Introduction
Tumor necrosis factor receptor-associated factor 6
(TRAF6) is an important E3 ubiquitin ligase (1) that is key to innate and adaptive
immunity (2). TRAF6 has been reported
to be involved in the invasive growth and metastasis of squamous
cell carcinoma (3) and gastric cancer
(4), as well as myelodysplastic
syndromes and acute myeloid leukemia (5). Due to the importance of the numerous
signal transduction pathways in which it is involved, TRAF6 may be
a potential target for the treatment of cancer (6).
Multiple myeloma (MM) is associated with a
dysregulated cell microenvironment, in which the marrow stromal
cells (MSCs) promote the growth of myeloma cells (7). In osteoclasts, TRAF6 mediates signal
transduction from the receptor activator of nuclear factor-κB
(NF-κB)/receptor activator of NF-κB ligand (RANK/RANKL) signaling
pathway (8); RANKL is primarily
expressed in stromal cells (9).
Inhibition of TRAF6 expression is able to suppress osteoclast
proliferation and induce apoptosis (10). Furthermore, macrophages (11) and dendritic cells (12) in the microenvironment of myeloma cells
express inflammatory factors, including TRAF6. The toll-like
receptor (TLR)-TRAF6-NF-κB signaling pathway may serve an important
role in the development and progression of myeloma (13,14).
However, the function of TRAF6 in myeloma cells has
yet to be elucidated. Chen et al (15) demonstrated that TRAF6 knockdown using
small interfering RNA (siRNA) molecules is able to inhibit the
proliferation of myeloma cells and induce apoptosis, suggesting
that TRAF6 may be a novel therapeutic target for myeloma. As TRAF6
is a signaling adapter molecule and patients with MM often
overexpress various cytokines (16),
the present study hypothesized that increased TRAF6 expression
levels may be affected by stimulation of microenvironment and
promote cell proliferation upon upstream signaling.
Materials and methods
Primary myeloma cells and human
myeloma cell lines (HMCL)
Bone marrow samples were obtained from 18 subjects
with recently diagnosed MM and 3 healthy donors at The Affiliated
Hospital of Nantong University (Nantong, China), from January 2014
to December 2014. Patient characteristics are presented in Table I.
 | Table I.Clinical features of patients with MM
and healthy donors. |
Table I.
Clinical features of patients with MM
and healthy donors.
| A, Patients with
MM. |
|---|
|
|---|
| Parameters | Patients with
myeloma |
|---|
| Number | 18 |
| Median age (range),
years | 57 (37–72) |
| Gender |
|
| Male | 15 |
|
Female | 3 |
| IgG positive | 8 |
| IgA positive | 3 |
| IgD positive | 2 |
| κ Fc positive | 2 |
| λ Fc positive | 3 |
| DS stage |
|
| I | 1 |
| II | 10 |
|
III | 7 |
| Organ involved |
|
| Renal
failure | 6 |
|
Osteolysis | 15 |
| Mean number of
plasma cells in the BM at diagnosis (%) | 41.2 (9–92) |
|
| B, Healthy
donors. |
|
| Parameters | Healthy donors |
|
| Number | 3 |
| Median age (range),
years | 38 (30–45) |
| Gender |
|
|
Male | 3 |
|
Female | 0 |
Bone marrow mononuclear cells (BMMCs) were obtained
using gradient centrifugation (716 × g; 20 min; room
temperature) in Ficoll®-Paque Premium media (no.
17-5442-02; GE Healthcare Life Sciences, Uppsala, Sweden). Primary
myeloma cells were purified using cluster of differentiation (CD)
138 microbeads, according to the manufacturer's protocol (Miltenyi
Biotec GmbH, Bergisch Gladbach, Germany). The U266 and RPMI-8226 MM
cell lines were purchased from the Beijing Cell Library of the
Chinese Academy of Sciences (Beijing, China). The Ethics Committee
of The Affiliated Hospital of Nantong University approved the study
protocol. Written informed consent was obtained from all
participants.
Bone marrow-derived MSCs
BMMCs were extracted from three patients and three
healthy donors (HD), suspended in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.). Non-adherent cells were removed after a
24-h incubation at 37°C in an atmosphere containing 5%
CO2 and the medium was subsequently changed every 3–4
days. Upon reaching >80% confluency, cells were detached using
0.125% trypsin and 0.01% EDTA and flow cytometry analysis (FACSAria
II; BD Biosciences, Franklin Lakes, NJ, USA) was performed. Cells
were subsequently split into new flasks and ~1×105 cells
from the fourth passage were resuspended in 24-well plates in 1 ml
DMEM and cultured until ~80% confluency was reached.
