Introduction
Ovarian cancer is one of the most common types of
gynecologic malignant tumor, whose incidence rate ranks third in
cancers of the female reproductive system, and mortality ranks
first (1). The etiology of ovarian
cancer remains unknown, and may be associated with a number of
internal and external factors. Early diagnosis of ovarian cancer is
difficult due to the complicated structure and function of the
ovary. In addition, late-stage tumors often recur and metastasize
subsequent to surgery, and eventually evolve resistance to
chemotherapy. The five-year survival rate of patients with ovarian
cancer is <30% (1,2). Therefore, it is necessary to identify
molecules that function in the occurrence, development and
metastasis of ovarian cancer, and to develop more effective
diagnosis and therapy in clinic.
Siva 1 is a pro-apoptotic protein first obtained by
Prasad et al from a HeLa cell library via yeast two-hybrid
screening with CD27, a member of the tumor necrosis factor receptor
superfamily, as the bait (3). Siva 1
exists in a wide variety of tissues and cells, and serves a crucial
role in certain extrinsic and intrinsic apoptosis signaling
pathways, for example: Siva 1 interacts with CD27 and induces
apoptosis via the caspase-independent mitochondrial pathway in T
lymphocytes (4); Siva 1 maybe
stimulated by thromboxane receptor and aggravates apoptosis of HeLa
cells induced by cisplatin (5). Siva
1 binds to B-cell lymphoma-extra large (Bcl-XL) and inhibits
Bcl-XL-mediated apoptosis protection from UV radiation in breast
cancer cells (6). Additionally, Siva
1 participates in virus infection-associated apoptosis: It has been
demonstrated that Siva 1 promotes apoptosis of A549 cells induced
by influenza A virus and sensitizes CD4+ cells to HIV-I
envelope-induced apoptosis in a caspase-dependent manner (7,8).
However, Siva 1 has been demonstrated to serve
opposite roles in other previous studies. In
KRASG12D-derived non-small cell lung cancer (NSCLC),
Siva 1 is highly expressed and facilitates tumorigenesis by
regulating metabolism and autophagy (9); the knockdown of Siva 1 inhibits human
fetal lung cell proliferation and induces cell cycle arrest via the
alternative reading frame tumor protein (p) 53 pathway (10); Siva 1 destabilizes p53 and promotes
its degradation, suppresses p53-mediated apoptosis in osteosarcoma
cells, and the knockdown of Siva 1 inhibits tumorigenesis (11). In addition, Li et al revealed
that Siva 1 phosphorylated Stathmin at Ser16, attenuated its
microtubule-destabilizing activity and suppressed
epithelial-mesenchymal transition and metastasis of breast cancer
cells (12). Stathmin is a
microtubule destabilizer, participating in cell mitosis and
migration by regulating microtubule stability. Additionally, it is
highly expressed in a number of malignant tumors, including ovarian
cancer (13), suggesting a
cancer-promoting effect.
In conclusion, the effect of Siva 1 is different in
a number of cell types, which is a result of the combined action of
multiple signaling pathways. At present, Siva 1 is pro-apoptotic
and carcinostatic in colorectal (14), cervical (5) and breast cancer (12) and acute leukemia (15), but anti-apoptotic and carcinogenic in
osteosarcoma (11) and NSCLC
(9). However, it remains unknown
whether Siva 1 functions in ovarian cancer. In the present study,
the effect of Siva 1 on ovarian cancer cells and the underlying
mechanism of its action in these cells were investigated.
Materials and methods
Cell culture, transfection and
establishment of stable cell line
Ovarian cancer SKOV3 and OVCAR-3 cell lines were
purchased from the Shanghai Cell Bank of Chinese Academy of
Sciences and cultured with RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA USA) supplemented with 10% fetal bone
serum (FBS; Hyclone; GE Healthcare; Logan, UT, USA) at 37°C in 5%
CO2. A2780 cells were purchased from Cell Preservation
Center of Wuhan University and cultured with Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
10% FBS at 37°C in 5% CO2. Siva 1 overexpression plasmid
pCMV3-Siva 1 or the control pCMV3 [China National Pharmaceutical
Group (CNPGC); Sinopharm, Beijing, China] were transfected into
A2780 cells with lipofectamine reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). A total of 24 h after transfection, 200 µg/ml
G418 (Invitrogen; Thermo Fisher Scientific, Inc.) was added to the
medium, and the culture medium was renewed every 2 days.
Approximately 1 week later, monoclonal cell clusters were observed
and selected for culture. Thereafter, the Siva 1 stably expressed
A2780 cell line and its control were used for subsequent
experiments.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cells by total RNA
rapid isolation kit (BioTeke Corporation, Beijing, China) according
to the manufacturer's protocol. The concentration was measured by
the OD260/OD280 ratio using a NanoDrop 2000
(Thermo Fisher Scientific, Inc., Wilmington, DE, USA). The RNA was
reverse transcribed with Moloney Murine leukemia virus (M-MLV)
reverse transcriptase (BioTeke Corporation), with
oligo-deoxy-thymidine nucleotides and random primers (Sangon
Biotech Co., Ltd., Shanghai, China) as the RT primers. The negative
control was the RNA in pCMV3-transfected cells. For the RT-PCR, the
following items were added with RNA samples into each tube: 1 µg
RNA, 1 µl oligo (dT) primers, 1 µl random primers, 2 µl dNTP and
ddH2O to make a final reaction volume of 14.5 µl. Each
tube was heated at 70°C for 5 min, cooled on ice for 2 min, and the
following items were added: 4 µl 5X buffer (250 mM Tris-HCl (pH
8.3), 375 mM KCl, 15 mM MgCl2, 50 mM DTT; Dongsheng
Biotech, Guangzhou, China), 0.5 µl RNasin ribonuclease inhibitor
(Tiandz, Inc., Beijing, China) and 1 µl M-MLV reverse
transcriptase. The thermocycler conditions were as follows: 25°C
for 10 min, then at 42°C for 50 min, heated at 95°C for 5 min, and
finally cooled on ice for several min. There was 20 µl cDNA sample
in each reaction. All the instruments used in this step were
treated with Surface RNase Erasol (Tiandz, Inc.) and all the
reagents used in this protocol were RNase-free.
