Introduction
Oral squamous cell carcinoma (OSCC) is the most type
of common head and neck cancer, representing 1–2% of all human
malignancies (1). Despite
advancements in diagnosis and treatment strategies in recent
decades, OSCC remains associated with a poor prognosis and low
survival rates (1). The major
characteristics of cancer cells, including the ability to invade
and metastasize, are associated with tumor progression, and
ultimately with patient survival. Identifying molecular changes in
cancer has been a research focus worldwide, with the aim of
developing potential therapeutic methods. Therefore, understanding
the molecular and biological characteristics of OSCC is essential
for the development of more efficacious therapies.
WNT1-inducible-signaling pathway protein-1 (WISP-1)
belongs to the cysteine rich 61 (Cyr61)/connective tissue growth
factor (CTGF)/nephroblastoma overexpressed (Nov) (CCN) family of
matricellular proteins, which are secreted proteins associated with
the extracellular matrix (ECM) (2–4). The ECM
modulates cellular responses, including cell growth,
differentiation and survival (2–4). Since the
first identification of human WISP-1 in an epithelial cell line in
1998, WISP-1 expression has been studied in various organs and
disease states, including malignant diseases, such as colorectal
cancer, hepatocellular carcinoma, lung carcinoma and breast cancer,
and nonmalignant diseases, including lung fibrosis (5–10). WISP-1
is strongly expressed in primary breast cancer and rectal cancer,
and increased WISP-1 expression is associated with more aggressive
features of cancer progression (8,10).
Although WISP-1 has been the focus of numerous studies in
identifying novel potential molecular therapeutic targets, the
function of WISP-1 in human OSCC remains unclear.
In the present study, WISP-1 expression in OSCC was
examined, and the association between its expression and
clinicopathological characteristics was evaluated in a well-defined
series of human OSCC samples. Furthermore, its prognostic value was
evaluated in OSCC. To the best of our knowledge, the present study
is the first to demonstrate the prognostic value of WISP-1 in OSCC.
In addition, the contribution of WISP-1 to cell invasion and
apoptosis, which are associated with tumor progression, and
survival, was analyzed in human OSCC cells. By considering the
clinicopathological characteristics and molecular findings, the
results of the present study may provide the basis for the
mechanism of WISP-1 function in human OSCC.
Materials and methods
Patients and tumor specimens
Formalin-fixed paraffin-embedded tumor and normal
stroma tissue sections were collected from 95 patients who
underwent surgical treatment for either diagnostic biopsy or
definitive surgery for OSCC at Chonnam National University Hwasun
Hospital (Gwangju, Korea) between June 2013 and May 2004. A total
of 61 men and 23 women participated in the present study with a
mean age of 63.03±12.51 years (range, 26–87 years). A total of 11
patients were excluded due to failure to follow-up, palliative
intent, or loss of paraffin-embedded tissue blocks. WISP-1
expression and clinicopathological parameters, including age, sex,
tumor location, overall, tumor (T) and node (N) stages, recurrence,
treatment failure and adjuvant treatment, were analyzed using the
collected OSCC tissue samples. Following primary treatment with
curative intent, patients who exhibited locoregional recurrence
underwent salvage surgery or concurrent chemoradiotherapy. Those
with inoperable locoregional progression or distant metastasis,
even following salvage treatment, were defined as treatment
failure. Tumor staging was performed according to the seventh
edition of the American Joint Committee on Cancer Staging system
(11). Survival was calculated using
the date of starting treatment and the date of mortality or last
follow-up. The present study was approved by the Ethics Committee
of the Institutional Review Board of Chonnam National University
Hwasun Hospital. Written informed consent was obtained from each
patient prior to tissue acquisition.
Immunohistochemistry
Tissue sections (thickness, 5 µm) were cut from the
paraffin blocks of OSCC tissue, and sections were mounted and dried
on glass slides. Tissues were deparaffinized and rehydrated using a
graded series of ethanol (100, 95, 90, 80, 70 and 60%), and antigen
retrieval was performed using 1X Citrate buffer (cat. no. S2031;
Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) for 10 min
using a steam cooker. Endogenous peroxidase activity was blocked
using peroxidase-blocking solution (Dako; Agilent Technologies,
Inc.) for 10 min at room temperature, followed by incubation with
polyclonal rabbit anti-human antibody directed against WISP-1 (cat.
no. sc-25441; 1:100; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) overnight at 4°C. Tissues were incubated with Polymer
horseradish peroxidase (HRP) antirabbit and antimouse IgG (cat. no.
D11-110; Ready-to-use; Golden Bridge International, Inc., Bothwell,
WA, USA) for 10 min at room temperature. Following washing in
TBS-Tween 20 buffer (Biosesang, Sungnam, Korea), tissues were
stained using a DAB 20X HRP detection kit (cat. no. C09-12; Golden
Bridge International, Inc.) for 3 min at room temperature. Tissues
were counterstained with hematoxylin and mounted with coverslips.
