Breast cancer is the most common type of cancer
diagnosed in females between the ages of 30 and 59 years, and is
the leading cause of cancer-associated mortality in females <45
years (1). Breast cancer is a
heterogeneous disease with 5 molecular subtypes: Luminal A, luminal
B, human epidermal growth factor receptor 2 (HER2)-enriched,
normal-like breast cancer and basal-like breast cancer. Each of
these subtypes exhibits important prognostic and predictive
information which enables the appropriate treatment to be
administered (2–4). Hormone receptor (HR) status serves a
notable role in selecting systemic hormonal therapy for patients
with early and advanced breast cancer (5–7). Endocrine
therapy is the preferred option for HR+ disease, even in
the presence of visceral disease, unless there is an indication of
endocrine resistance or rapidly progressive disease (8).
Estrogen receptor (ER) and/or progesterone receptor
(PR) expression occurs in ~50% of patients with HER2+
breast cancer and patients with ER+/HER2+
metastatic disease typically obtain less benefit from endocrine
therapy, compared with patients with
HER2−/HR+ disease (9–14).
Previous studies have suggested that cross-talk between the ER and
HER2 pathways is implicated in resistance to endocrine therapy and
supports tumor progression (15–18).
Furthermore, several clinical studies indicate that simultaneous
inhibition of the HER2 and ER signaling pathways is more effective
than ER inhibition alone (19–21). These
studies suggest that a combination of endocrine therapy and HER2
inhibition may be the optimal treatment for
HR+/HER2+ breast cancer; however, this
remains unclear.
The interaction between the phosphoinositide
3-kinase (PI3K)-protein kinase B-mammalian target of rapamycin
(mTOR) and ER signaling pathways is an additional emerging
mechanism of endocrine resistance (22–24). mTOR
is a downstream target in the HER2 signaling pathway and is
associated with the activity of the ER signaling pathway. A number
of clinical studies have identified the value of using allosteric
mTOR inhibitors in combination with anti-estrogen therapy in
advanced endocrine-resistant tumors. Everolimus, an mTOR inhibitor,
is effective in treating tumors which exhibit increased activity of
the mTOR signaling pathway and therefore is a potential agent for
the reversal of endocrine resistance (25,26).
To the best of our knowledge, there have been no
previous studies on the co-suppression of the ER, HER2 and mTOR
signaling pathways. The present case report describes a patient
with metastatic breast cancer who has received systemic treatment
with a combination of an estrogen inhibitor, trastuzumab and
everolimus. Progression-free survival (PFS) of the patient has
currently extended to 27 months and continues at the time of
writing. Thus, the inhibition of ER, HER2 and mTOR may produce an
improved clinical outcome, suggesting a combination strategy for
patients with HR+/HER2+ breast cancer.
In March 2002, a 48-year-old female identified a
mass in her right breast. The patient underwent a modified radical
mastectomy with axillary lymph node dissection in the same month.
Pathological examination revealed a 2×2 cm infiltrative ductal
carcinoma that was ER+, PR+ and
HER2−, with no metastases to the axillary lymph nodes at
that time (pT1N0M0, using the pathological tumor-node-metastasis
staging). The patient was administered adjuvant chemotherapy
comprising cyclophosphamide (500 mg/m2), epriubicin (100
mg/m2) and 5-fluouracil (500 mg/m2) every 3
weeks over 18 weeks (for 6 cycles). Subsequently, the patient
received anti-estrogen therapy (tamoxifen at 20 mg/day) for 5 years
and regular medical examinations.