Co-cultivation of HMCLs with MSCs
The U266 and RMPI-8226 cell lines were maintained in
RPMI-1640 medium supplemented with 10% FBS, 2 mmol/l glutamine and
1% penicillin/streptomycin (all from Gibco; Thermo Fisher
Scientific, Inc.). To evaluate the effect of MSCs obtained from MM
patients on myeloma cells, the U266 and RMPI-8226 cell lines
(including siRNA transfected cells) were washed in PBS twice,
cultured at 37°C in an atmosphere containing 5% CO2 in
24-well plates with RPMI-1640 media without FBS for 4 h and
recollected. Approximately 1×106 cells were subsequently
resuspended in 24-well plates containing MSCs (6×105
cells) with >80% confluency.
Western blot analysis
Cells were lysed using 5X Laemmli sample buffer
consisting of 50 mM Tris buffer (pH 6.8), 2% sodium dodecyl sulfate
and 10% glycerol, and containing the following protease inhibitors:
Leupeptin (1 µg/ml), aprotinin (1 µg/ml) and 2 mM
phenylmethylsulfonyl fluoride (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany). Whole cell lysates (40 mg) were separated by
10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF)
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). PVDF
membranes were blocked using PBS with Tween-20 (PBST; 10 mM
Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween-20) containing 5% skimmed
milk, and then incubated overnight at 4°C with antibodies against
TRAF6 (dilution, 1:1,000; no. ab40675; Abcam, Cambridge, UK) or
antibodies against p-p65 or p-p100 (dilution, 1:1,000; p-p65, no.
3033; p-p100, no. 4810; Cell Signaling Technology, Inc., Danvers,
MA, USA). The PVDF membranes were washed three times in PBST and
incubated with a peroxidase-conjugated AffiniPure Goat Anti-Rabbit
Immunoglobulin G (H+L) (dilution, 1:5,000; no. 111-035-003; Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 2 h at
room temperature. β-actin was detected using the above method but
with the anti-β-actin antibody (dilution, 1:1,000; no. 4970; Cell
Signaling Technology, Inc., Danvers, MA, USA). Following washing
with PBST, the proteins were visualized using
BioSpectrum® MultiSpectral Imaging System (Ultra-Violet
Products Ltd., Cambridge, UK), and quantified using Quantity One
1-D Analysis software (version 4.6.9; Bio-Rad Laboratories, Inc.)
and the developer 20X LumiGLO® reagent and 20X peroxide,
(no. 7003; Cell Signaling Technology, Inc.). Relative expression
levels of the target protein were measured as the gray value ratio
of target protein content to β-actin content.
Transfection of myeloma cell lines
with TRAF6 siRNA
Prior to transfection, 6×105 cells were
seeded in 6-well plates in RPMI-1640 supplemented with 10% FBS at
37°C. The following day, 50 nM of TRAF6 siRNA (Shanghai GenePharma
Co, Ltd., Shanghai, China) was added to each well with
Entranster™-R (Engreen Biosystem Co., Ltd., Beijing, China) and
cultured for a further 48 h at 37°C in an atmosphere containing 5%
CO2. RNA interference oligomers were complementary to
the TRAF6 mRNA, and were as follows: Sense
5′-GCAGAUGGGGCAUUCAUATT-3′; antisense 5′-UAUGAAUGCCCCAUCUGCTT-3′).
The negative control siRNAs were as follows: Sense
5′-UUCUCCGAACGUGUCACGUTT-3′; antisense 5′-ACGUGACACGUUCGGAGAATT-3′.
Scrambled siRNAs were used as controls (Shanghai GenePharma Co.,
Ltd.).
Cell proliferation assay
Transfected cells were seeded in 96-well plates at a
density of 5×103/well, and cultured at 37°C in a
humidified incubator for 48 h. Subsequently, 10 µl Cell Counting
Kit-8 (CCK-8) solution (no. CK04; Dojindo Molecular Technologies,
Inc., Kamamoto, Japan) was added to each well and the cells were
incubated for a further 2 h. Optical density (OD) values were
determined at a wavelength of 450 nm using a microplate reader
(Synergy HT; BioTek Instruments, Inc., Winooski, VT, USA).