Stained tissues were observed and imaged using a light microscope.
Assessment of staining was interpreted by two independent observers
who were blinded to the clinical information. Assessment of
staining intensity was performed as follows: 0, no staining of
tumor cells; 1+, weak to comparable staining in the cytoplasm
and/or nucleus compared with that of non-tumor cells; 2+, readily
appreciable or dark brown staining distinctly marking the tumor
cell cytoplasm and/or nucleus. Specimens with 0 or 1+ staining were
regarded as low expression and those with 2+ staining were regarded
as high expression.
Cell culture and transfection
The human OSCC SCC-1483 cell line was provided by
Dr. Kim CH (Ajou University, Suwon, Korea). The cells were cultured
in RPMI-1640 medium supplemented with 10% fetal bovine serum (both
from HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 50 U/ml
penicillin and 50 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in a humidified atmosphere
containing 5% CO2 at 37°C. SCC-1483 cells were seeded
into 6-well plates at a density of 2×105 cells/well and
transfected with 100 pmol WISP-1-specific small interfering (si)RNA
(cat. no., 1164842; Bioneer Corporation, Daejeon, Korea) or
negative control siRNA (AllStar Neg. Control; cat. no., 1027281;
Qiagen, Inc., Valencia, CA, USA) using Lipofectamine RNA iMAX
(Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h at 37°C.
RNA isolation and reverse
transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Total RNA (1 µg) was mixed with 1 µl 10 mM
dNTP mix (Enzynomics, Daejeon, Korea), 1 µl oligo dT (500 µg/ml;
Promega Corporation, Madison, WI, USA), 2 µl DTT (0.1 M), 4 µl 5X
First-strand buffer, 2 µl M-MLV reverse transcriptase (both
Invitrogen; Thermo Fisher Scientific, Inc.) and 1 µl RNase
inhibitor (Promega Corporation). Gene-specific primers were
subsequently used to amplify the complementary DNA using PCR. A
total of 0.1 µl GoTaq DNA polymerase and 4 µl 5X Green GoTaq DNA
polymerase reaction buffer (both from Promega Corporation) were
used for amplification. The primer sequences were as follows:
WISP-1 forward, 5′-CTCAGCAGCTTGGGGACAAC-3′ and reverse,
5′-GATGCCTCTGGCTGGTACAC-3′; and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) forward, 5′-ACCACAGTCCATGCCATCAC-3′ and
reverse primer, 5′-TCCACCACCCTGTTGCTGTA-3′ (Bioneer Corporation).
WISP-1 expression was evaluated using the following thermocycling
conditions: 94°C for 5 min; 32 cycles of 94°C for 30 sec, 55°C for
30 sec, 72°C for 30 sec and a final elongation step at 72°C for 7
min. Following separating PCR products using electrophoresis on 1%
agarose gels (Lonza, Rockland, ME, USA) containing ethidium bromide
(Bioneer Corporation). Multi Gauge software (version 3.2; Fujifilm,
Tokyo, Japan) was used to quantify the signal using densitometric
analysis.
Protein isolation and western blot
analysis
The cells and tissue samples were lysed in RIPA
buffer (1 M Tris-HCl, 150 mM NaCl, 1% Triton X-100, 2 mM EDTA) with
1 mM phenylmethanesulfonyl fluoride, 1 mM Halt phosphatase
inhibitor and 1 mM Halt protease inhibitor cocktail(Thermo Fisher
Scientific, Inc.). The resolved protein concentrations were
measured using a BCA kit, and 20–30 µg protein/lane was separated
through SDS-PAGE on 10–12% gels and transferred to polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). Following
incubation of the membranes for 1 h at room temperature in blocking
solution [5% bovine serum albumin (BSA); BioShop, Inc., Burlington,
ON, Canada] and washing four times for 15 min with TBS-Tween 20
buffer, specific proteins were sequentially blotted with primary
antibodies. The following antibodies were used: Rabbit anti-human
polyclonal antibody directed against WISP-1 (cat. no. sc-25441) and
rabbit anti-human antibody directed against GAPDH (cat. no.
sc-25778) (Santa Cruz Biotechnology, Inc.). Antibodies were diluted
at 1:1,000 and incubated with the membranes for 24 h at 4°C. An
enhanced chemiluminescence detection system with 1X horseradish
peroxidase substrate (cat. no. WBKL S00 50; EMD Millipore) was used
to visualize immunoreactive proteins for 30 sec at room
temperature, and an LAS-4000 luminescent image analyzer (Fujifilm,
Tokyo, Japan) was used to analyze the proteins.