In March 2010, the patient identified a hard lump in
a right supraclavicular lymph node. The subsequent computed
tomography (CT) scan of the chest revealed multiple nodules in the
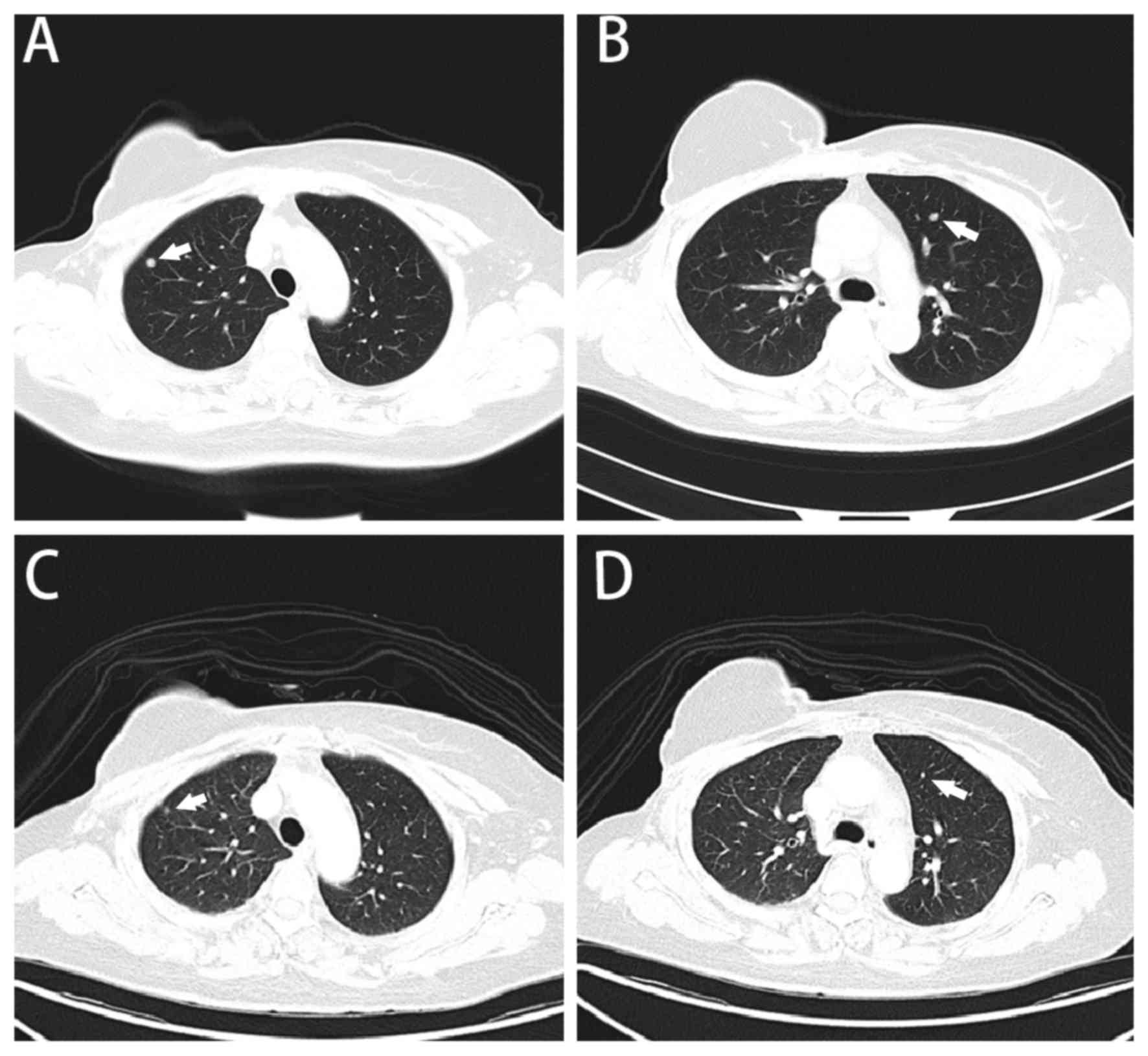
lungs (Fig. 1A and B). The subsequent
excision biopsy revealed metastatic adenocarcinoma, and
immunohistochemistry identified it to be ER+,
PR− and HER2++-HER2+++. Tumor
sections were deparaffinized and stained with antibodies against
ER, PR, HER2, using standard protocols (27,28).
Fluorescent in situ hybridization (FISH) determined HER2
gene amplification. Docetaxel (75 mg/m2 on day 1) and
capecitabine (1,250 mg/m2 twice daily on days 1–14) was
administered in combination with trastuzumab (8 mg/kg loading dose,
6 mg/kg subsequently) every 3 weeks for 3 cycles until the patient
developed hand-foot syndrome. The patient experienced reddening,
desquamation and numbness of the palms of the hands and soles of
the feet, due to the side effects of capecitabine. Subsequently,
capecitabine was replaced by gemcitabine (1,000 mg/m2 on
days 1 and 8). The regimen was carried on for 3 cycles until August
2010, when a CT scan indicated partial remission of the pulmonary
metastasis (Fig. 1C and D).
Subsequently, the patient was administered anastrozole (1 mg/day)
combined with trastuzumab (6 mg/kg) every 3 weeks. The
administration of trastuzumab was stopped after 1 year for
financial reasons.
