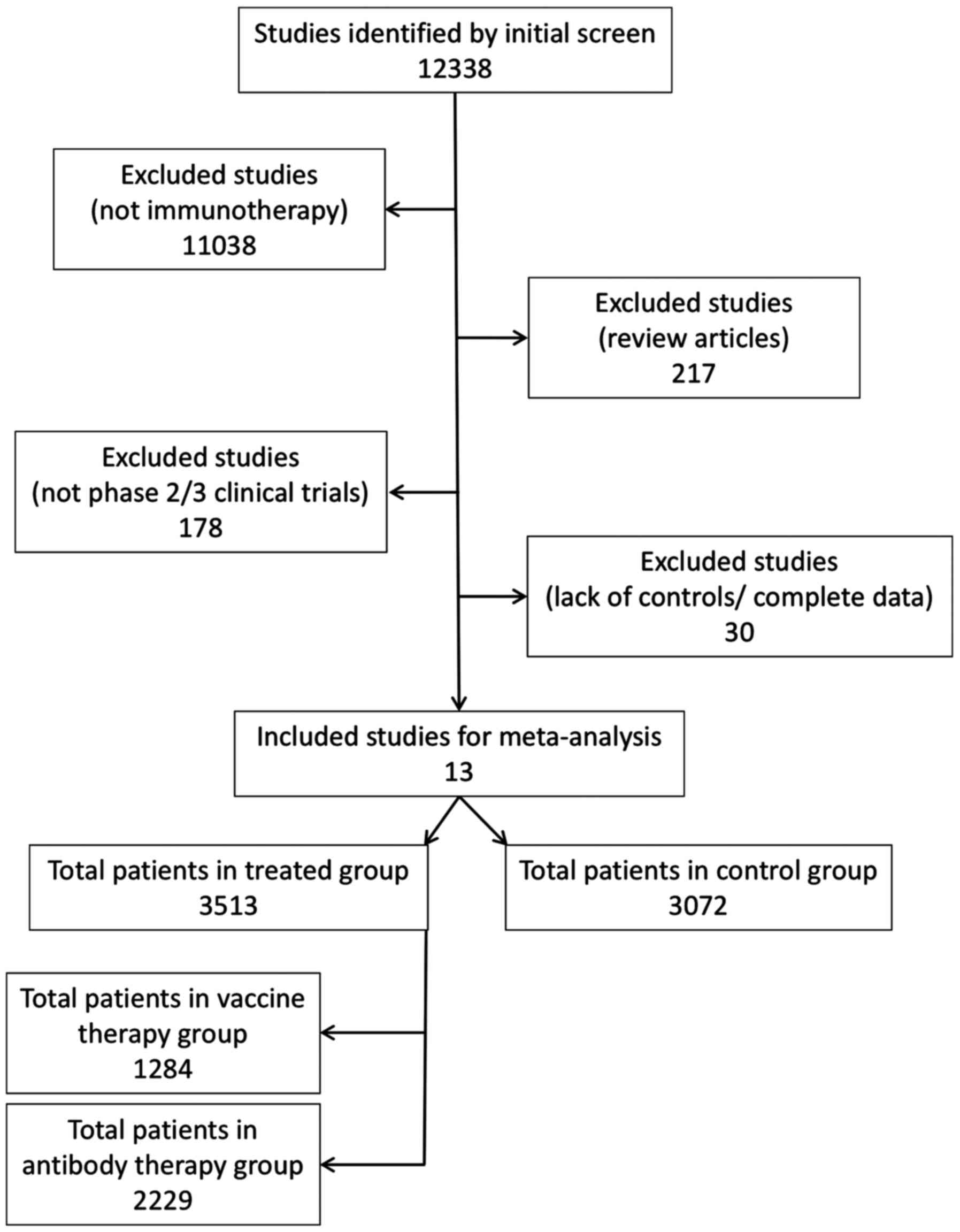
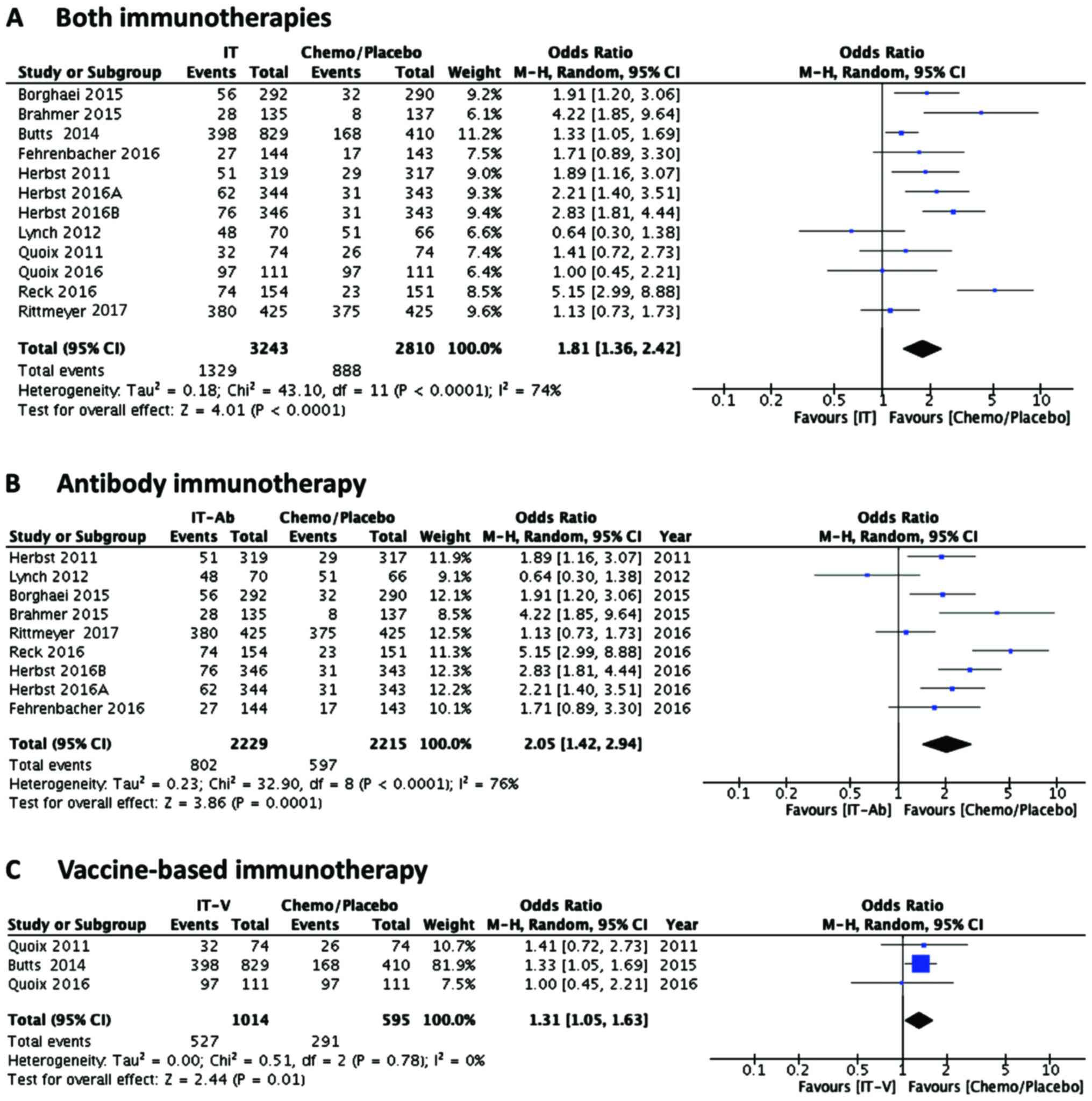
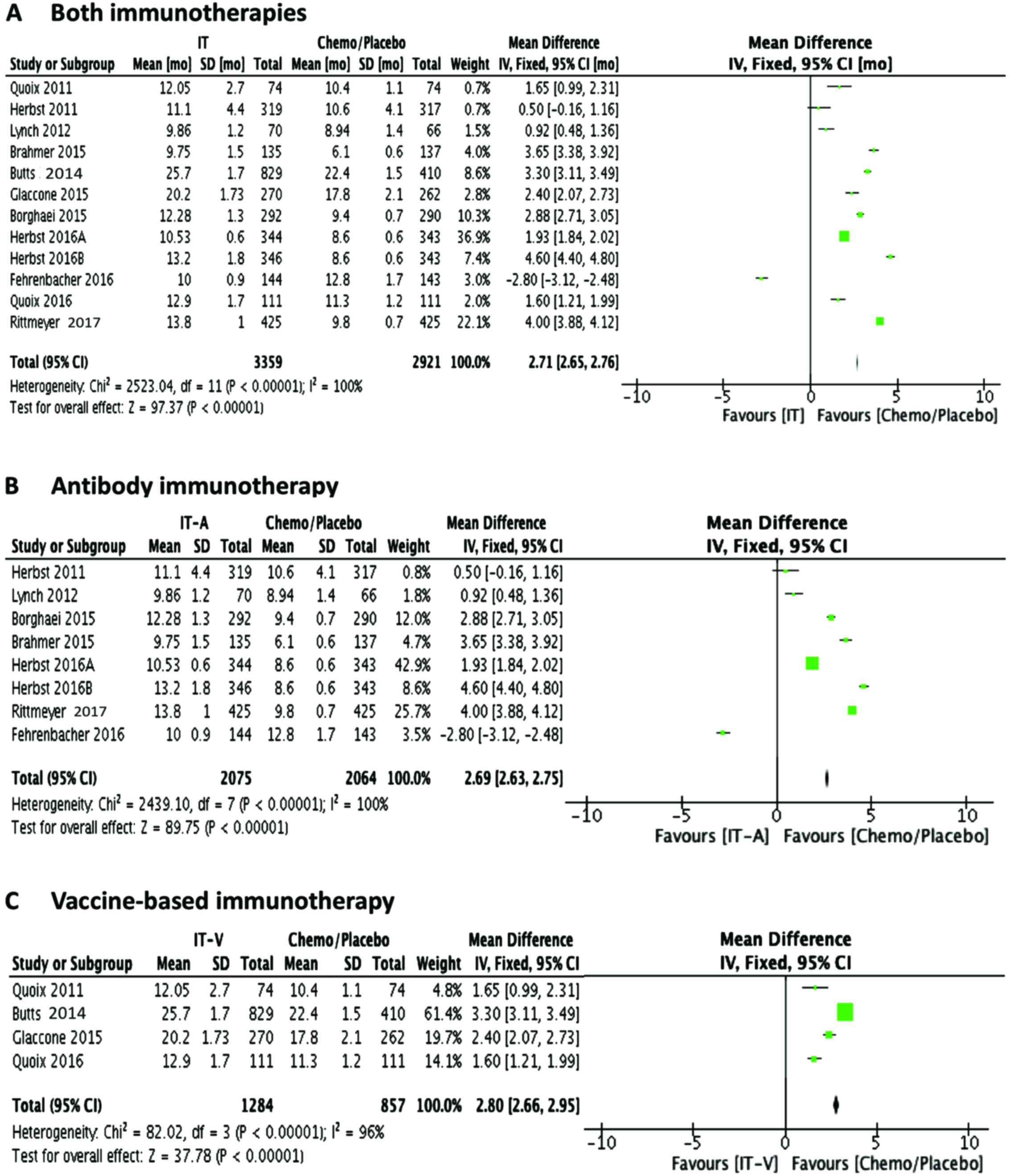
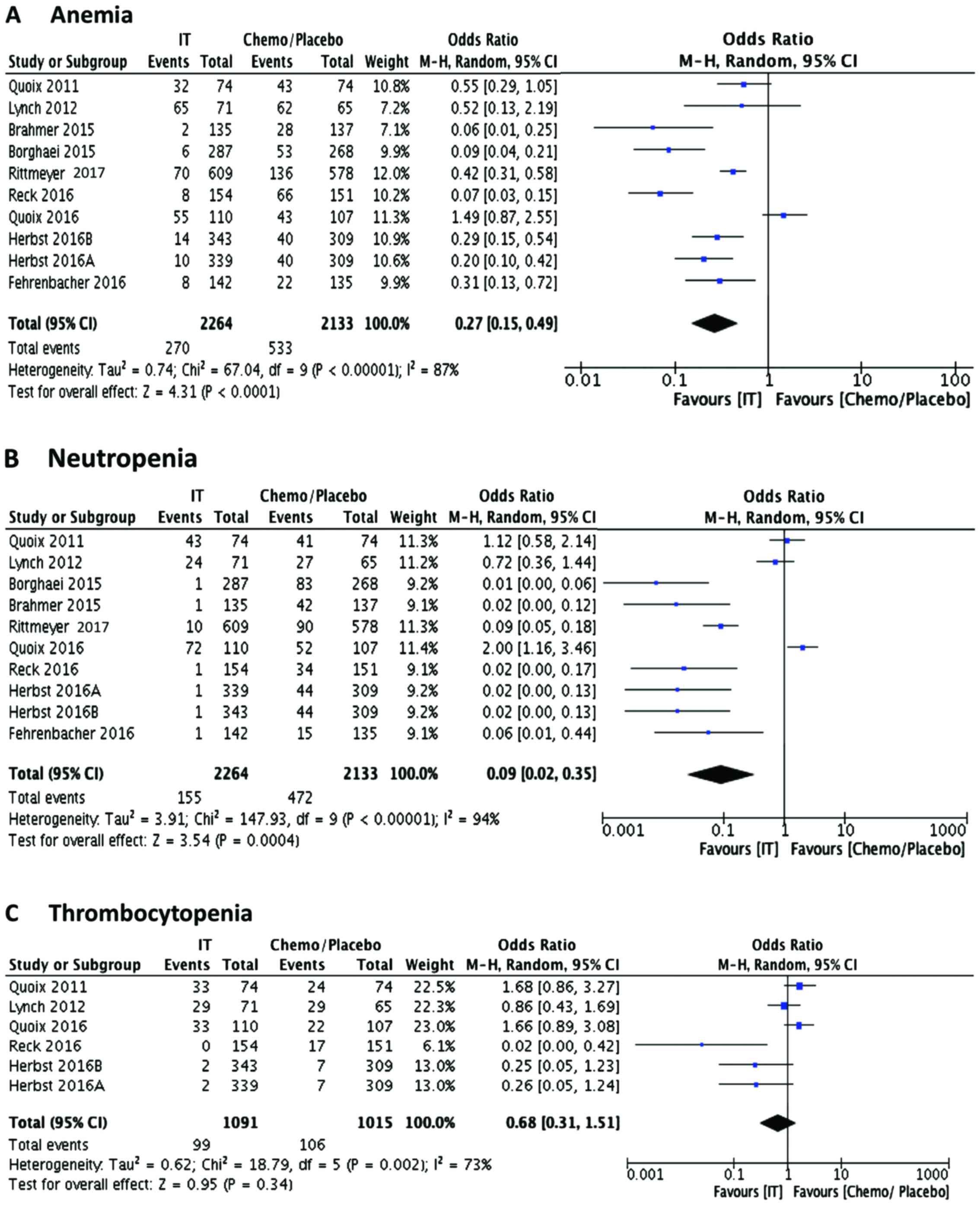
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gordon SB, Bruce NG, Grigg J, Hibberd PL,
Kurmi OP, Lam KB, Mortimer K, Asante KP, Balakrishnan K, Balmes J,
et al: Respiratory risks from household air pollution in low and
middle income countries. Lancet Respir Med. 2:823–860. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haghgoo SM, Allameh A, Mortaz E, Garssen
J, Folkerts G, Barnes PJ and Adcock IM: Pharmacogenomics and
targeted therapy of cancer: Focusing on non-small cell lung cancer.