Cell invasion assay
Cell invasion ability was calculated according to
the total number of cells invading through a Matrigel-coating
Transwell invasion apparatus with 8 µm pores. The cells transfected
with WISP-1 siRNA or negative control siRNA (3×105
cells/well) were seeded into the upper chambers with 120 µl
RPMI-1640 medium containing 0.2% BSA. Next, 400 µl 0.2% BSA
containing 7 µg/ml fibronectin (EMD Millipore) was added to the
lower chamber as the chemoattractant. Following incubation for 24 h
at room temperature, Diff Quik solution (Sysmex Corporation, Kobe,
Japan) was used to stain the bottom Transwell surface containing
the invaded cells. Five random squares in the light microscopic
field of view were measured, and the results are presented as the
means ± standard error of the number of cells/field in three
individual experiments.
Apoptosis assay
Annexin V-fluorescein isothiocyanate (FITC) assays
were used to determine apoptotic rates. The cells transfected with
WISP-1 siRNA or negative control siRNA were collected with trypsin
for 5 min at room temperature (179 × g), washed twice in
phosphate-buffered saline and resuspended in binding buffer (BD
Biosciences, San Jose, CA, USA) following 48 h. Subsequently,
annexin V-FITC and 7-amino-actino mycin D (BD Biosciences) were
added. The cells were incubated in the dark for 15 min at room
temperature and resuspended in 400 µl binding buffer. A FACSCalibur
flow cytometer was used, and the data were analyzed with BD Cell
Quest (version 3.3; BD Biosciences) and WinMDI (version 2.9; The
Scripps Research Institute, San Diego, CA, USA).
Statistical analysis
Associations between WISP-1 expression and various
clinicopathological parameters were compared using the
χ2 test and Fisher's exact test. Survival curves were
calculated using the Kaplan-Meier estimator method and compared
using the log-rank test. Student's t-tests were used to assess the
significance of experimental differences. Analyses were performed
using SPSS software (version 21.0; IBM Corp., Armonk, NY, USA).
P<0.05 were considered to indicate a statistically significant
difference.
Results
Immunoreactivity for WISP-1 is
increased in OSCC tissues compared with adjacent normal
tissues
Table I summarizes the
clinicopathological characteristics of 84 patients with OSCC
included in the present study group. WISP-1 protein expression was
examined immunohistochemically in formalin-fixed paraffin-embedded
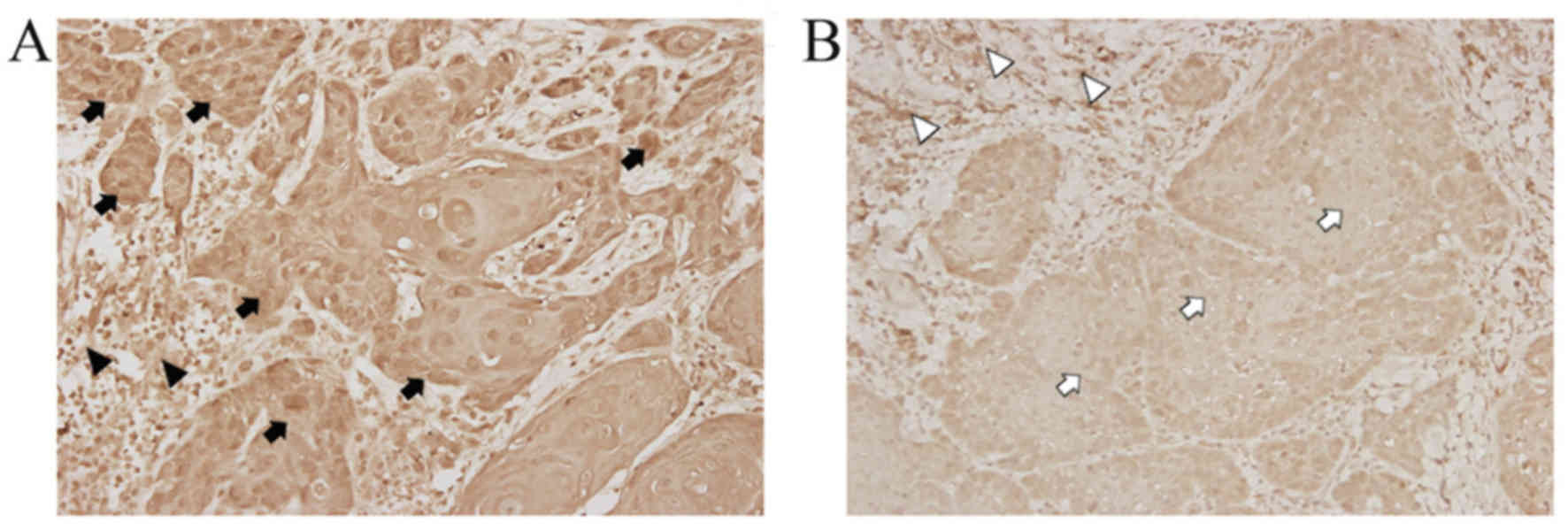
blocks of specimens from patients with OSCC.WISP-1 immunoreactivity
in OSCC cells demonstrated heterogeneous patterns with stronger
immunostaining in the cytoplasm and/or nuclei compared with the
cells in the normal stromal tissue (Fig.