In May 2012, the patient identified another lump in
the right supraclavicular fossa; however, a CT revealed no
progression of the lesions in the lungs. Radiation therapy of 24 Gy
in 12 fractions was administered to the right supraclavicular lymph
nodes with a complete response. Subsequently, the patient received
endocrine therapy with fulvestrant (250 mg every 4 weeks) followed
by stable disease for 19 months. In December 2013, a CT scan
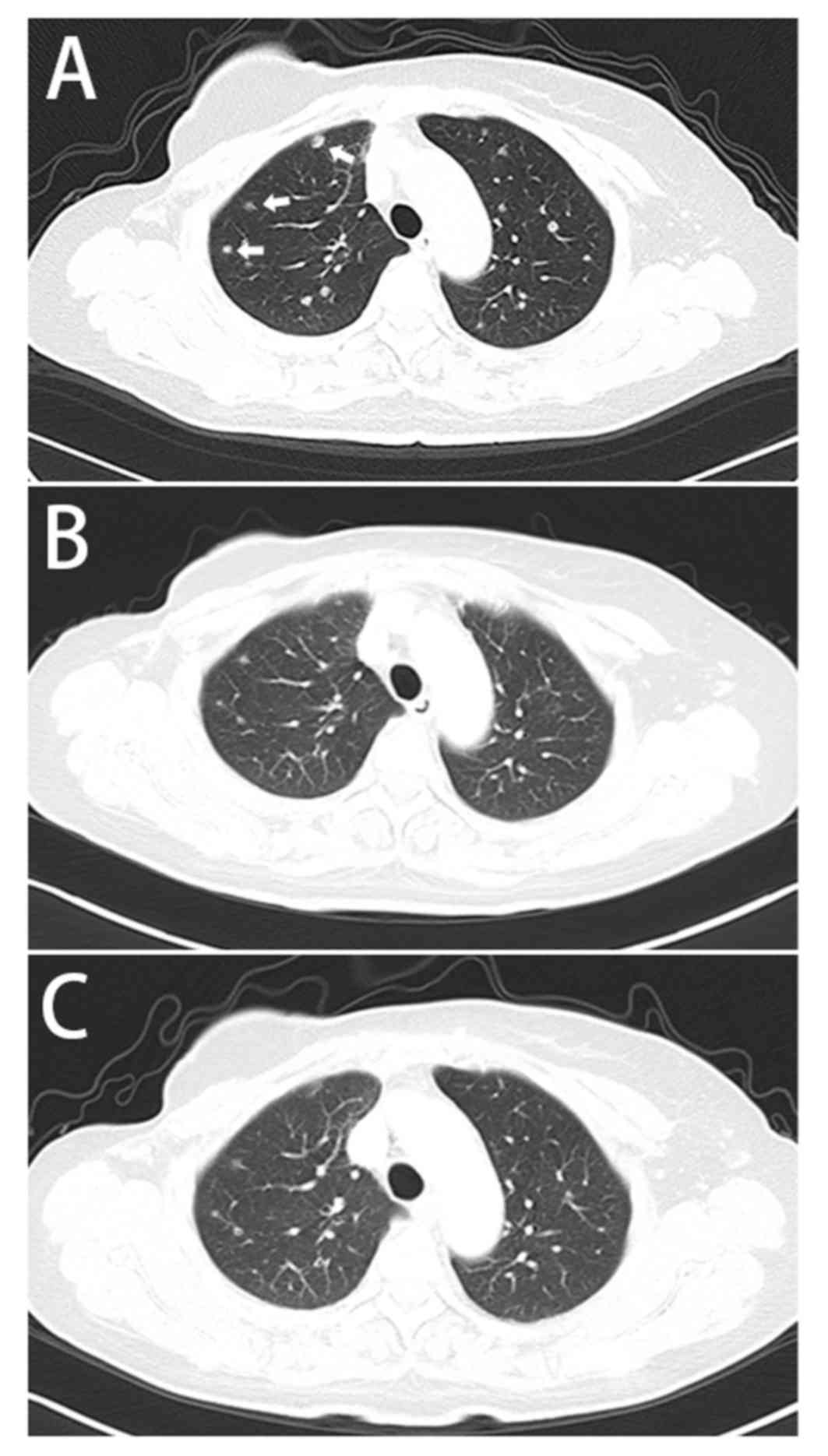
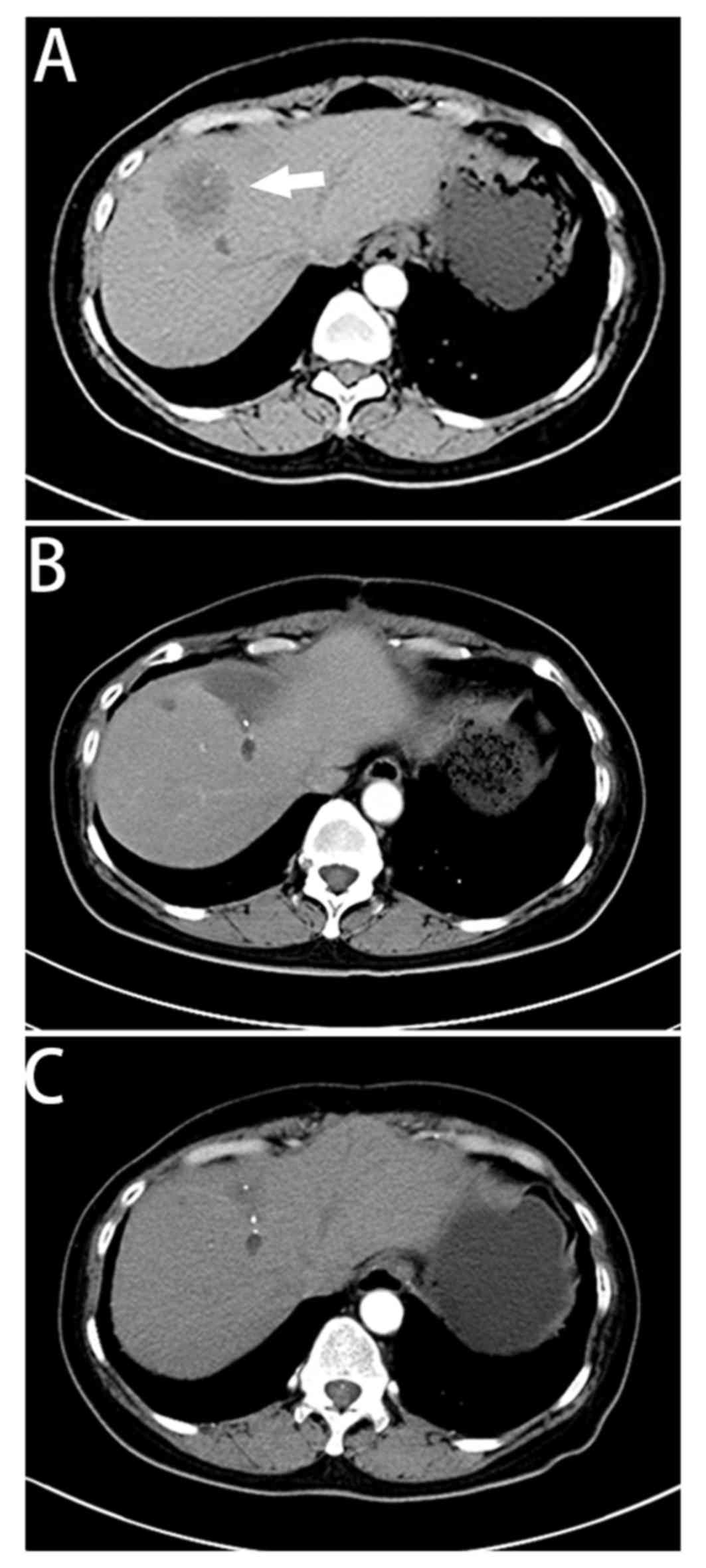
identified novel nodules in the lungs (Fig. 2) and a single 4×4 cm low-intensity
lesion in the liver (Fig. 3). In
addition, the level of the tumor marker CA153 was revealed to be
>300 U/l. The pulmonary metastases were stable. The patient
underwent surgical resection of the liver metastasis 1 week later
and whole-exome sequencing was performed on a partially resected
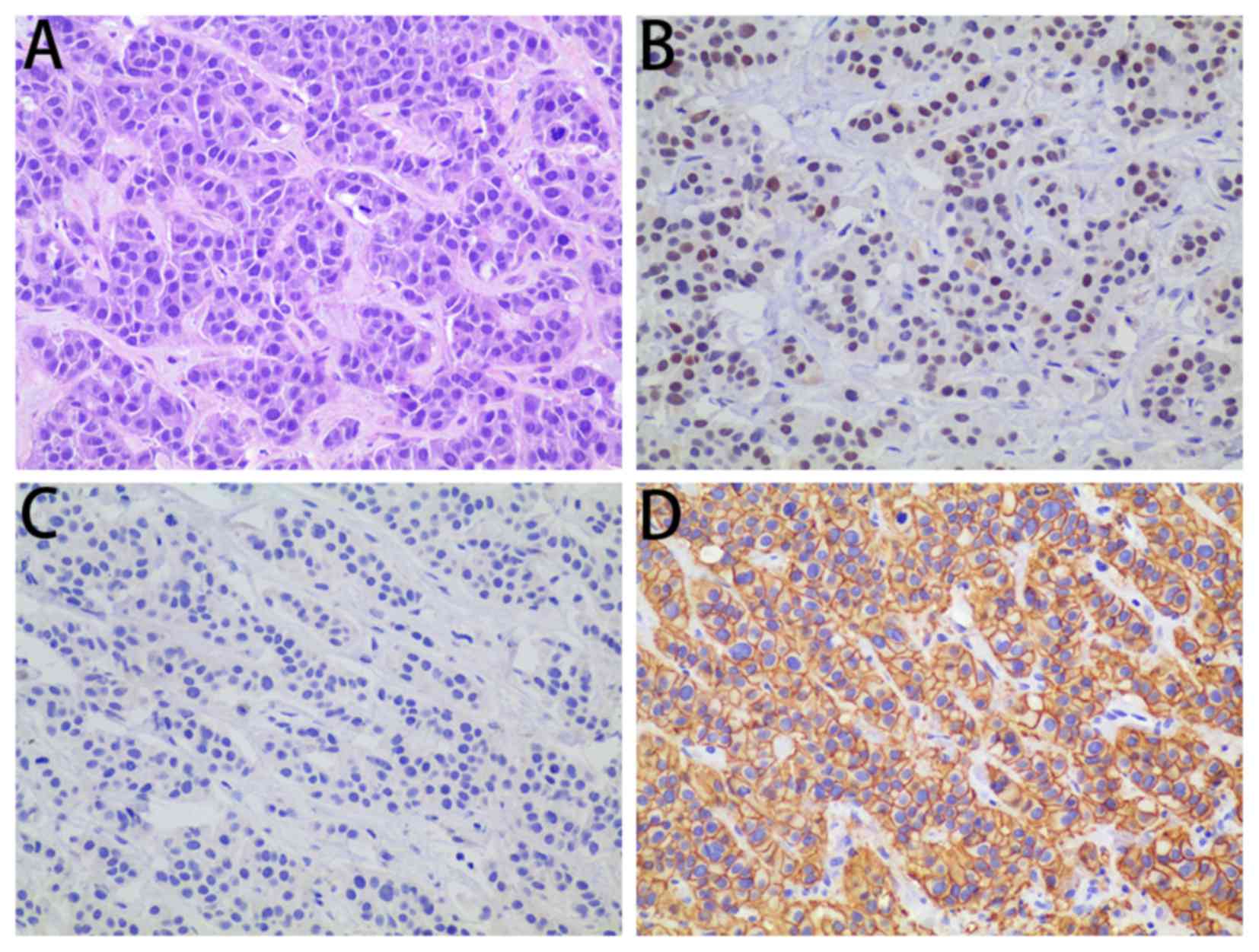
specimen of the liver. Postoperative pathology revealed liver
adenocarcinoma derived from the breast; immunohistochemistry
indicated that they were ER+, PR− and
HER2+++ (Fig. 4). The
patient was administered everolimus (5 mg/day) and exemestane (25
mg/day) in combination with trastuzumab every 3 weeks. After 5
months of treatment, there was partial remission of the lesions in
the lungs and liver (Figs. 2B and
3B) with a marked decrease in levels
of CA153. The patient currently remains on the combined regimen of
everolimus, trastuzumab and exemestane, and regular medical
examinations have identified no recurrence or additional metastases
for >27 months (Figs. 2C and
3C).
All procedures performed in the present case report
were in accordance with The Declaration of Helsinki (1964) and its
later amendments or comparable ethical standards. Written informed