Clinical correlation study
Blood samples were obtained from 18 patients
(Table I) with MM on the second day
following admission to The Affiliated Hospital of Nantong
University. Blood levels of hemoglobin (Hb), lactate dehydrogenase
(LDH), β2Microglobulin (β2M) and albumin were evaluated in
biochemical analysis department using standard clinical
protocols.
Statistical analysis
SPSS version 19.0 (IBM SPSS, Armonk, NY, USA) was
used for statistical analyses, and the results were presented as
the mean ± standard deviation. The Student's t-test was used to
compare the expression levels of TRAF6 between the groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression levels of TRAF6 in myeloma
cells are correlated with prognosis
BMMCs from 18 patients with MM were purified using
CD138 microbeads, and CD56 and CD38 surface phenotypes were
subsequently detected using standard clinical protocols. All
purified primary myeloma cells (C138+ CD56+
CD38+) were >90% pure (Fig.
1A). Immunoblotting was used to determine TRAF6 protein levels
in BMMCs obtained from patients, as well as in U266 and RPMI-8226
cells (Fig. 1B). The correlations
between the expression levels of TRAF6 protein in primary myeloma
cells and the Hb, LDH, β2M and albumin levels were examined. TRAF6
expression levels were not significantly correlated with the blood
levels of LDH (r2=0.021; P=0.56) or Hb
(r2=0.197; P=0.065) protein. However, the expression
levels of TRAF6 were significantly and positively correlated with
blood β2M levels (r2=0.3472; P=0.01) and negatively
correlated with albumin levels (r2=0.5494; P=0.0004;
Fig. 1C). The International Staging
System (ISS) of the International Myeloma Working Group (IMWG) was
used to assess patients with MM (17)
compared with the combination of the two objective prognostic
variables, serum β2M and serum albumin, from this source. Although