1A). By contrast, minimal staining for WISP-1 immunoreactivity
was observed in OSCC cells compared with normal stromal tissue as a
negative control (Fig. 1B). These
immunohistochemical analyses revealed that 24/84 (28.57%) OSCC
specimens exhibited high WISP-1 expression.
 | Table I.Association between WISP-1 expression
and clinicopathological parameters in patients with oral squamous
cell carcinoma (n=84). |
Table I.
Association between WISP-1 expression
and clinicopathological parameters in patients with oral squamous
cell carcinoma (n=84).
|
| WISP-1
expression |
|
|---|
|
|
|
|
|---|
| Parameters | Low (n=60) | High (n=24) | P-value |
|---|
| Age, years |
|
| 0.890 |
|
<63.03 | 29 | 12 |
|
|
≥63.03 | 31 | 12 |
|
| Sex |
|
| 0.757 |
| Male | 43 | 18 |
|
|
Female | 17 | 6 |
|
| Location |
|
| 0.651 |
| Oral
tongue | 43 | 16 |
|
| FOM, BM
or RMT | 17 | 8 |
|
| Stage |
|
| 0.885 |
| I,
II | 39 | 16 |
|
| III,
IV | 21 | 8 |
|
| T stage |
|
| 0.413 |
| T1,
T2 | 51 | 22 |
|
| T3,
T4 | 9 | 2 |
|
| N stage |
|
| 0.940 |
| N0 | 42 | 17 |
|
| N1,
N2 | 18 | 7 |
|
| CRT |
|
| 1.000 |
| No | 35 | 14 |
|
| Yes | 25 | 10 |
|
| Recurrence |
|
| 0.283 |
|
Negative | 40 | 13 |
|
|
Positive | 20 | 11 |
|
| Treatment
failure |
|
| 0.042 |
|
Negative | 46 | 13 |
|
|
Positive | 14 | 11 |
|
WISP-1 expression is associated with
treatment failure and survival in OSCC
To elucidate the prognostic role of WISP-1 protein
in OSCC, the associations between WISP-1 expression and
clinicopathological parameters were analyzed. WISP-1 expression in
OSCC was not significantly associated with age, sex, primary tumor
location, overall stage, T stage, N stage, chemoradiotherapy and
recurrence (P>0.05; Table I).
Treatment failure was identified to be significantly associated
with WISP-1 expression (P=0.042; Table
I).
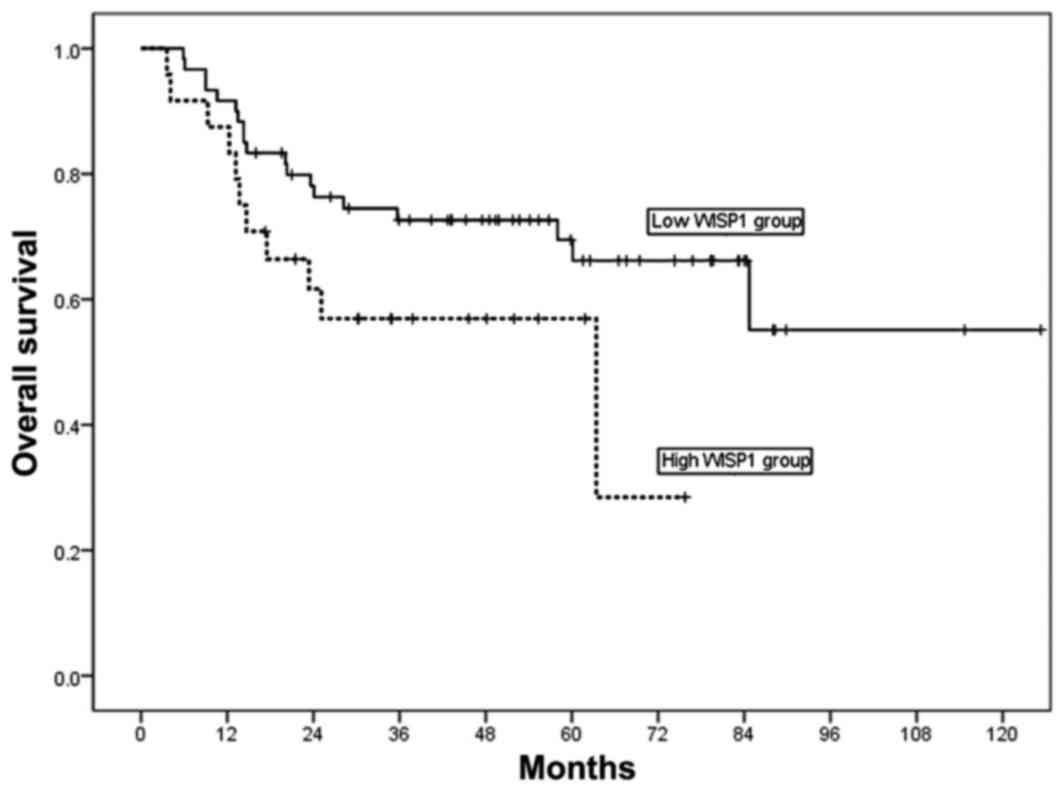
For the 84 patients enrolled in the present study,
the 3- and 5-year overall survival (OS) rates were 68 and 59%,
respectively. The 5-year OS rate was 33% in patients with high
WISP-1 expression, and 66% in patients with low WISP1 expression
(Fig. 2). High WISP-1 expression
tended to decrease OS in patients with OSCC; however, analysis of
Kaplan-Meier curves for OS using log-rank tests did not demonstrate
a significant difference between these two groups of patients
(P=0.075; Fig. 2).