consent was obtained from the patient for inclusion in the present
case report.
Cross-talk between the ER and HER2 signaling
pathways is implicated in resistance to endocrine therapy and
therefore supports tumor progression (15,16).
Massarweh et al (29)
identified that tamoxifen resistance is mediated by the activation
of HER family signaling which may be a result of increased
expression of HER ligands and the release of membrane-bound HER
ligands which act in an autocrine manner; however, this may be
inhibited by the HER inhibitor gefitinib. In addition, Evans et
al (35) demonstrated that
AEE788, an epithelial growth factor receptor/HER2 inhibitor,
increased ER-mediated transcription in
HER2+/ER+ breast cancer cells. This indicated
that letrozole in combination with AEE78 may be superior to
letrozole alone for the treatment of acquired
ER+/HER2+ endocrine-resistant breast cancer.
A previous study provided a rationale for the dual inhibition of ER
and HER2; for instance, the Trastuzumab and Anastrozole Directed
Against ER-Positive HER2-Positive Mammary Carcinoma study, the
first randomized Phase III study to combine a hormonal agent with
trastuzumab without chemotherapy to treat
HR+/HER2+ metastatic breast cancer, has
identified that the combination of trastuzumab and anastrozole
improves outcomes, compared with anastrozole alone (19). An additional study has demonstrated
that the combination of letrozole and trastuzumab produces durable
responses in HER2+ and ER+ advanced breast
cancers: The median time to progression was 5.8 months and the
duration of response was >20.6 months (9). These observations suggest that the
optimal treatment for HR+/HER2+ breast
cancers may be a combination of endocrine therapy and HER2
inhibitors (36). On the basis of the