The produced cDNA (1 µg) was used for qPCR by Taq
PCR MasterMix (BioTeke Corporation) supplemented with SYBR Green
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) to detect Siva 1 or Stathmin using the following procedure:
95°C for 10 min, 40 cycles of 95°C for 10 sec, 60°C for 20 sec and
72°C for 30 sec, and finally 4°C for 5 min, with β-actin as the
internal control. The primer sequences are provided in Table I. The data were analyzed by the
2−ΔΔCq method (16), with
β-actin as the reference gene.
 | Table I.Sequences of real-time PCR
primers. |
Table I.
Sequences of real-time PCR
primers.
| Name | Sequence (5′ to
3′) |
|---|
| Siva 1 | F:
CCAAGCGACTCCTGTTCCTC |
|
| R:
CCAATCAGCATCTGCCCAC |
| Stathmin | F:
TCGCTTGTCTTCTATTCACCA |
|
| R:
CTTCTTTCTCGTGCTCTCGTT |
| β-actin | F:
CTTAGTTGCGTTACACCCTTTCTTG |
|
| R:
TGTCACCTTCACCGTTCCAGTTT |
Western blot analysis
Protein samples from SKOV3, OVCAR-3 and A2780 cell
lines were extracted using a total protein extraction kit
(Wanleibio Co., Ltd., Shenyang, China) according to the
manufacturer's protocol. Subsequent to quantification of the
content using a bicinchoninic acid protein quantification kit
(Wanleibio Co., Ltd.), the protein was isolated by SDS-PAGE using
40 µg protein in each lane and transferred to polyvinylidene
fluoride (PVDF) membrane (EMD Millipore, Billerica, MA, USA).
Subsequent to blocking with 5% skim milk (YILI, Hohhot, Inner
Mongolia, China) at room temperature for 1 h, diluted by TBS Tween
(TBST), the PVDF membrane was incubated with the following
antibodies at 4°C overnight: rabbit anti-human anti-Siva 1
polyclonal antibody (dilution, 1:200; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA, cat. no., sc-48767), rabbit anti-human
anti-cleaved caspase-3 polyclonal antibody (dilution, 1:1,000;
Abcam, Cambridge, UK, cat. no., ab2302), goat anti-human
anti-cleaved caspase-9 polyclonal antibody (dilution, 1:200; Santa
Cruz Biotechnology, Inc., cat. no., sc-22182), rabbit anti-human
anti-Bcl-2-like protein 4 (Bax) polyclonal antibody (dilution,
1:400; Boster, Hubei, Wuhan, China, cat. no., BA0315), rabbit
anti-human anti-Bcl-2 polyclonal antibody (dilution, 1:400; Boster,
cat. no., BA0412), rabbit anti-human anti-Stathmin polyclonal
antibody (dilution, 1:500; Bioss Antibodies, Beijing, China, cat.
no., bs-1902R), rabbit anti-human anti-phosphorylated Stathmin
polyclonal antibody (dilution, 1:500; Bioss Antibodies, cat. no.,
bs-3431R), rabbit anti-human anti-α-tubulin polyclonal antibody
(dilution, 1:1,000; Abcam, cat. no., ab178484) or mouse anti-human
anti-acetyl-α-tubulin monoclonal antibody (dilution, 1:1,000;
Abcam, cat. no., ab24610). Subsequent to washing with TBST 4 times
for 5 min each, the PVDF membrane was incubated with goat
anti-rabbit polyclonal IgG-horseradish peroxidase (HRP; dilution,
1:5,000; Wanleibio Co., Ltd., cat. no., WLA023), goat anti-mouse
polyclonal IgG-HRP (dilution, 1:5,000; Wanleibio Co., Ltd., cat.
no., WLA024) or donkey anti-goat polyclonal IgG-HRP (dilution,
1:5,000; Beyotime Institute of Biotechnology, Haimen, Jiangsu,
China, cat. no., A0181) at 37°C for 45 min, followed treatment with
a ECL reagent (Wanleibio Co., Ltd.). Subsequent to the removal of
antibodies by stripping buffer (Wanleibio Co., Ltd.), the PVDF
membrane was incubated with rabbit anti-human anti-β-actin antibody
(dilution, 1:1,000; Santa Cruz Biotechnology, Inc., cat. no.,
sc-47778) and goat anti-rabbit polyclonal IgG-HRP (dilution,
1:5,000; Wanleibio Co., Ltd.) to detect the internal control,
β-actin. The bands were analyzed with Gel-Pro-Analyzer software
(version 4, Media Cybernatics, Silver Spring, MD, USA), and
experiments were performed in triplicate.
Immunofluorescence
A2780 cells were seeded onto glass slides prior to
this protocol. When cell confluence reached 70–80%, the cells were
fixed in 4% paraformaldehyde (CNPGC; Sinopharm) at room temperature
for 15 min, and permeated with 0.1% TritonX-100 (Amresco, LLC.,
Solon, OH, USA) for 30 min. Subsequent to blocking with goat serum
(Beijing Solarbio Science & Technology Co., Ltd., cat. no.,
SL2-10) for 15 min, the cells were incubated with rabbit anti-human
anti-Siva 1 polyclonal antibody (dilution, 1:50; Santa Cruz
Biotechnology, Inc., cat. no., sc-48767) at 4°C overnight.
Subsequent to rinsing with PBS, the cells were incubated with goat
anti-rabbit polyclonal IgG-Cy3 (1:200) (Beyotime Institute of
Biotechnology, cat. no., A0516) for 60 min, stained with DAPI
(Biosharp, Hefei, China) for 5 min, and mounted with an anti-fade
solution (Beijing Solarbio Science & Technology Co., Ltd.) in
the dark. The slides were observed and images were captured with a
fluorescence microscope (Olympus Corporation, Tokyo, Japan) at
magnification, ×400, and analyzed with Image pro-plus v6.0 (IPP)
software (Media Cybernetics, Rockville, MD, USA).