WISP-1 knockdown suppresses invasion
in OSCC cells
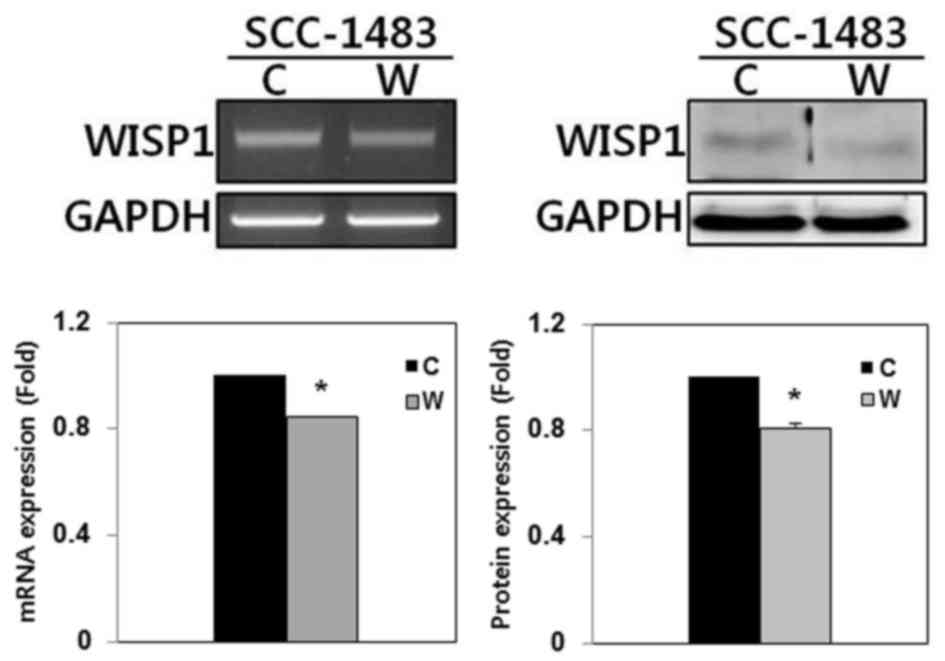
The expression of WISP-1 mRNA and protein was
examined using RT-PCR and western blotting, respectively, in
SCC-1483 cells. RT-PCR and western blotting demonstrated that
WISP-1 was expressed in SCC-1483 cells (Fig. 3). To elucidate the function of WISP-1
in tumor progression in human OSCC cells, WISP-1 siRNA was used to
suppress endogenous WISP-1 expression in SCC-1483 cells. WISP-1
mRNA and protein expression levels were reduced following WISP-1
siRNA transfection as compared with the cells transfected with
negative control siRNA (Fig. 3).