results of previous studies (9,19,35), endocrine therapy combined with
trastuzumab in the first- and second-line treatment of advanced
breast cancer was selected in the present case report. When the
first progression was assessed in a right supraclavicular lymph
node and the lungs, trastuzumab in combination with chemotherapy
was recommended to control the disease. Subsequently, trastuzumab
was combined with anastrozole for maintenance therapy. When the
second progression was observed, following a complete response to
radiation therapy, anastrozole was replaced by fulvestrant to
stabilize the disease for a longer period of time. However, the
combination of trastuzumab and endocrine therapy may only be
recommended for patients with less extensive or asymptomatic
metastatic disease. For young patients, or those with
life-threatening or symptomatic disease, chemotherapy-based
HER2-targeted combination therapy may be preferred.
In the present report, after 19 months of PFS, a
single liver metastasis was identified by CT. Although liver
resection in patients with breast cancer exhibiting extrahepatic
metastases has been debated, a number of studies determining the
role of therapeutic hepatic metastasectomy have demonstrated
encouraging survival statistics for patients with tumors of low
histological grade, long disease-free interval and patients with
well-controlled extrahepatic metastases (37–39). Chua
et al (40) identified a
median survival time following partial hepatectomy of 40 months and
a 5-year survival rate of 40%, which suggested that surgery for
liver metastases, due to breast cancer, may be an effective
therapeutic strategy. However, a number of studies have
demonstrated that hormone-refractory liver metastasis is a negative
predictor of overall survival rate (38,41).