Eur J Pharmacol. 754:82–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madureira P, de Mello RA, de Vasconcelos A
and Zhang Y: Immunotherapy for lung cancer: For whom the bell
tolls? Tumour Biol. 36:1411–1422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun JY, Shi L, Gao XD and Xu SF: Physical
activity and risk of lung cancer: A meta-analysis of prospective
cohort studies. Asian Pac J Cancer Prev. 13:3143–3147. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Zou ZH, Xia HL, He JX, Zhong NS
and Tao AL: Strengths and weaknesses of immunotherapy for advanced
non-small-cell lung cancer: A meta-analysis of 12 randomized
controlled trials. PLoS One. 7:e326952012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin ZY, Zhao XT, Zhang LN, Wang Y, Yue WT
and Xu SF: Effects of polymorphisms in the XRCC1, XRCC3, and XPG
genes on clinical outcomes of platinum-based chemotherapy for
treatment of non-small cell lung cancer. Genet Mol Res.
13:7617–7625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gridelli C, Ascierto PA, Barberis MC,
Felip E, Garon EB, O'Brien M, Senan S, Casaluce F, Sgambato A,
Papadimitrakopoulou V and De Marinis F: Immunotherapy of non-small
cell lung cancer: Report from an international experts panel
meeting of the Italian Association of Thoracic Oncology. Expert
Opin Biol Ther. 16:1479–1489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forde PM, Kelly RJ and Brahmer JR: New
strategies in lung cancer: Translating immunotherapy into clinical
practice. Clin Cancer Res. 20:1067–1073. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kimura H, Matsui Y, Ishikawa A, Nakajima
T, Yoshino M and Sakairi Y: Randomized controlled phase III trial
of adjuvant chemo-immunotherapy with activated killer T cells and
dendritic cells in patients with resected primary lung cancer.
Cancer Immunol Immunother. 64:51–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dammeijer F, Lievense LA, Veerman GD,
Hoogsteden HC, Hegmans JP, Arends LR and Aerts JG: Efficacy of
tumor vaccines and cellular immunotherapies in non-small-cell lung
cancer: A systematic review and meta-analysis. J Clin Oncol.
34:3204–3212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramalingam S, Crawford J, Chang A,
Manegold C, Perez-Soler R, Douillard JY, Thatcher N, Barlesi F,
Owonikoko T, Wang Y, et al: Talactoferrin alfa versus placebo in
patients with refractory advanced non-small-cell lung cancer
(FORTIS-M trial). Ann Oncol. 24:2875–2880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Shi GL, Song CX and Xu SF:
Relationship between genetic polymorphism of MCP-1 and
non-small-cell lung cancer in the Han nationality of North China.
Genet Mol Res. 9:765–771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang L, Wang LJ, Shi GL, Ni L, Song CX,
Zhang ZX and Xu SF: Analysis of HLA-A, HLA-B and HLA-DRB1 alleles
in Chinese patients with lung cancer. Genet Mol Res. 9:750–755.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou L, Wang XL, Deng QL, Du YQ and Zhao
NQ: The efficacy and safety of immunotherapy in patients with
advanced NSCLC: A systematic review and meta-analysis. Sci Rep.
6:320202016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cuppens K and Vansteenkiste J: Vaccination
therapy for non-small-cell lung cancer. Curr Opin Oncol.