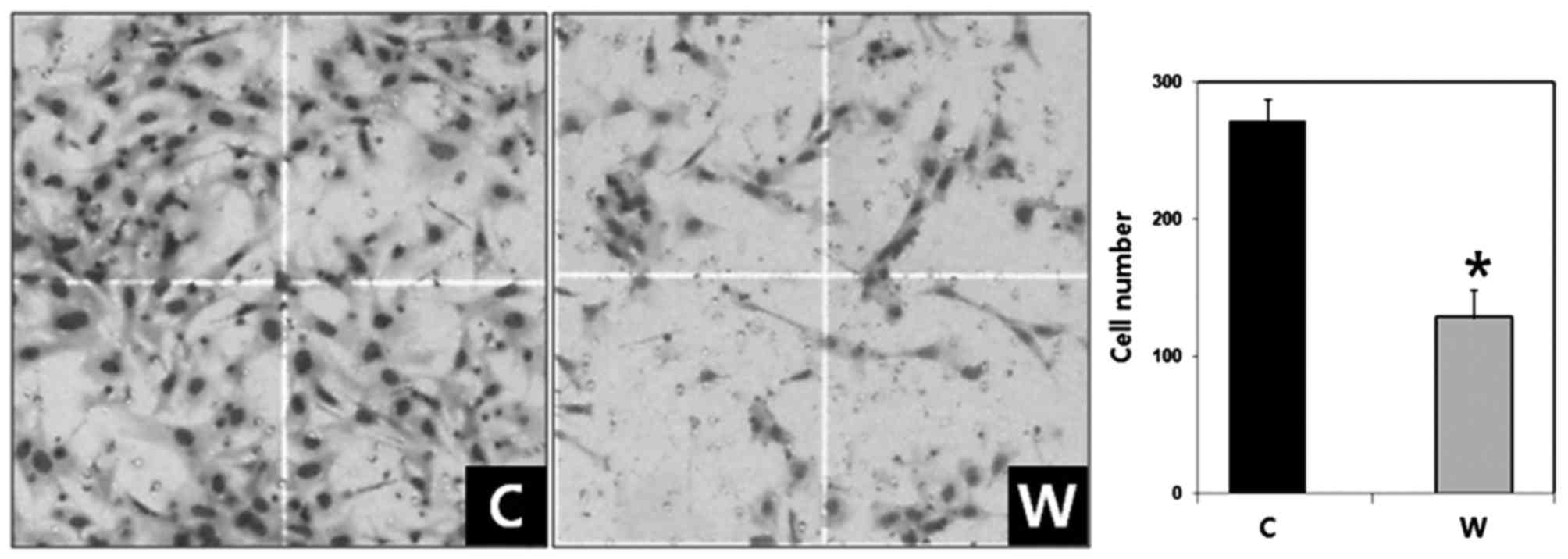
The number of invaded cells in the WISP-1-knockdown
group was 128.30±20.48 compared with 271.80±15.50 cells in the
negative control group (Fig. 4). The
difference between the two groups was statistically significant
(P<0.05). Therefore, WISP-1 knockdown resulted in reduced cell
invasiveness in human OSCC cells.
WISP-1 knockdown induces apoptosis in
OSCC cells
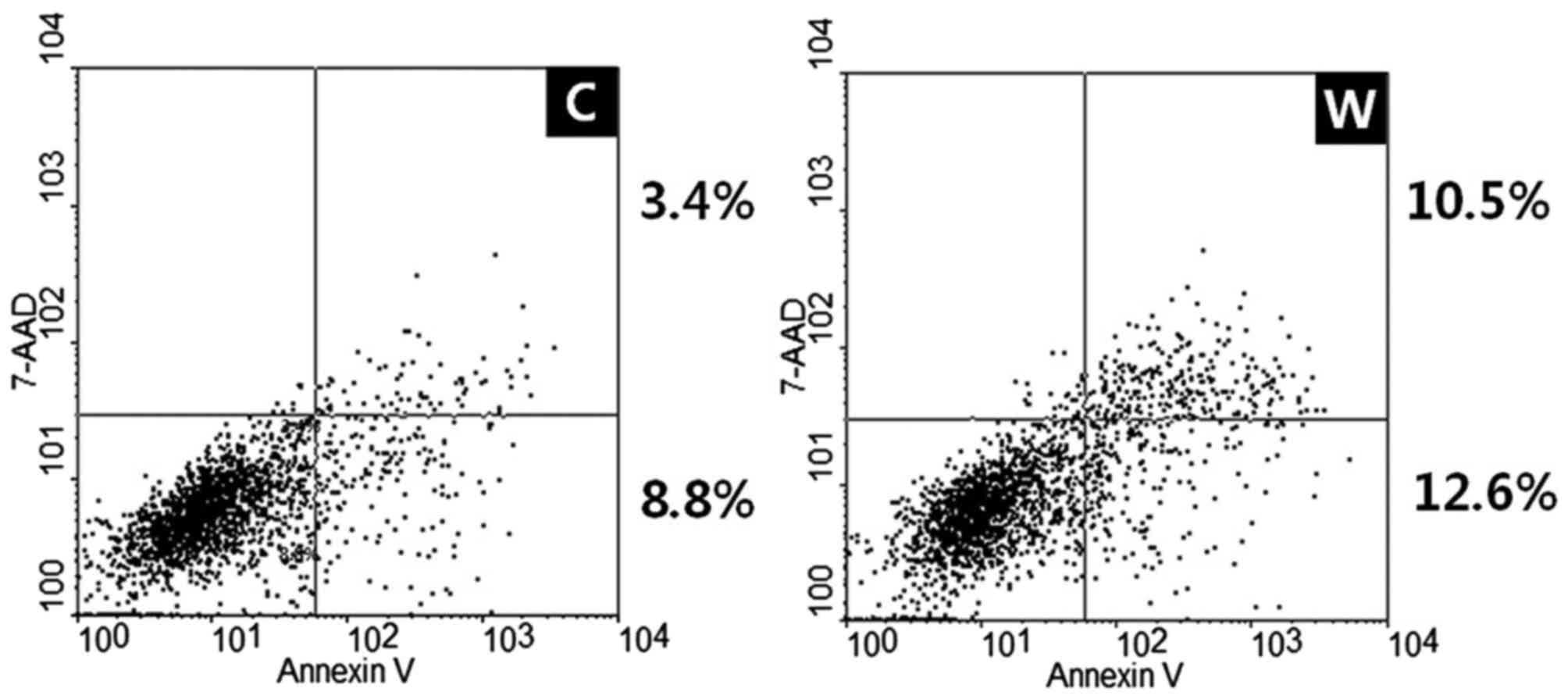
Annexin V-FITC apoptosis assays were used to
evaluate the effects of WISP-1 on cell apoptosis. Transfection with
WISP-1 siRNA and negative control siRNA in SCC-1483 cells induced
early and late apoptosis, with an increased apoptotic rate observed
in cells transfected with WISP-1 siRNA compared with cells
transfected with negative control siRNA (12.2 vs. 23.1%,
respectively; Fig. 5). Therefore,
these results revealed that WISP-1 knockdown increased apoptosis in
human OSCC cells.