CCK-8 assay
A2780 cells were seeded in a 96-well plate with
3×103cells/well. Cell viability was detected at 24, 48,
72, 96 and 120 h after seeding. The CCK-8 reagent (Wanleibio Co.,
Ltd.) was added into the culture medium at10 µl/100 µl medium/well,
and incubated at 37°C for 1 h. Then, the optical density at 450 nm
of culture medium was determined by microplate reader (Biotek
Instruments, Inc., Winooski, VT, USA).
Colony formation
A2780 cells were seeded onto 35-mmpetri dishes with
500 cells per dish, and cultured at 37°C in 5% CO2.
Approximately 2 weeks later, the majority of the clones were able
to be seen by the naked eye. The cells were fixed with 4%
paraformaldehyde (CNPGC; Sinopharm) at room temperature for 20 min
and stained with Wright-Giemsa stain reagent (Nanjing Jiancheng
Bioengineering Research Institute, Nanjing, China) for 5–8 min.
Subsequent to rinsing with PBS 2–3 times, cell clones were counted
and the clone formation rate was calculated as follows: clone
number/number of cells seeded. Clones with >50 cells were
considered positive.
Hoechst staining
A2780 cells were seeded on microscope slides in a
12-well plate with 3×105 per well. When cell confluence
reached 60–70%, the cells were fixed with 4% paraformaldehyde
(CNPGC; Sinopharm) at room temperature for 20 min and stained with
Hoechst 33258 stain reagent (Wanleibio Co., Ltd.) for 5 min in the
dark. Cells were blocked with Antifade Mounting Medium
(BeyotimeInstitute of Biotechnology) and observed with fluorescence
microscopy (Olympus Corporation) at magnification, ×400, and
analyzed with CellSens version 1.6 (Olympus Corporation).
Wound healing assay
A wound healing assay was performed to measure cell
migration ability. A3780 cells were cultured with serum-free medium
supplemented with 1 µg/ml mitomycin C (Sigma-Aldrich, Merck KGaA,
Darmstadt, Germany) for 1 h. The wound was caused by 200 µl pipette
tip, and images of the wound sizes 0, 24 and 48 h subsequent to
wounding were captured with an inverted phase contrast microscope
(Motic, Xiamen, Fujian, China) at magnification, ×100. The cell
migration rate at 24 h was calculated as (wound size at 0 h-wound
size at 24 h)/wound size at 0 h; the cell migration rate at 48 h
was calculated as (wound size at 0 h-wound size at 48 h)/wound size
at 0 h.
Transwell assay
A2780 cell invasion was detected by Transwell assay
supplemented with Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA) in vitro, with A2780 cells transfected with pCMV3
vector used as the negative control. All experiments were performed
in triplicate. A total of 40 µl 2.5 mg/ml Matrigel was added into
the Transwell chamber (Corning Incorporated, Corning, NY, USA) and
preheated at 37°C to solidify. A total of 200 µl serum-free cell