TRAF6 expression levels in myeloma cells tended to be higher in
patients with MM at ISS stage III, compared with those at ISS stage
II (Fig. 1D), no significant
differences were observed, possibly due to the small sample size of
patients.
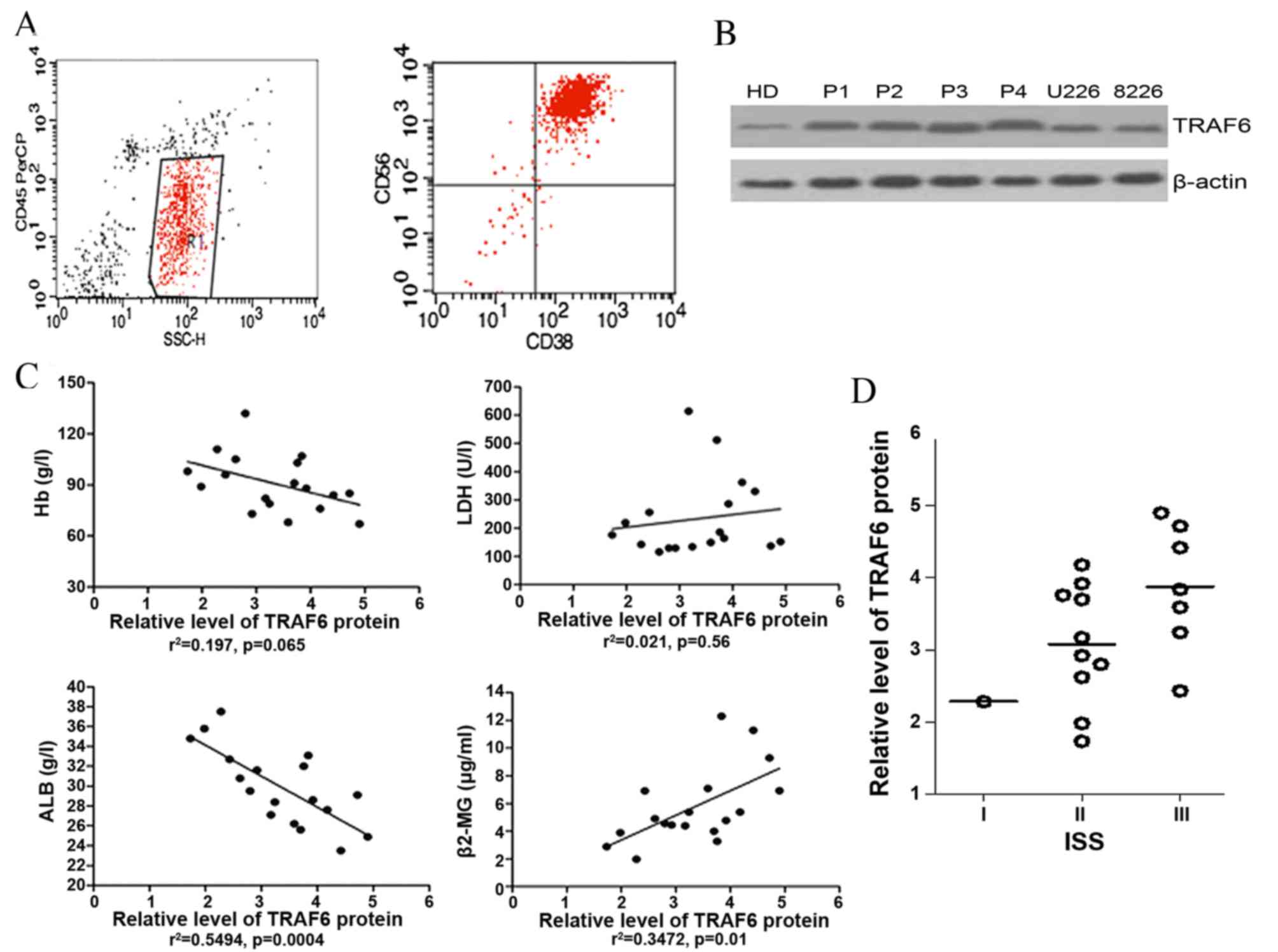 | Figure 1.Expression of TRAF6 in primary myeloma
cells and HMCLs. (A) Representative flow cytometry profile of
purified CD138+ primary myeloma cells, presenting the primary
myeloma cells (C138+ CD56+ CD38+)
were >90% pure. (B) Western blot analysis of TRAF6 protein
expression in myeloma cells. (C) Correlation between TRAF6
expression levels in primary myeloma cells from 18 patients with MM
and various prognostic indices. (D) TRAF6 expression in plasma
cells from patients with MM with various ISS classifications.
TRAF6, tumor necrosis factor receptor-associated factor 6; CD,
cluster of differentiation; Hb, hemoglobin; ALB, albumin; LDH,
lactate dehydrogenase; β2-MG, β2Microglobulin; ISS, International
Scoring System; MM, multiple myeloma; HMCL, human myeloma cell
line; HD, healthy donor; P1-4, patients 1–4. |
Immunophenotypic characteristics of
MSCs
The immunophenotype of MSCs from three patients was
detected using flow cytometry, with all cell samples positive for
CD90 (85.75±2.35%), CD44 (96.53±3.79%), CD73 (90.21±1.09%) and
CD105 (94.46±3.59%) expression, but negative for CD45, CD34 and
human leukocyte antigen-antigen D related (HLA-DR) expression
(Fig. 2A). Concordant with the
results of previous study (18), the
immunophenotype of the MSCs from the three HDs was similar to that
of the patients with MM, expressing CD90 (84.23±3.85%), CD44
(96.76±3.9%), CD73 (91.67±2.36%) and CD105 (95.15±2.76%), and ≤5%
of MSCs expressing CD45, CD34 and HLA-DR (data not shown).
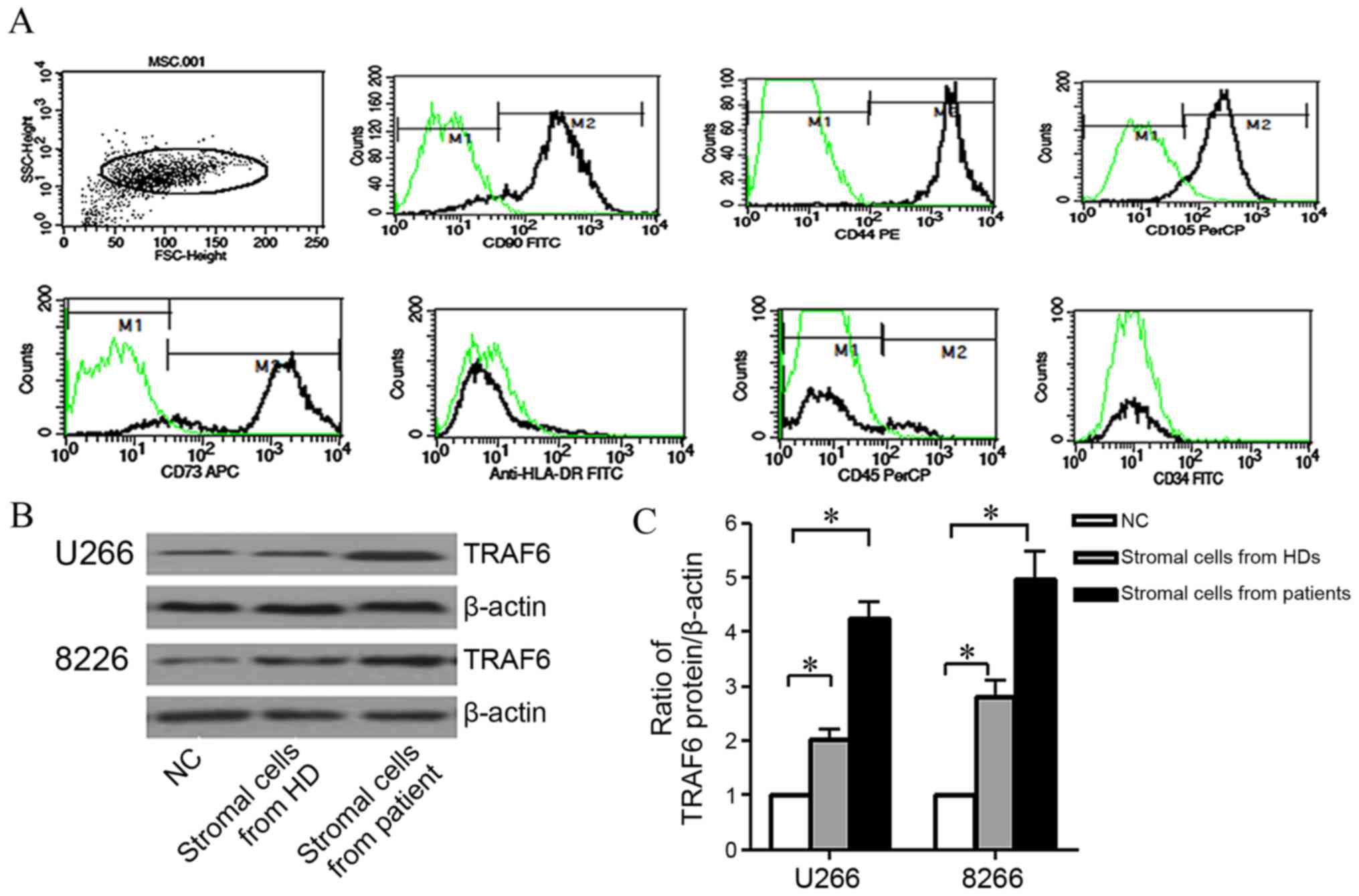 | Figure 2.Bone marrow stromal cells induced
TRAF6 expression in human myeloma cell lines. (A)