Therefore, continued treatment following the resection of liver
metastases is of importance.
The question remains whether, following long PFS
owing to dual inhibition of ER and HER, there is a way of improving
the regimen for patients confronted with progression. An emerging
mechanism of endocrine resistance is the interaction between the
PI3K-protein kinase B-mTOR and ER signaling pathways (22–24). In a
previous study involving patients with newly diagnosed breast
cancer, neoadjuvant everolimus combined with letrozole improved the
clinical response rate and decreased tumor cell viability, compared
with letrozole alone (42). The
Tamoxifen Plus Everolimus randomized Groupe d'Investigateurs
Nationaux pour l'Etude des Cancers de l'Ovaire et du Sein trial
(43) identified an improved time to
progression: 4.5 months with tamoxifen alone vs. 8.6 months with
tamoxifen plus everolimus. The Breast Cancer Trials of Oral
Everolimus-2 (BOLERO-2) study (44)
revealed that the addition of everolimus to exemestane markedly
improved PFS; the median PFS time, on the basis of central
assessment, was 10.6 and 4.1 months, respectively (hazard ratio,
0.36; 95% confidence interval, 0.27–0.47; P<0.001). Notably,
everolimus was additionally identified to reverse trastuzumab in
metastatic breast cancer. The results of the BOLERO-3 study
(26) demonstrated that the addition
of everolimus to trastuzumab with vinorelbine markedly prolonged
PFS time in patients with trastuzumab-resistant and
taxane-pretreated HER2+ advanced breast cancer; however,
their hormone receptor status was not assessed. Patients with
ER− breast cancer exhibit an increased benefit compared
with patients with ER+ breast cancer, indicating the
importance of dual inhibition of the HER2 signal and the ER
signaling pathway. Patients with a loss of phosphatase and tensin
homolog (PTEN) and mutations to phosphatidylinositol
4,5-bisphosphate 3-kinase catalytic subunit-α isoform (PIK3CA) have
a greater sensitivity to everolimus; although next-generation
sequencing of the resected liver specimen revealed no loss of PTEN
and or PIK3CA mutations, it remains likely that the patient would
benefit from everolimus (45,46).
The patient was recommended to continue to follow
the principle of dual inhibition of ER and HER2. Everolimus was
introduced into the regimen on the assumption that it may
strengthen the therapeutic effect. Although there was no clinical
evidence of the efficacy of exemestane with everolimus and
trastuzumab, the patient provided written informed consent for the
treatment. The patient's response was continually monitored during
treatment. The patient achieved a long-term remission and is in
good health at the time of writing.
The combined regimen of exemestane with everolimus
and trastuzumab may be used for similar cases. To the best of our
knowledge, the present case report is the first in which the
patient's PFS reached >27 months. Additional observation has
been undertaken and case series or small-scale clinical trials
would be welcome to increase understanding on this topic.
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sorlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:pp. 10869–10874. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parker JS, Mullins M, Cheang MC, Leung S,
Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, et al:
Supervised risk predictor of breast cancer based on intrinsic
subtypes. J Clin Oncol. 27:1160–1167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldhirsch A, Coates AS, Gelber RD, Glick
JH, Thürlimann B and Senn HJ: St Gallen Expert Panel Members:
First-select the target: Better choice of adjuvant treatments for
breast cancer patients. Ann Oncol. 17:1772–1776. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buzdar AU: Endocrine therapy in the
treatment of metastatic breast cancer. Semin Oncol. 28:291–304.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jordan C: Historical perspective on
hormonal therapy of advanced breast cancer. Clin Ther. 24 Suppl
A:A3–A16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cardoso F, Costa A, Norton L, Cameron D,
Cufer T, Fallowfield L, Francis P, Gligorov J, Kyriakides S, Lin N,
et al: 1st International consensus guidelines for advanced breast
cancer (ABC 1). Breast. 21:242–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marcom PK, Isaacs C, Harris L, Wong ZW,
Kommarreddy A, Novielli N, Mann G, Tao Y and Ellis MJ: The
combination of letrozole and trastuzumab as first or second-line
biological therapy produces durable responses in a subset of HER2
positive and ER positive advanced breast cancers. Breast Cancer Res
Treat. 102:43–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ellis M: Overcoming endocrine therapy
resistance by signal transduction inhibition. Oncologist. 9 Suppl
3:S20–S26. 2004. View Article : Google Scholar
|
|
11
|
Dowsett M, Allred C, Knox J, Quinn E,
Salter J, Wale C, Cuzick J, Houghton J, Williams N, Mallon E, et
al: Relationship between quantitative estrogen and progesterone
receptor expression and human epidermal growth factor receptor 2
(HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or
in Combination trial. J Clin Oncol. 26:1059–1065. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jones A: Combining trastuzumab (Herceptin)
with hormonal therapy in breast cancer: What can be expected and
why? Ann Oncol. 14:1697–1704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lipton A, Ali SM, Leitzel K, Demers L,
Chinchilli V, Engle L, Harvey HA, Brady C, Nalin CM, Dugan M, et
al: Elevated serum Her-2/neu level predicts decreased response to
hormone therapy in metastatic breast cancer. J Clin Oncol.