Discussion
Identifying altered molecular mechanisms responsible
for tumor invasion and metastasis is essential for developing
potential therapeutic methods. By extension, identifying molecular
markers that can detect cancer earlier or monitor cancer
progression would enable personalization of medicine and improve
the survival rate of patients with cancer (12). WISP-1 is a member of the CCN protein
family (2–4). The CCN family includes CYR61 (CCN1),
CTGF (CCN2), NOV (CCN3), WISP-1 (CCN4), WISP-2 (CCN5) and WISP-3
(CCN6) (2–4). The majority of CCN family proteins are
secreted and expression of these proteins are associated with the
ECM, which has been suggested to serve essential roles in
tumorigenesis-associated processes, including tumor survival,
proliferation, migration, and invasion (2). These findings have increased the
interest towards investigating the potential of CCN proteins as
therapeutic targets.
In adult human tissue, WISP-1 mRNA has been
demonstrated to be expressed in number of organs, including the
heart, kidney, lung, pancreas, placenta, ovary, small intestine and
spleen (5). Notably, increased WISP-1
expression has been identified in several cancer types, including
colorectal cancer, hepatocellular carcinoma, lung carcinoma,
breast, esophageal and endometrial cancer (5–8,10,13–15).
Furthermore, WISP-1 expression has also been revealed to be low in
healthy lung epithelial cells, but significantly upregulated in
lung cancer tissue (15).
In the present study, 24/84 (28.57%) OSCC specimens
demonstrated high WISP-1 expression. In addition, it was revealed
that high WISP-1 expression was significantly associated with
treatment failure. This finding is important as treatment failure
leads to poor prognosis and survival in patients with OSCC. The
5-year OS rate was 33% in patients with high WISP-1 expression and
66% in patients with low WISP-1 expression. Although the number of
patients with OSCC in the current study was not sufficient to
demonstrate a significant difference between the two groups, high
WISP-1 expression tended to be associated with reduced survival
rates in patients with OSCC. In esophageal and endometrial cancer,
high WISP-1 expression has also been revealed to be associated with
poor survival and clinicopathological parameters indicative of
aggressive disease (13,14). Furthermore, in a previous study,
Chuang et al (16) reported
that high WISP-1 expression was associated with an increased tumor
stage in patients with OSCC. Taken together, these results and the
findings of the present study suggest that WISP-1 expression may be
used to predict poor clinical outcomes in patients with OSCC.
The dysregulation of cell invasion and apoptosis is
a principle characteristic of cancer cells (17). OSCC is characterized by high rates of
local invasion, and depth of invasion is a significant prognostic
factor in patients with OSCC (18).
Therefore, the role of WISP-1 in cell invasion and apoptosis was
investigated using the human OSCC SCC-1483 cell line.
siRNA-mediated WISP-1 knockdown significantly decreased cell
invasion compared with siRNA-control transfected cells. In
addition, WISP-1 knockdown induced apoptosis in SCC-1483 cells.
Furthermore, a previous study reported that WISP-1 increased the
invasive activity of SCC4 cells, another OSCC cell line (16). Additionally, You et al
(19) and Su et al (20) demonstrated that WISP-1 suppresses
c-myc-induced and p53-mediated apoptotic signaling pathways. These
studies are consistent with the results of the present study and
suggest that WISP-1 promotes aggressive phenotypes in OSCC
cells.
In conclusion, although additional studies are
warranted to support the findings of the present study, these
results suggest that WISP-1 is associated with tumor progression
and poor prognosis by increasing tumor cell invasion, and
inhibiting cell apoptosis in human OSCC.