suspension with 1×104 cells were seeded into the upper
chamber, and 800 µl medium with 20% FBS (Hyclone; GE Healthcare;
Logan, UT, USA) was added into the lower chamber. Subsequent to
culturing for 24 h, the Transwell chamber was removed and the cells
in upper chamber were wiped away. The cells in lower chamber were
fixed with 4% paraformaldehyde (CNPGC; Sinopharm) at room
temperature for 20 min and stained with 0.5% crystal violet reagent
(Amresco, LLC) for 5 min. Subsequent to rinsing with water, the
cells in the lower chambers were counted with an inverted phase
contrast microscope (Motic, Xiamen, Fujian, China) at
magnification, ×200.
Flow cytometry
A2780 cells were cultured in 6-well plates until the
cell confluence reached 90%. The cells were collected and treated
with Annexin V-fluorescein isothiocyanate/propidium iodide
apoptosis detection kit (Wanleibio Co., Ltd.) according to the
manufacturer's protocol, and detected by flow cytometry (BD
Biosciences).
Statistical analysis
The data in the present study are presented as the
mean ± standard deviation, with three individual experiments, and
were analyzed with one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Establishment of Siva 1 stably
overexpressed cell line
In order to study the roles of Siva 1 in ovarian
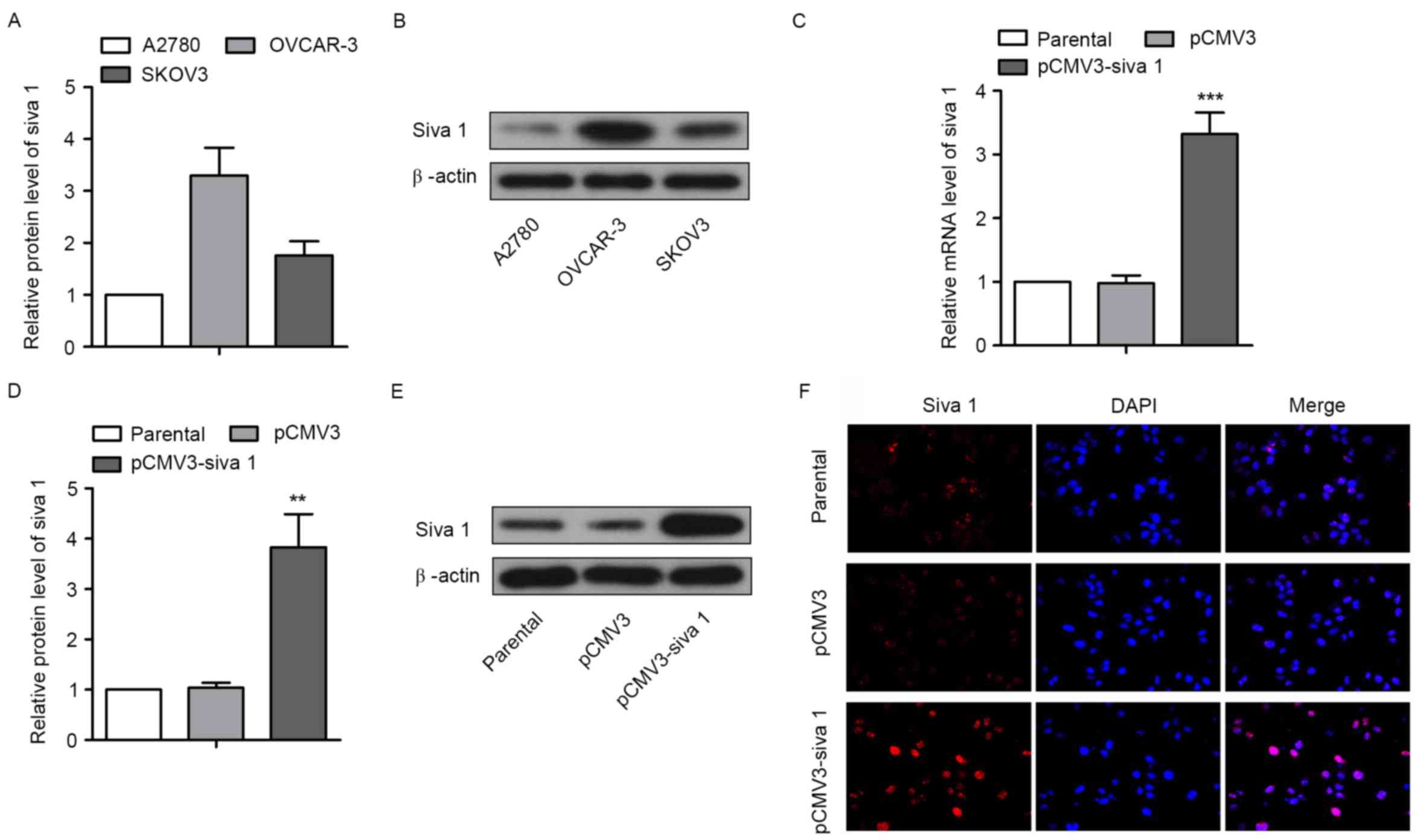
cancer cells, Siva 1 expression level was first detected in three
ovarian cancer cell lines, A2780, OVCAR-3 and SKOV3. The western
blot analyses results demonstrated that the expression level of
Siva 1 in A2780 cells was markedly lower compared with OVCAR-3
cells and SKOV3 cells (Fig. 1A and
B). The overexpression plasmid pCMV3-Siva 1 was then
constructed and transfected into A2780 cells. By screening with
G418, a monoclonal cell line was obtained, with A2780 cells
transfected with pCMV3 as the control and untreated A2780 cells as
the parental line. qPCR and western blot analyses showed that mRNA
and protein levels of Siva 1 in pCMV3-Siva 1 cells increased
3.39-fold (P=0.0004) and 3.68-fold (P=0.0019), respectively,
compared with pCMV3 (Fig. 1C-E). The
immunofluorescence assay results demonstrated the same increase,
and Siva 1 was distributed in the cytoplasm and in the nuclei
(Fig. 1F).
Siva 1 inhibits the growth and
proliferation of ovarian cancer cells
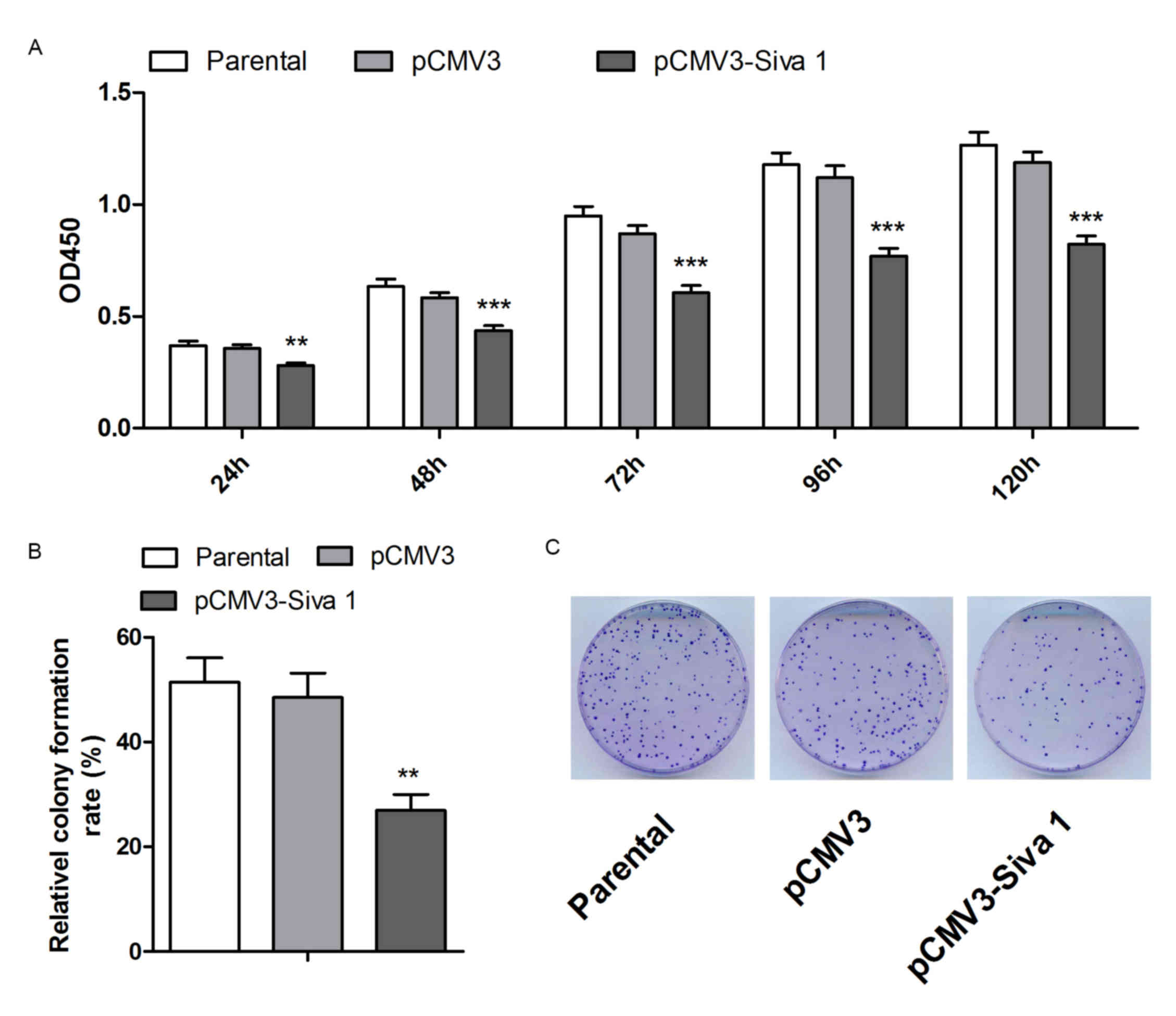
CCK-8 and colony formation assays were performed to
detect cell viability and colony formation abilities of the cell
lines. As shown in Fig. 2A, Siva 1
impaired cell viability by 21 (P=0.0056), 25 (P=0.0005), 30
(P<0.0001), 32 (P=0.0001) and 31% (P<0.0001) in 24, 48, 72,
96 and 120 h, respectively. The colony formation assay indicated
that Siva 1 decreased the colony formation ability of A2780 cells
by 44% (P=0.0025) (Fig. 2B).