26:165–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quoix E, Ramlau R, Westeel V, Papai Z,
Madroszyk A, Riviere A, Koralewski P, Breton JL, Stoelben E, Braun
D, et al: Therapeutic vaccination with TG4010 and first-line
chemotherapy in advanced non-small-cell lung cancer: A controlled
phase 2B trial. Lancet Oncol. 12:1125–1133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lynch TJ, Bondarenko I, Luft A,
Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H,
Cuillerot JM and Reck M: Ipilimumab in combination with paclitaxel
and carboplatin as first-line treatment in stage IIIB/IV
non-small-cell lung cancer: Results from a randomized,
double-blind, multicenter phase II study. J Clin Oncol.
30:2046–2054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quoix E, Lena H, Losonczy G, Forget F,
Chouaid C, Papai Z, Gervais R, Ottensmeier C, Szczesna A,
Kazarnowicz A, et al: TG4010 immunotherapy and first-line
chemotherapy for advanced non-small-cell lung cancer (TIME):
Results from the phase 2b part of a randomised, double-blind,
placebo-controlled, phase 2b/3 trial. Lancet Oncol. 17:212–223.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: KEYNOTE-024 Investigators: Pembrolizumab versus
chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl
J Med. 375:1823–1833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: OAK Study Group: Atezolizumab versus docetaxel in
patients with previously treated non-small-cell lung cancer (OAK):
A phase 3, open-label, multicentre randomised controlled trial.
Lancet. 389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Butts C, Socinski MA, Mitchell PL,
Thatcher N, Havel L, Krzakowski M, Nawrocki S, Ciuleanu TE, Bosquée
L, Trigo JM, et al: START trial team: Tecemotide (L-BLP25) versus
placebo after chemoradiotherapy for stage III non-small-cell lung
cancer (START): A randomised, double-blind, phase 3 trial. Lancet
Oncol. 15:59–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Herbst RS, Ansari R, Bustin F, Flynn P,
Hart L, Otterson GA, Vlahovic G, Soh CH, O'Connor P and Hainsworth
J: Efficacy of bevacizumab plus erlotinib versus erlotinib alone in
advanced non-small-cell lung cancer after failure of standard
first-line chemotherapy (BeTa): A double-blind, placebo-controlled,
phase 3 trial. Lancet. 377:1846–1854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giaccone G, Bazhenova LA, Nemunaitis J,
Tan M, Juhász E, Ramlau R, Van den Heuvel MM, Lal R, Kloecker GH,
Eaton KD, et al: A phase III study of belagenpumatucel-L, an
allogeneic tumour cell vaccine, as maintenance therapy for
non-small cell lung cancer. Eur J Cancer. 51:2321–2329. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vesely MD, Kershaw MH, Schreiber RD and
Smyth MJ: Natural innate and adaptive immunity to cancer. Annu Rev
Immunol. 29:235–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Emens LA and Middleton G: The interplay of
immunotherapy and chemotherapy: Harnessing potential synergies.
Cancer Immunol Res. 3:436–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Noguchi M, Sasada T and Itoh K:
Personalized peptide vaccination: A new approach for advanced
cancer as therapeutic cancer vaccine. Cancer Immunol Immunother.
62:919–929. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pol J, Bloy N, Buqué A, Eggermont A,
Cremer I, Sautès-Fridman C, Galon J, Tartour E, Zitvogel L, Kroemer
G, et al: Trial Watch: Peptide-based anticancer vaccines.
Oncoimmunology. 4:e9744112015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ettinger DS: Non-small cell lung cancer
treatment-related bone marrow toxicities. Semin Oncol. 32:S81–S85.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gelao L, Criscitiello C, Esposito A,
Goldhirsch A and Curigliano G: Immune checkpoint blockade in cancer
treatment: A double-edged sword cross-targeting the host as an
‘innocent bystander’. Toxins (Basel). 6:914–933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weber JS, Yang JC, Atkins MB and Disis ML:
Toxicities of immunotherapy for the practitioner. J Clin Oncol.
33:2092–2099. 2015. View Article : Google Scholar : PubMed/NCBI
|