Acknowledgements
The present study was supported by the Chonnam
National University Hwasun Hospital Institute of Biomedical Science
(grant no. HCRI 16922-21). The authors would like to thank Dr CH
Kim (Ajou University) for providing the SCC-1483 cell line.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kleer CG, Zhang Y, Pan Q and Merajver SD:
WISP3 (CCN6) is a secreted tumor-suppressor protein that modulates
IGF signaling in inflammatory breast cancer. Neoplasia. 6:179–185.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holbourn KP, Acharya KR and Perbal B: The
CCN family of proteins: Structure-function relationships. Trends
Biochem Sci. 33:461–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perbal B: NOV (nephroblastoma
overexpressed) and the CCN family of genes: Structural and
functional issues. Mol Pathol. 54:57–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pennica D, Swanson TA, Welsh JW, Roy MA,
Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M, et al:
WISP genes are members of the connective tissue growth factor
family that are up-regulated in wnt-1-transformed cells and
aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA.
95:pp. 14717–14722. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calvisi DF, Conner EA, Ladu S, Lemmer ER,
Factor VM and Thorgeirsson SS: Activation of the canonical
Wnt/beta-catenin pathway confers growth advantages in c-Myc/E2F1
transgenic mouse model of liver cancer. J Hepatol. 42:842–849.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Margalit O, Eisenbach L, Amariglio N,
Kaminski N, Harmelin A, Pfeffer R, Shohat M, Rechavi G and Berger
R: Overexpression of a set of genes, including WISP-1, common to
pulmonary metastases of both mouse D122 Lewis lung carcinoma and
B16-F10.9 melanoma cell lines. Br J Cancer. 89:314–319. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie D, Nakachi K, Wang H, Elashoff R and
Koeffler HP: Elevated levels of connective tissue growth factor,
WISP-1, and CYR61 in primary breast cancers associated with more
advanced features. Cancer Res. 61:8917–8923. 2001.PubMed/NCBI
|
|
9
|
Konigshoff M, Kramer M, Balsara N, Wilhelm
J, Amarie OV, John A, Rose F, Fink L, Seeger W, Schaefer L, et al:
WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in
mice and is upregulated in humans with idiopathic pulmonary
fibrosis. J Clin Invest. 119:772–787. 2009.PubMed/NCBI
|
|
10
|
Tian C, Zhou ZG, Meng WJ, Sun XF, Yu YY,
Li L, Luo HZ, Yang L, Zhou B and Gu J: Overexpression of connective
tissue growth factor WISP-1 in Chinese primary rectal cancer
patients. World J Gastroenterol. 13:3878–3882. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Tritti A: American joint committee on cancer-cancer
staging manual. 7th. New York: Springer; 2010
|
|
12
|
Myung DS, Park YL, Chung CY, Park HC, Kim
JS, Cho SB, Lee WS, Lee KH, Lee JH and Joo YE: Expression of Livin
in colorectal cancer and its relationship to tumor cell behavior
and prognosis. PLoS One. 8:e732622013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagai Y, Watanabe M, Ishikawa S, Karashima
R, Kurashige J, Iwagami S, Iwatsuki M, Baba Y, Imamura Y, Hayashi N
and Baba H: Clinical significance of Wnt-induced secreted protein-1
(WISP-1/CCN4) in esophageal squamous cell carcinoma. Anticancer
Res. 31:991–997. 2011.PubMed/NCBI
|
|
14
|
Tang Q, Jiang X, Li H, Lin Z, Zhou X, Luo
X, Liu L and Chen G: Expression and prognostic value of WISP-1 in
patients with endometrial adenocarcinoma. J Obstet Gynaecol Res.
37:606–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen PP, Li WJ, Wang Y, Zhao S, Li DY,
Feng LY, Shi XL, Koeffler HP, Tong XJ and Xie D: Expression of
Cyr61, CTGF, and WISP-1 correlates with clinical features of lung
cancer. PLoS One. 2:e5342007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chuang JY, Chang AC, Chiang IP, Tsai MH
and Tang CH: Apoptosis signal-regulating kinase 1 is involved in
wisp-1-promoted cell motility in human oral squamous cell carcinoma
cells. PLoS One. 8:e780222013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Igney FH and Krammer PH: Death and
anti-death: Tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung J, Cho NH, Kim J, Choi EC, Lee SY,
Byeon HK, Park YM, Yang WS and Kim SH: Significant invasion depth
of early oral tongue cancer originated from the lateral border to
predict regional metastases and prognosis. Int J Oral Maxillofac
Surg. 38:653–660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
You Z, Saims D, Chen S, Zhang Z, Guttridge
DC, Guan KL, MacDougald OA, Brown AM, Evan G, Kitajewski J and Wang
CY: Wnt signaling promotes oncogenic transformation by inhibiting
c-Myc-induced apoptosis. Cell Biol. 157:429–440. 2002. View Article : Google Scholar
|
|
20
|
Su F, Overholtzer M, Besser D and Levine
AJ: WISP-1 attenuates p53-mediated apoptosis in response to DNA
damage through activation of the Akt kinase. Genes Dev. 16:46–57.
2002. View Article : Google Scholar : PubMed/NCBI
|