Therefore, these data suggest that overexpression of Siva 1
inhibited the proliferation of ovarian cancer cells.
Siva 1 promotes apoptosis of ovarian
cancer cells
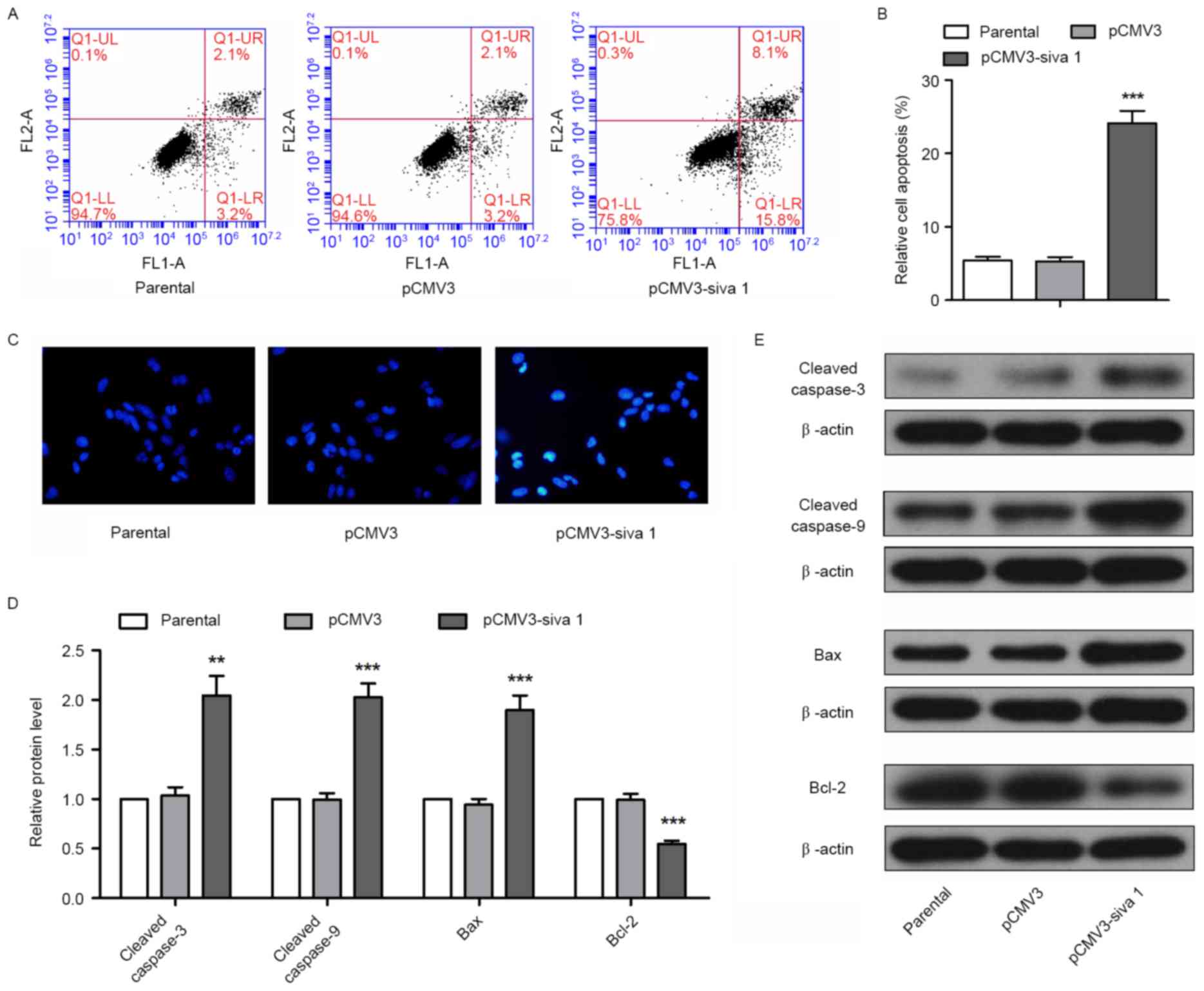
To determine the effect of Siva 1 on apoptosis of
ovarian cancer cells, flow cytometry and Hoechst staining were
performed. Flow cytometry detection demonstrated that Siva 1
increased the apoptosis rate by 4.55-fold (P<0.0001) (Fig. 3A and B). Using Hoechst staining,
bright nuclei in were observed in the pCMV3-Siva 1 cells (Fig. 3C), which indicates chromosome breakage
and nucleus shrinkage in apoptosis progress. In addition, there are
a number of signaling molecules participating in intrinsic
apoptosis, which may be detected as apoptosis markers. In the
present study, the expression levels of executioners of apoptosis,
cleaved caspase-3 and cleaved caspase-9, pro-apoptotic protein Bax
and anti-apoptotic protein Bcl-2 were detected. The western blot
analyses results demonstrated that in cells that expressed stable
levels of Siva 1, the protein levels of cleaved caspase-3 increased
1.96-fold (P=0.0016), cleaved caspase-9 increased 2.03-fold
(P=0.0002), Bax increased 2.02-fold (P=0.0005) and Bcl-2 decreased
by 44% (P=0.0004) (Fig. 3D and E).
