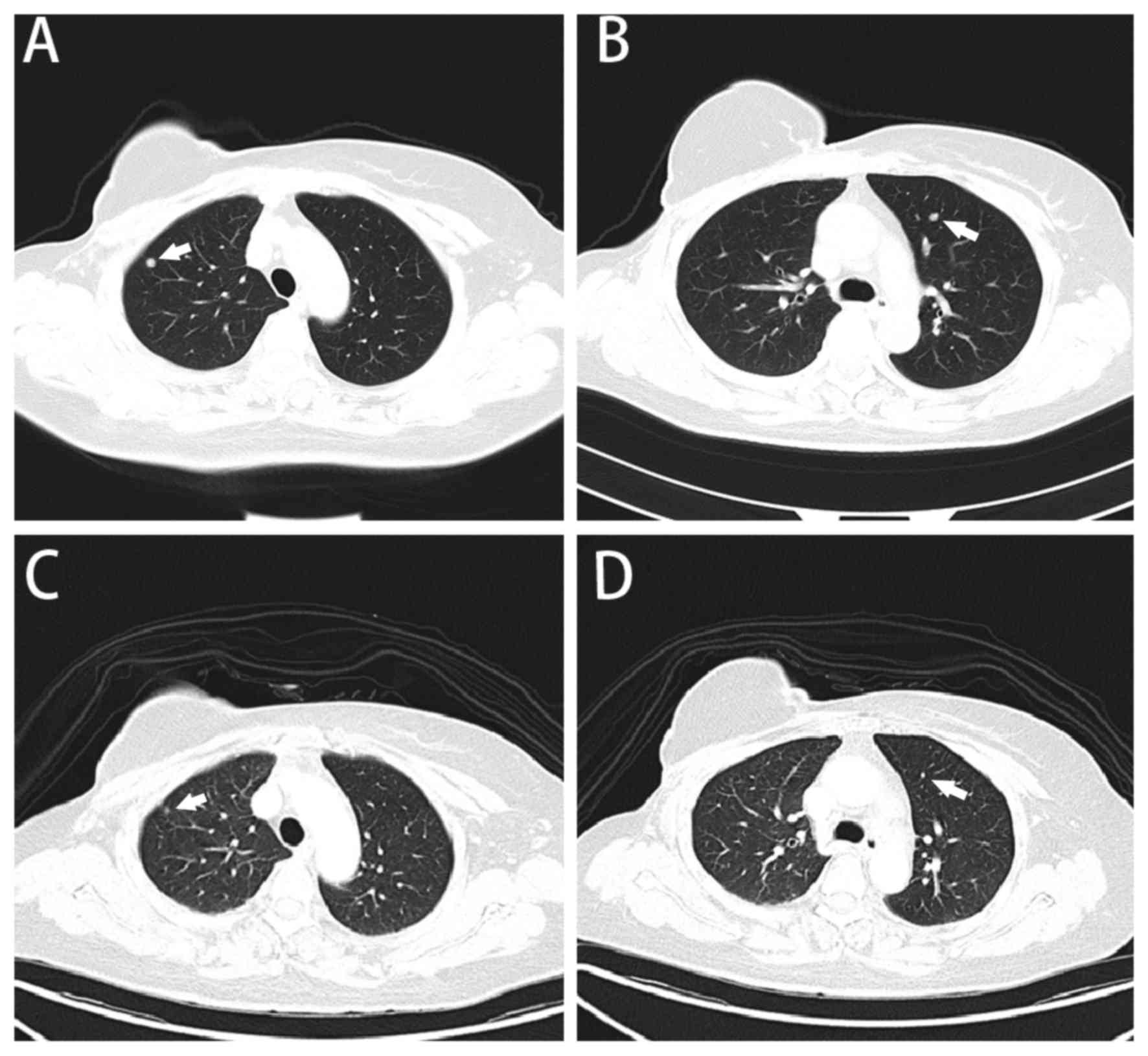
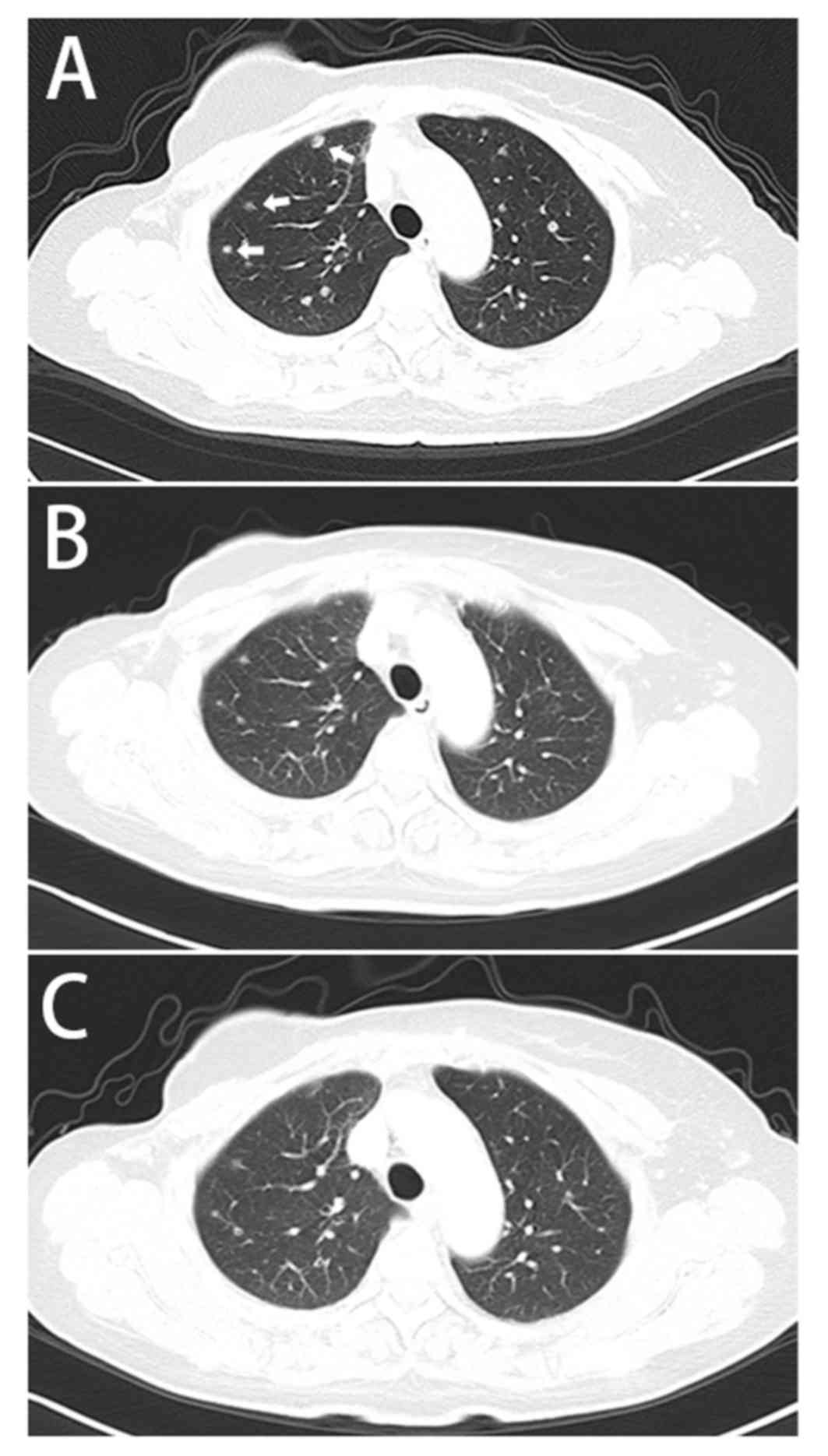
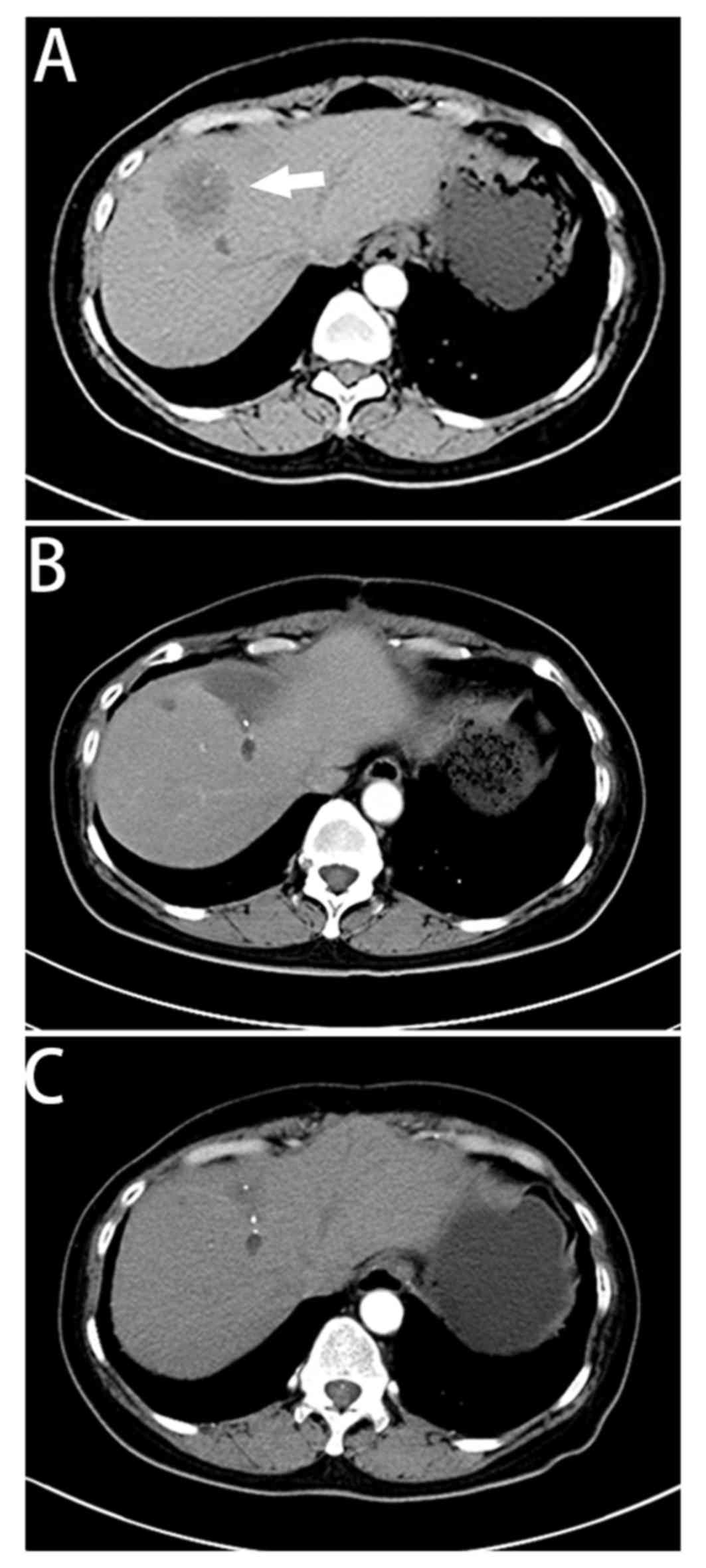
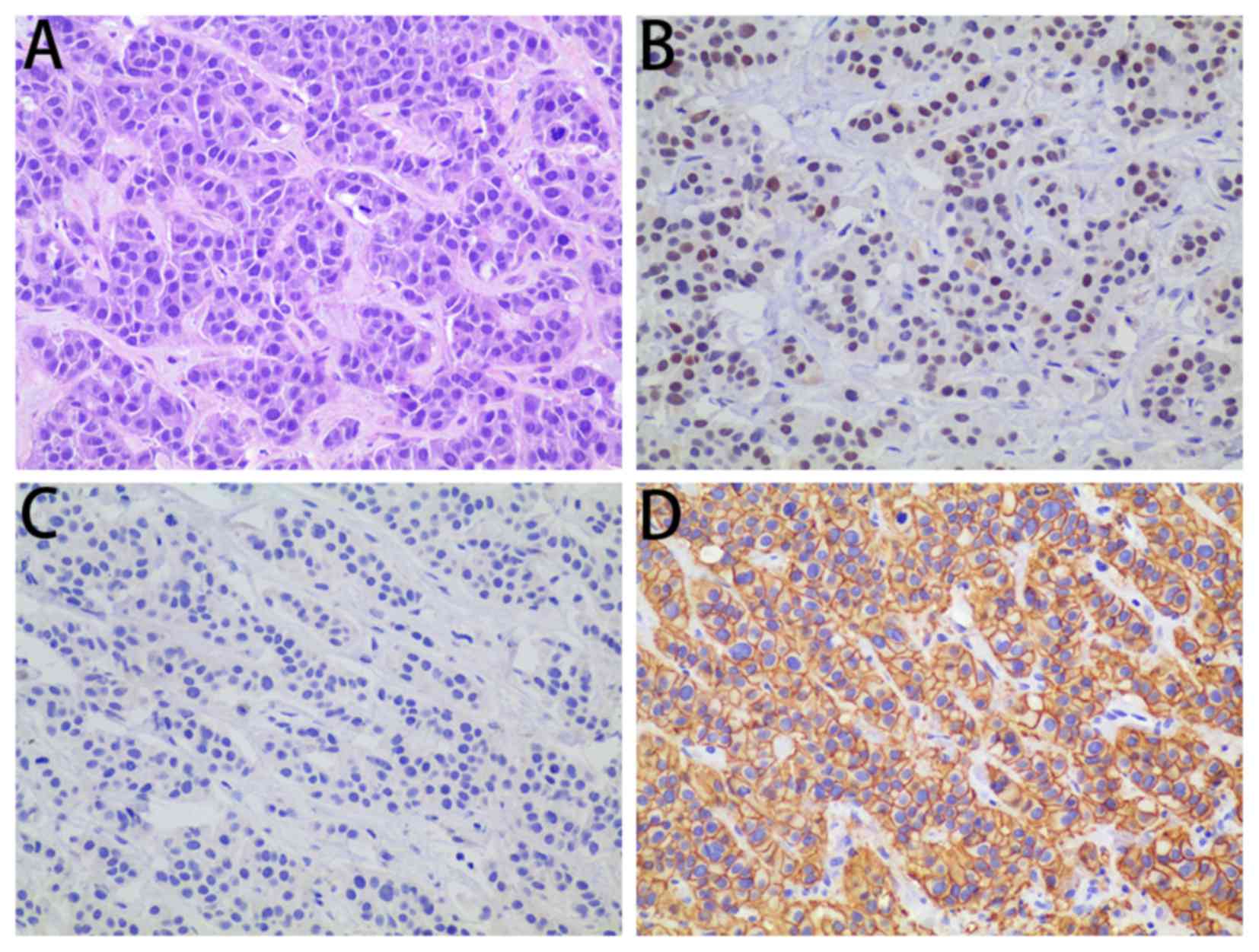
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sorlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:pp. 10869–10874. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parker JS, Mullins M, Cheang MC, Leung S,
Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, et al:
Supervised risk predictor of breast cancer based on intrinsic
subtypes. J Clin Oncol. 27:1160–1167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldhirsch A, Coates AS, Gelber RD, Glick
JH, Thürlimann B and Senn HJ: St Gallen Expert Panel Members:
First-select the target: Better choice of adjuvant treatments for
breast cancer patients. Ann Oncol. 17:1772–1776. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buzdar AU: Endocrine therapy in the
treatment of metastatic breast cancer. Semin Oncol. 28:291–304.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jordan C: Historical perspective on
hormonal therapy of advanced breast cancer. Clin Ther. 24 Suppl
A:A3–A16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cardoso F, Costa A, Norton L, Cameron D,
Cufer T, Fallowfield L, Francis P, Gligorov J, Kyriakides S, Lin N,
et al: 1st International consensus guidelines for advanced breast
cancer (ABC 1). Breast. 21:242–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marcom PK, Isaacs C, Harris L, Wong ZW,
Kommarreddy A, Novielli N, Mann G, Tao Y and Ellis MJ: The
combination of letrozole and trastuzumab as first or second-line
biological therapy produces durable responses in a subset of HER2
positive and ER positive advanced breast cancers. Breast Cancer Res
Treat. 102:43–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ellis M: Overcoming endocrine therapy
resistance by signal transduction inhibition. Oncologist. 9 Suppl
3:S20–S26. 2004. View Article : Google Scholar
|
|
11
|
Dowsett M, Allred C, Knox J, Quinn E,
Salter J, Wale C, Cuzick J, Houghton J, Williams N, Mallon E, et
al: Relationship between quantitative estrogen and progesterone
receptor expression and human epidermal growth factor receptor 2
(HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or
in Combination trial. J Clin Oncol. 26:1059–1065. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jones A: Combining trastuzumab (Herceptin)
with hormonal therapy in breast cancer: What can be expected and
why? Ann Oncol. 14:1697–1704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lipton A, Ali SM, Leitzel K, Demers L,
Chinchilli V, Engle L, Harvey HA, Brady C, Nalin CM, Dugan M, et
al: Elevated serum Her-2/neu level predicts decreased response to
hormone therapy in metastatic breast cancer. J Clin Oncol.