20:1467–1472. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Laurentiis M, Arpino G, Massarelli E,
Ruggiero A, Carlomagno C, Ciardiello F, Tortora G, D'Agostino D,
Caputo F, Cancello G, et al: A meta-analysis on the interaction
between HER-2 expression and response to endocrine treatment in
advanced breast cancer. Clin Cancer Res. 11:4741–4748. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Z, Wang Y, Warden C and Chen S:
Cross-talk between ER and HER2 regulates c-MYC-mediated glutamine
metabolism in aromatase inhibitor resistant breast cancer cells. J
Steroid Biochem Mol Biol. 149:118–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui J, Germer K, Wu T, Wang J, Luo J, Wang
SC, Wang Q and Zhang X: Cross-talk between HER2 and MED1 regulates
tamoxifen resistance of human breast cancer cells. Cancer Res.
72:5625–5634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shou J, Massarweh S, Osborne CK, Wakeling
AE, Ali S, Weiss H and Schiff R: Mechanisms of tamoxifen
resistance: Increased estrogen receptor-HER2/neu cross-talk in
ER/HER2-positive breast cancer. J Natl Cancer Inst. 96:926–935.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Osborne CK, Shou J, Massarweh S and Schiff
R: Crosstalk between estrogen receptor and growth factor receptor
pathways as a cause for endocrine therapy resistance in breast
cancer. Clin Cancer Res. 11:865s–870s. 2005.PubMed/NCBI
|
|
19
|
Kaufman B, Mackey JR, Clemens MR, Bapsy
PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova
A, et al: Trastuzumab plus anastrozole versus anastrozole alone for
the treatment of postmenopausal women with human epidermal growth
factor receptor 2-positive, hormone receptor-positive metastatic
breast cancer: Results from the randomized phase III TandEM study.
J Clin Oncol. 27:5529–5537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huober J, Fasching PA, Barsoum M,
Petruzelka L, Wallwiener D, Thomssen C, Reimer T, Paepke S, Azim
HA, Ragosch V, et al: Higher efficacy of letrozole in combination
with trastuzumab compared to letrozole monotherapy as first-line
treatment in patients with HER2-positive, hormone-receptor-positive
metastatic breast cancer-results of the eLEcTRA trial. Breast.
21:27–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnston S, Pippen J Jr, Pivot X,
Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A,
Kennedy MJ, et al: Lapatinib combined with letrozole versus
letrozole and placebo as first-line therapy for postmenopausal
hormone receptor-positive metastatic breast cancer. J Clin Oncol.
27:5538–5546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnston SR: Clinical efforts to combine
endocrine agents with targeted therapies against epidermal growth
factor receptor/human epidermal growth factor receptor 2 and
mammalian target of rapamycin in breast cancer. Clin Cancer Res.
12:1061s–1068s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bostner J, Karlsson E, Pandiyan MJ,
Westman H, Skoog L, Fornander T, Nordenskjöld B and Stål O:
Activation of Akt, mTOR, and the estrogen receptor as a signature
to predict tamoxifen treatment benefit. Breast Cancer Res Treat.
137:397–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grunt TW and Mariani GL: Novel approaches
for molecular targeted therapy of breast cancer: Interfering with
PI3K/AKT/mTOR signaling. Curr Cancer Drug Targets. 13:188–204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano
MS, Neciosup SP, Tseng LM, Zhang Q, Shen K, Liu D, et al:
Combination of everolimus with trastuzumab plus paclitaxel as
first-line treatment for patients with HER2-positive advanced
breast cancer (BOLERO-1): A phase 3, randomised, double-blind,
multicentre trial. Lancet Oncol. 16:816–829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
André F, O'Regan R, Ozguroglu M, Toi M, Xu
B, Jerusalem G, Masuda N, Wilks S, Arena F, Isaacs C, et al:
Everolimus for women with trastuzumab-resistant, HER2-positive,
advanced breast cancer (BOLERO-3): A randomised, double-blind,
placebo-controlled phase 3 trial. Lancet Oncol. 15:580–591. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hammond ME, Hayes DF, Dowestt M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer (unabridged version). Arch Pathol Lab Med.