These data indicate that Siva 1 promoted the apoptosis of ovarian
cancer cells.
Siva 1 suppresses the migration and
invasion of ovarian cancer cells
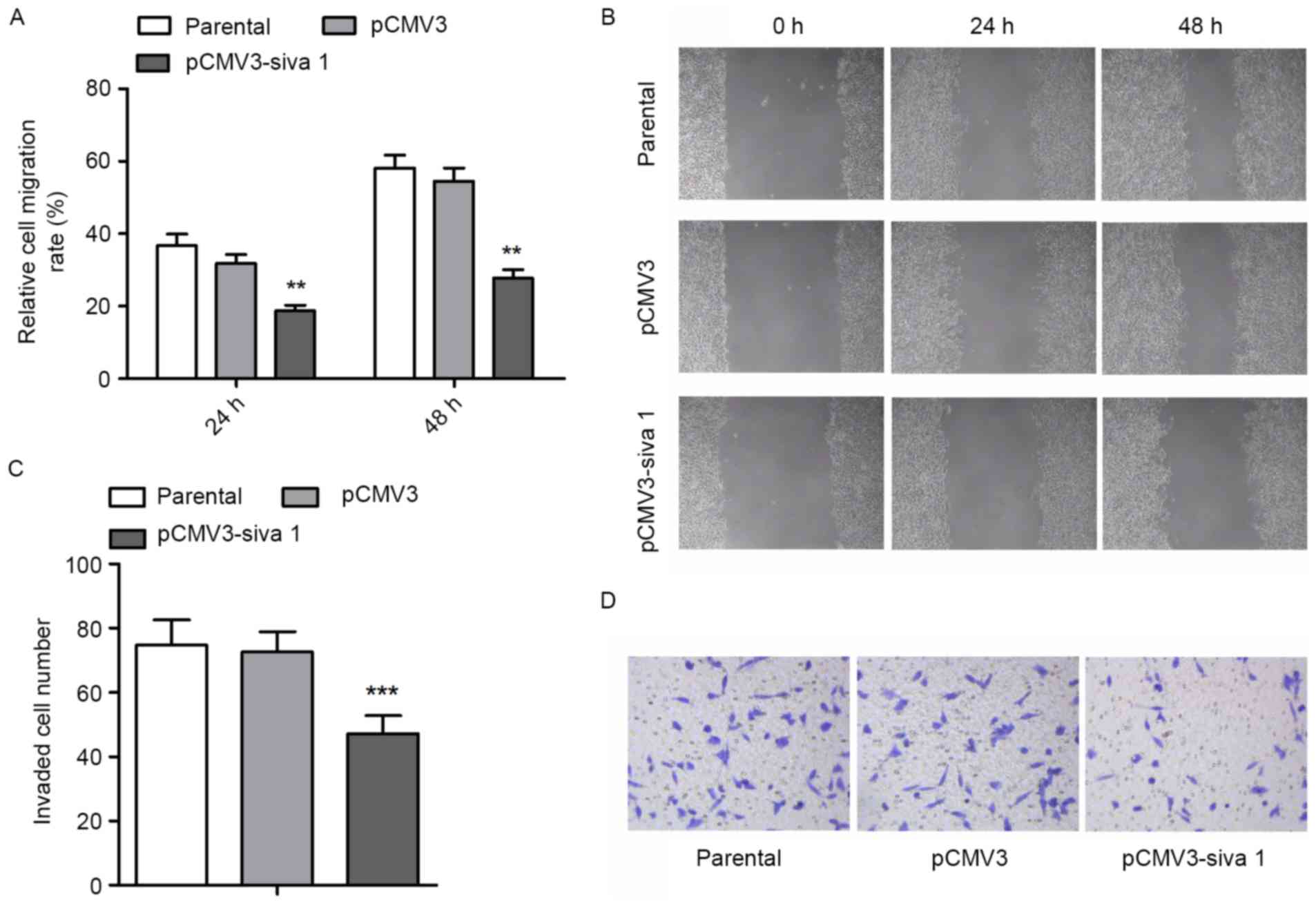
Since migration and invasion are other vital aspects
of cancer cell malignancies, the migration and invasion potential
of ovarian cancer cells was measured. The wound healing assay
demonstrated that subsequent to Siva 1 overexpression, migration
rate decreased by 41% (P=0.0052) and 49% (P=0.001) after 24 and 48
h, respectively (Fig. 4A and B). The
Transwell assay with Matrigel found that Siva 1 decreased the
invasion potential of ovarian cancer cells by 35% (P<0.0001)
(Fig. 4C and D).