20:1467–1472. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Laurentiis M, Arpino G, Massarelli E,
Ruggiero A, Carlomagno C, Ciardiello F, Tortora G, D'Agostino D,
Caputo F, Cancello G, et al: A meta-analysis on the interaction
between HER-2 expression and response to endocrine treatment in
advanced breast cancer. Clin Cancer Res. 11:4741–4748. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Z, Wang Y, Warden C and Chen S:
Cross-talk between ER and HER2 regulates c-MYC-mediated glutamine
metabolism in aromatase inhibitor resistant breast cancer cells. J
Steroid Biochem Mol Biol. 149:118–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui J, Germer K, Wu T, Wang J, Luo J, Wang
SC, Wang Q and Zhang X: Cross-talk between HER2 and MED1 regulates
tamoxifen resistance of human breast cancer cells. Cancer Res.
72:5625–5634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shou J, Massarweh S, Osborne CK, Wakeling
AE, Ali S, Weiss H and Schiff R: Mechanisms of tamoxifen
resistance: Increased estrogen receptor-HER2/neu cross-talk in
ER/HER2-positive breast cancer. J Natl Cancer Inst. 96:926–935.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Osborne CK, Shou J, Massarweh S and Schiff
R: Crosstalk between estrogen receptor and growth factor receptor
pathways as a cause for endocrine therapy resistance in breast
cancer. Clin Cancer Res. 11:865s–870s. 2005.PubMed/NCBI
|
|
19
|
Kaufman B, Mackey JR, Clemens MR, Bapsy
PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova
A, et al: Trastuzumab plus anastrozole versus anastrozole alone for
the treatment of postmenopausal women with human epidermal growth
factor receptor 2-positive, hormone receptor-positive metastatic
breast cancer: Results from the randomized phase III TandEM study.
J Clin Oncol. 27:5529–5537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huober J, Fasching PA, Barsoum M,
Petruzelka L, Wallwiener D, Thomssen C, Reimer T, Paepke S, Azim
HA, Ragosch V, et al: Higher efficacy of letrozole in combination
with trastuzumab compared to letrozole monotherapy as first-line
treatment in patients with HER2-positive, hormone-receptor-positive
metastatic breast cancer-results of the eLEcTRA trial. Breast.
21:27–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnston S, Pippen J Jr, Pivot X,
Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A,
Kennedy MJ, et al: Lapatinib combined with letrozole versus
letrozole and placebo as first-line therapy for postmenopausal
hormone receptor-positive metastatic breast cancer. J Clin Oncol.
27:5538–5546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnston SR: Clinical efforts to combine
endocrine agents with targeted therapies against epidermal growth
factor receptor/human epidermal growth factor receptor 2 and
mammalian target of rapamycin in breast cancer. Clin Cancer Res.
12:1061s–1068s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bostner J, Karlsson E, Pandiyan MJ,
Westman H, Skoog L, Fornander T, Nordenskjöld B and Stål O:
Activation of Akt, mTOR, and the estrogen receptor as a signature
to predict tamoxifen treatment benefit. Breast Cancer Res Treat.
137:397–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grunt TW and Mariani GL: Novel approaches
for molecular targeted therapy of breast cancer: Interfering with
PI3K/AKT/mTOR signaling. Curr Cancer Drug Targets. 13:188–204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano
MS, Neciosup SP, Tseng LM, Zhang Q, Shen K, Liu D, et al:
Combination of everolimus with trastuzumab plus paclitaxel as
first-line treatment for patients with HER2-positive advanced
breast cancer (BOLERO-1): A phase 3, randomised, double-blind,
multicentre trial. Lancet Oncol. 16:816–829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
André F, O'Regan R, Ozguroglu M, Toi M, Xu
B, Jerusalem G, Masuda N, Wilks S, Arena F, Isaacs C, et al:
Everolimus for women with trastuzumab-resistant, HER2-positive,
advanced breast cancer (BOLERO-3): A randomised, double-blind,
placebo-controlled phase 3 trial. Lancet Oncol. 15:580–591. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hammond ME, Hayes DF, Dowestt M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer (unabridged version). Arch Pathol Lab Med.
134:e48–e72. 2010.PubMed/NCBI
|
|
28
|
Wolff AC, Hammond EA, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Massarweh S, Osborne CK, Creighton CJ, Qin
L, Tsimelzon A, Huang S, Weiss H, Rimawi M and Schiff R: Tamoxifen
resistance in breast tumors is driven by growth factor receptor
signaling with repression of classic estrogen receptor genomic
function. Cancer Res. 68:826–833. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Donders F, Kuypers D, Wolter P and Neven
P: Everolimus in acute kidney injury in a patient with breast
cancer: A case report. J Med Case Rep. 8:3862014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, V.3.