134:e48–e72. 2010.PubMed/NCBI
|
|
28
|
Wolff AC, Hammond EA, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Massarweh S, Osborne CK, Creighton CJ, Qin
L, Tsimelzon A, Huang S, Weiss H, Rimawi M and Schiff R: Tamoxifen
resistance in breast tumors is driven by growth factor receptor
signaling with repression of classic estrogen receptor genomic
function. Cancer Res. 68:826–833. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Donders F, Kuypers D, Wolter P and Neven
P: Everolimus in acute kidney injury in a patient with breast
cancer: A case report. J Med Case Rep. 8:3862014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, V.3.
2015.http://www.nccn.org/professionals/physician_gls/pdf/breast.pdfAugust
30–2015
|
|
32
|
Dowsett M, Cuzick J, Wale C, Howell T,
Houghton J and Baum M: Retrospective analysis of time to recurrence
in the ATAC trial according to hormone receptor status: An
hypothesis-generating study. J Clin Oncol. 23:7512–7517. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ellis MJ, Coop A, Singh B, Mauriac L,
Llombert-Cussac A, Jänicke F, Miller WR, Evans DB, Dugan M, Brady
C, et al: Letrozole is more effective neoadjuvant endocrine therapy
than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen
receptor-positive primary breast cancer: Evidence from a phase III
randomized trial. J Clin Oncol. 19:3808–3816. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Robertson JF, Llombart-Cussac A, Rolski J,
Feltl D, Dewar J, Macpherson E, Lindemann J and Ellis MJ: Activity
of fulvestrant 500 mg versus anastrozole 1 mg as first-line
treatment for advanced breast cancer: Results from the FIRST study.
J Clin Oncol. 27:4530–4535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Evans AH, Pancholi S, Farmer I, Thornhill
A, Evans DB, Johnston SR, Dowsett M and Martin LA: EGFR/HER2
inhibitor AEE788 increases ER-mediated transcription in
HER2/ER-positive breast cancer cells but functions synergistically
with endocrine therapy. Br J Cancer. 102:1235–1243. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Glück S, Arteaga CL and Osborne CK:
Optimizing chemotherapy-free survival for the ER/HER2-positive
metastatic breast cancer patient. Clin Cancer Res. 17:5559–5561.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abbott DE, Brouquet A, Mittendorf EA,
Andreou A, Meric-Bernstam F, Valero V, Green MC, Kuerer HM, Curley
SA, Abdalla EK, et al: Resection of liver metastases from breast
cancer: Estrogen receptor status and response to chemotherapy
before metastasectomy define outcome. Surgery. 151:710–716. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hoffmann K, Franz C, Hinz U, Schirmacher
P, Herfarth C, Eichbaum M, Büchler MW and Schemmer P: Liver
resection for multimodal treatment of breast cancer metastases:
Identification of prognostic factors. Ann Surg Oncol. 17:1546–1554.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singletary SE, Walsh G, Vauthey JN, Curley
S, Sawaya R, Weber KL, Meric F and Hortobágyi GN: A role for
curative surgery in the treatment of selected patients with
metastatic breast cancer. Oncologist. 8:241–251. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chua TC, Saxena A, Liauw W, Chu F and
Morris DL: Hepatic resection for metastatic breast cancer: A
systematic review. Eur J Cancer. 47:2282–2290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Martinez SR, Young SE, Giuliano AE and
Bilchik AJ: The utility of estrogen receptor, progesterone
receptor, and Her-2/neu status to predict survival in patients
undergoing hepatic resection for breast cancer metastases. Am J
Surg. 191:281–283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baselga J, Semiglazov V, van Dam P,
Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R,
Bianchi G, et al: Phase II randomized study of neoadjuvant
everolimus plus letrozole compared with placebo plus letrozole in
patients with estrogen receptor-positive breast cancer. J Clin
Oncol. 27:2630–2637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bachelot T, Bourgier C, Cropet C,
Ray-Coquard I, Ferrero JM, Freyer G, Abadie-Lacourtoisie S, Eymard
JC, Debled M, Spaëth D, et al: Randomized phase II trial of
everolimus in combination with tamoxifen in patients with hormone
receptor-positive, human epidermal growth factor receptor
2-negative metastatic breast cancer with prior exposure to
aromatase inhibitors: A GINECO study. J Clin Oncol. 30:2718–2724.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Baselga J, Campone M, Piccart M, Burris HA
III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun
F, et al: Everolimus in postmenopausal hormone-receptor-positive
advanced breast cancer. N Engl J Med. 366:520–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gonzalez-Angulo AM and Blumenschein GR Jr:
Defining biomarkers to predict sensitivity to PI3K/Akt/mTOR pathway
inhibitors in breast cancer. Cancer Treat Rev. 39:313–320. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hurvitz SA, Kalous O, Conklin D, Desai AJ,
Dering J, Anderson L, O'Brien NA, Kolarova T, Finn RS, Linnartz R,
et al: In vitro activity of the mTOR inhibitor everolimus, in a
large panel of breast cancer cell lines and analysis for predictors
of response. Breast Cancer Res Treat. 149:669–680. 2015. View Article : Google Scholar : PubMed/NCBI
|