Siva 1 enhances the phosphorylation of
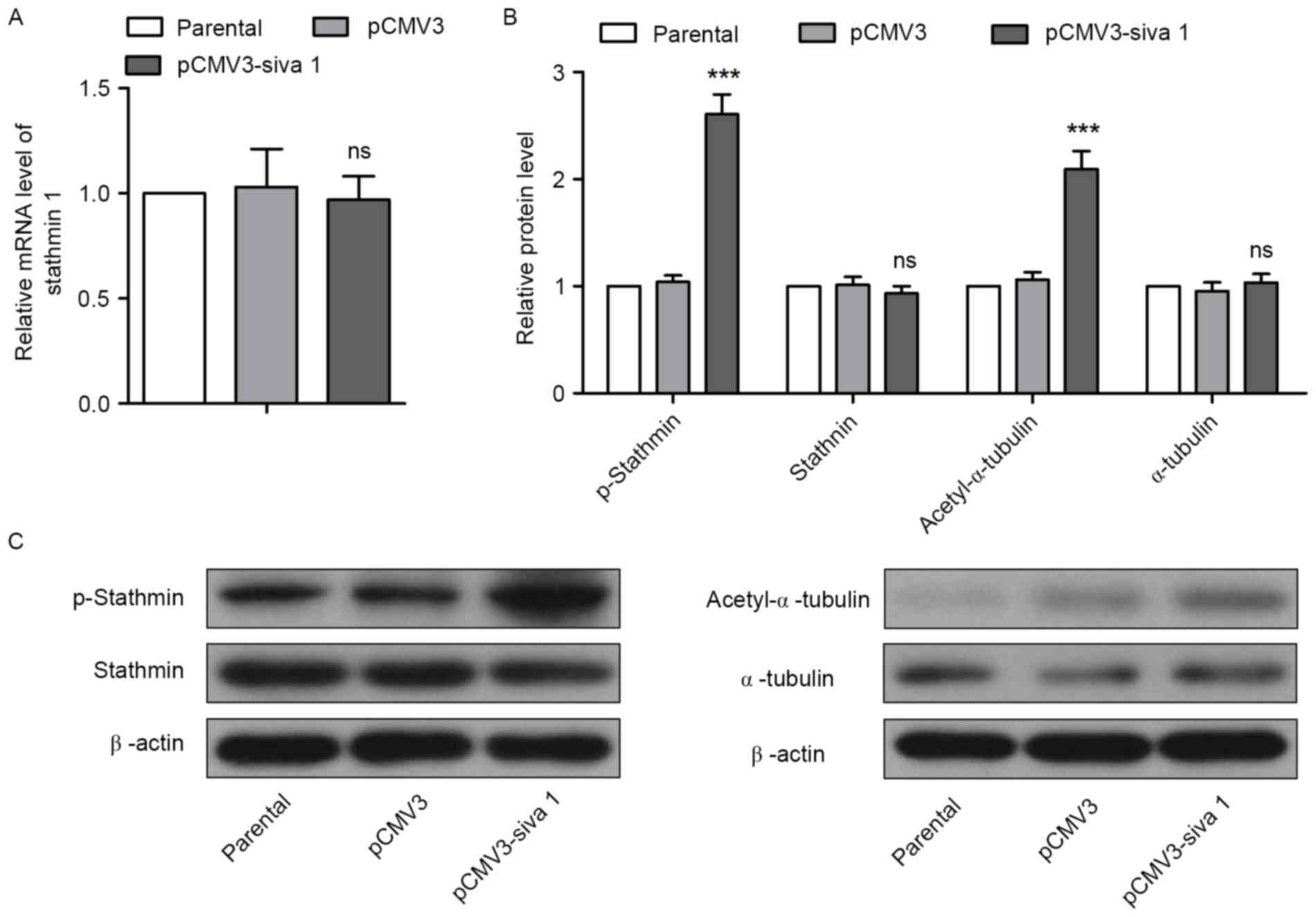
Stathmin
The effects of Siva 1 on Stathmin in ovarian cancer
cells were then investigated. qPCR and western blot analyses
results demonstrated that overexpression of Siva 1 did not increase
Stathmin expression, but promoted its phosphorylation (Fig. 5A-C). As the polymerized tubulin was
acetylated, the level of acetyl-α-tubulin was also measured to
determine whether the microtubule-destabilizing activity of
Stathmin was impaired. Western blot analyses revealed that
acetyl-α-tubulin levels were elevated, but the levels of α-tubulin
were not in cells with an overexpression of Siva 1, which indicates
that Siva 1 stabilized microtubules by inhibiting Stathmin
activity.
Taken together, these data suggest that Siva 1
inhibited cell growth and proliferation, promoted apoptosis and
suppressed migration and invasion by promoting Stathmin
phosphorylation and stabilizing microtubules in ovarian cancer
cells.
Discussion
There have been several studies demonstrating that
Siva 1 facilitated or suppressed apoptosis in different types of
cancer cells previously. In the present study, it was revealed that
Siva 1 promoted intrinsic apoptosis and inhibited the proliferation
of ovarian cancer cells. In this process, the levels of apoptosis
executors activated caspase-3, caspase-9 and pro-apoptosis protein
Bax increased, and the level of the anti-apoptosis protein Bcl-2
decreased. Additionally, the phosphorylation of Stathmin and
acetylation of α-tubulin increased, which represented an increase
in the number of microtubules produced.
Stathmin, also known as oncoprotein 18, is a
microtubule destabilizing protein. It binds to tubulin and
maintains the dynamic process of continuous microtubule synthesis
and degradation (17). Microtubules,
composed of α- and β-tubulin, constitute cytoskeleton, spindle,
cilia and flagella, and participate in support, material transport,
cell movement, mitosis and other functions. During the mitosis
process, tubulin is rapidly depolymerized and re-polymerized into
spindle fibers. Once Stathmin was phosphorylated and inactivated,
cell proliferation was inhibited (18,19). In
the present study, Siva 1 inhibited cell proliferation and promoted
apoptosis by facilitating the phosphorylation of Stathmin and
decreasing its activity.
Metastasis is another important contributor to the
high mortality rates and difficulty incuring malignant tumors.
Stathmin was highly expressed in a number of malignant tumors
(20). It reduced the stability of
microtubules and provided tumor cells with improved motility.
Stathmin has been used as a therapeutic target and prognostic
indicator in prostate and cervical cancer, oral squamous cell
carcinoma and medulloblastoma (21–24). In
the present study, it was revealed that Siva 1 promoted the
phosphorylation of Stathmin, increased the level of acetylated
α-tubulin, which was present only in polymerized tubulin (25), and therefore inhibited the migration
and invasion of ovarian cancer cells.