2015.http://www.nccn.org/professionals/physician_gls/pdf/breast.pdfAugust
30–2015
|
|
32
|
Dowsett M, Cuzick J, Wale C, Howell T,
Houghton J and Baum M: Retrospective analysis of time to recurrence
in the ATAC trial according to hormone receptor status: An
hypothesis-generating study. J Clin Oncol. 23:7512–7517. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ellis MJ, Coop A, Singh B, Mauriac L,
Llombert-Cussac A, Jänicke F, Miller WR, Evans DB, Dugan M, Brady
C, et al: Letrozole is more effective neoadjuvant endocrine therapy
than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen
receptor-positive primary breast cancer: Evidence from a phase III
randomized trial. J Clin Oncol. 19:3808–3816. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Robertson JF, Llombart-Cussac A, Rolski J,
Feltl D, Dewar J, Macpherson E, Lindemann J and Ellis MJ: Activity
of fulvestrant 500 mg versus anastrozole 1 mg as first-line
treatment for advanced breast cancer: Results from the FIRST study.
J Clin Oncol. 27:4530–4535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Evans AH, Pancholi S, Farmer I, Thornhill
A, Evans DB, Johnston SR, Dowsett M and Martin LA: EGFR/HER2
inhibitor AEE788 increases ER-mediated transcription in
HER2/ER-positive breast cancer cells but functions synergistically
with endocrine therapy. Br J Cancer. 102:1235–1243. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Glück S, Arteaga CL and Osborne CK:
Optimizing chemotherapy-free survival for the ER/HER2-positive
metastatic breast cancer patient. Clin Cancer Res. 17:5559–5561.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abbott DE, Brouquet A, Mittendorf EA,
Andreou A, Meric-Bernstam F, Valero V, Green MC, Kuerer HM, Curley
SA, Abdalla EK, et al: Resection of liver metastases from breast
cancer: Estrogen receptor status and response to chemotherapy
before metastasectomy define outcome. Surgery. 151:710–716. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hoffmann K, Franz C, Hinz U, Schirmacher
P, Herfarth C, Eichbaum M, Büchler MW and Schemmer P: Liver
resection for multimodal treatment of breast cancer metastases:
Identification of prognostic factors. Ann Surg Oncol. 17:1546–1554.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singletary SE, Walsh G, Vauthey JN, Curley
S, Sawaya R, Weber KL, Meric F and Hortobágyi GN: A role for
curative surgery in the treatment of selected patients with
metastatic breast cancer. Oncologist. 8:241–251. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chua TC, Saxena A, Liauw W, Chu F and
Morris DL: Hepatic resection for metastatic breast cancer: A
systematic review. Eur J Cancer. 47:2282–2290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Martinez SR, Young SE, Giuliano AE and
Bilchik AJ: The utility of estrogen receptor, progesterone
receptor, and Her-2/neu status to predict survival in patients
undergoing hepatic resection for breast cancer metastases. Am J
Surg. 191:281–283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baselga J, Semiglazov V, van Dam P,
Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R,
Bianchi G, et al: Phase II randomized study of neoadjuvant
everolimus plus letrozole compared with placebo plus letrozole in
patients with estrogen receptor-positive breast cancer. J Clin
Oncol. 27:2630–2637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bachelot T, Bourgier C, Cropet C,
Ray-Coquard I, Ferrero JM, Freyer G, Abadie-Lacourtoisie S, Eymard
JC, Debled M, Spaëth D, et al: Randomized phase II trial of
everolimus in combination with tamoxifen in patients with hormone
receptor-positive, human epidermal growth factor receptor
2-negative metastatic breast cancer with prior exposure to
aromatase inhibitors: A GINECO study. J Clin Oncol. 30:2718–2724.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Baselga J, Campone M, Piccart M, Burris HA
III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun
F, et al: Everolimus in postmenopausal hormone-receptor-positive
advanced breast cancer. N Engl J Med. 366:520–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gonzalez-Angulo AM and Blumenschein GR Jr:
Defining biomarkers to predict sensitivity to PI3K/Akt/mTOR pathway
inhibitors in breast cancer. Cancer Treat Rev. 39:313–320. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hurvitz SA, Kalous O, Conklin D, Desai AJ,
Dering J, Anderson L, O'Brien NA, Kolarova T, Finn RS, Linnartz R,
et al: In vitro activity of the mTOR inhibitor everolimus, in a
large panel of breast cancer cell lines and analysis for predictors
of response. Breast Cancer Res Treat. 149:669–680. 2015. View Article : Google Scholar : PubMed/NCBI
|