Siva 1 serves different roles in various cells, and
the mechanism of action also relies on the specific cell types. In
the present study, Siva 1 was overexpressed in the ovarian cancer
A2780 cell line, the changes in cell phenotype were detected, and
the potential mechanism was studied. The results of the present
study demonstrated that Siva 1 inhibited proliferation, promoted
apoptosis, suppressed migration and invasion by facilitating
phosphorylation of Stathmin and polymerization of tubulin in
ovarian cancer cells. These data provide novel insights into the
molecular mechanism of ovarian cancer pathogenesis, and provide a
basis for additional studies investigating the clinical diagnosis
and treatment methods of ovarian cancer.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeung TL, Leung CS, Li F, Wong SS and Mok
SC: Targeting stromal-cancer cell crosstalk networks in ovarian
cancer treatment. Biomolecules. 6:32016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prasad KV, Ao Z, Yoon Y, Wu MX, Rizk M,
Jacquot S and Schlossman SF: CD27, a member of the tumor necrosis
factor receptor family, induces apoptosis and binds to Siva, a
proapoptotic protein. Proc Natl Acad Sci USA. 94:pp. 6346–6351.
1997; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Py B, Slomianny C, Auberger P, Petit PX
and Benichou S: Siva-1 and an alternative splice form lacking the
death domain, Siva-2, similarly induce apoptosis in T lymphocytes
via a caspase-dependent mitochondrial pathway. J Immunol.
172:4008–4017. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iorio-Morin C, Germain P, Roy S, Genier S,
Labrecque P and Parent JL: Thromboxane A2 modulates
cisplatin-induced apoptosis through a Siva1-dependent mechanism.
Cell Death Differ. 19:1347–1357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gudi R, Barkinge J, Hawkins S, Chu F,
Manicassamy S, Sun Z, Duke-Cohan JS and Prasad KV: Siva-1
negatively regulates NF-kappaB activity: Effect on T-cell
receptor-mediated activation-induced cell death (AICD). Oncogene.
25:3458–3462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shiozaki T, Iwai A, Kawaoka Y, Takada A,
Kida H and Miyazaki T: Requirement for Siva-1 for replication of
influenza A virus through apoptosis induction. J Gen Virol.
92:315–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Py B, Bouchet J, Jacquot G, Sol-Foulon N,
Basmaciogullari S, Schwartz O, Biard-Piechaczyk M and Benichou S:
The Siva protein is a novel intracellular ligand of the CD4
receptor that promotes HIV-1 envelope-induced apoptosis in
T-lymphoid cells. Apoptosis. 12:1879–1892. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Nostrand JL, Brisac A, Mello SS,
Jacobs SB, Luong R and Attardi LD: The p53 target gene siva enables
non-small cell lung cancer development. Cancer Discov. 5:622–635.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Zha M, Zhao X, Jiang P, Du W, Tam
AY, Mei Y and Wu M: Siva1 inhibits p53 function by acting as an ARF
E3 ubiquitin ligase. Nat Commun. 4:15512013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du W, Jiang P, Li N, Mei Y, Wang X, Wen L,
Yang X and Wu M: Suppression of p53 activity by Siva1. Cell Death
Differ. 16:1493–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li N, Jiang P, Du W, Wu Z, Li C, Qiao M,
Yang X and Wu M: Siva1 suppresses epithelial-mesenchymal transition
and metastasis of tumor cells by inhibiting stathmin and
stabilizing microtubules. Proc Natl Acad Sci USA. 108:pp.
12851–12856. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei SH, Lin F, Wang X, Gao P and Zhang HZ:
Prognostic significance of stathmin expression in correlation with
metastasis and clinicopathological characteristics in human ovarian
carcinoma. Acta Histochem. 110:59–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ray RM, Bhattacharya S and Johnson LR:
Mdm2 inhibition induces apoptosis in p53 deficient human colon
cancer cells by activating p73- and E2F1-mediated expression of
PUMA and Siva-1. Apoptosis. 16:35–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Machado-Neto JA, Lazarini M, Favaro P, de
Melo Campos P, Scopim-Ribeiro R, Junior GC Franchi, Nowill AE, Lima
PR, Costa FF, Benichou S, et al: ANKHD1 silencing inhibits Stathmin
1 activity, cell proliferation and migration of leukemia cells.
Biochim Biophys Acta. 1853:583–593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gupta KK, Li C, Duan A, Alberico EO, Kim
OV, Alber MS and Goodson HV: Mechanism for the
catastrophe-promoting activity of the microtubule destabilizer
Op18/stathmin. Proc Natl Acad Sci USA. 110:pp. 20449–20454. 2013;
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alli E, Yang JM and Hait WN: Silencing of
stathmin induces tumor-suppressor function in breast cancer cell
lines harboring mutant p53. Oncogene. 26:1003–1012. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Price DK, Ball JR, Bahrani-Mostafavi Z,
Vachris JC, Kaufman JS, Naumann RW, Higgins RV and Hall JB: The
phosphoprotein Op18/stathmin is differentially expressed in ovarian
cancer. Cancer Invest. 18:722–730. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roos G, Brattsand G, Landberg G, Marklund
U and Gullberg M: Expression of oncoprotein 18 in human leukemias
and lymphomas. Leukemia. 7:1538–1546. 1993.PubMed/NCBI
|
|
21
|
Mistry SJ, Bank A and Atweh GF: Targeting
stathmin in prostate cancer. Mol Cancer Ther. 4:1821–1829. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kouzu Y, Uzawa K, Koike H, Saito K,
Nakashima D, Higo M, Endo Y, Kasamatsu A, Shiiba M, Bukawa H, et
al: Overexpression of stathmin in oral squamous-cell carcinoma:
Correlation with tumour progression and poor prognosis. Br J
Cancer. 94:717–723. 2006.PubMed/NCBI
|
|
23
|
Xi W, Rui W, Fang L, Ke D, Ping G and
Hui-Zhong Z: Expression of stathmin/op18 as a significant
prognostic factor for cervical carcinoma patients. J Cancer Res
Clin Oncol. 135:837–846. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuo MF, Wang HS, Kuo QT, Shun CT, Hsu HC,
Yang SH and Yuan RH: High expression of stathmin protein predicts a
fulminant course in medulloblastoma. J Neurosurg Pediatr. 4:74–80.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schulze E, Asai DJ, Bulinski JC and
Kirschner M: Posttranslational modification and microtubule
stability. J Cell Biol. 105:2167–2177. 1987. View Article : Google Scholar : PubMed/NCBI
|