Immunophenotypical characteristics of bone marrow stromal cells
from a patient with myeloma. (B) Representative TRAF6 western
blots. (C) Relative intensity of TRAF6 protein expression following
co-culture with bone marrow stromal cells. The presented data are
the mean ± standard deviation of three experiments. *P<0.05, as
compared with cells cultured in Dulbecco's modified Eagle's medium.
TRAF6, tumor necrosis factor receptor-associated factor 6; FSC,
front scatter; SSC, side scatter; CD, cluster of differentiation;
FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP,
peridinin chlorophyll protein; APC, allophycocyanin; HLA-DR, human
leukocyte antigen-antigen D related; HD, healthy donor; MSC, bone
marrow stromal cell. |
TRAF6 expression is enhanced in
myeloma cells by MSCs
U266 and RPMI-8226 cells were serum-starved for 4 h
and then cultured in DMEM containing MSCs from a patient with MM
and a HD, or without the stromal cells [(negative control (NC)] for
48 h. TRAF6 protein expression levels in HMCLs were subsequently
evaluated. As compared with NCs, the expression levels of TRAF6
protein were higher in U266 and RPMI-8226 cells co-cultured with
stromal cells from a HD; however, the highest TRAF6 expression
levels were observed in cells co-cultured with MSCs from a patient
with MM (Fig. 2B). These experiments
were performed with three myeloma patients and three HDs, and the
mean and standard deviation were derived from the experiments in
which the gray value ratios were evaluated three times. The data
revealed that TRAF6 protein levels in myeloma cells increased
significantly when co-cultured with HD stromal cells or MM patient
stromal cells, as compared with NCs (P<0.05; Fig. 2C).
Effects of TRAF6 silencing on myeloma
cell proliferation when co-cultured with MSCs
The transfection efficiency of 50 nmol/l siTRAF6 was
92 and 87% in U266 and RPMI-8226 cells, respectively, as determined
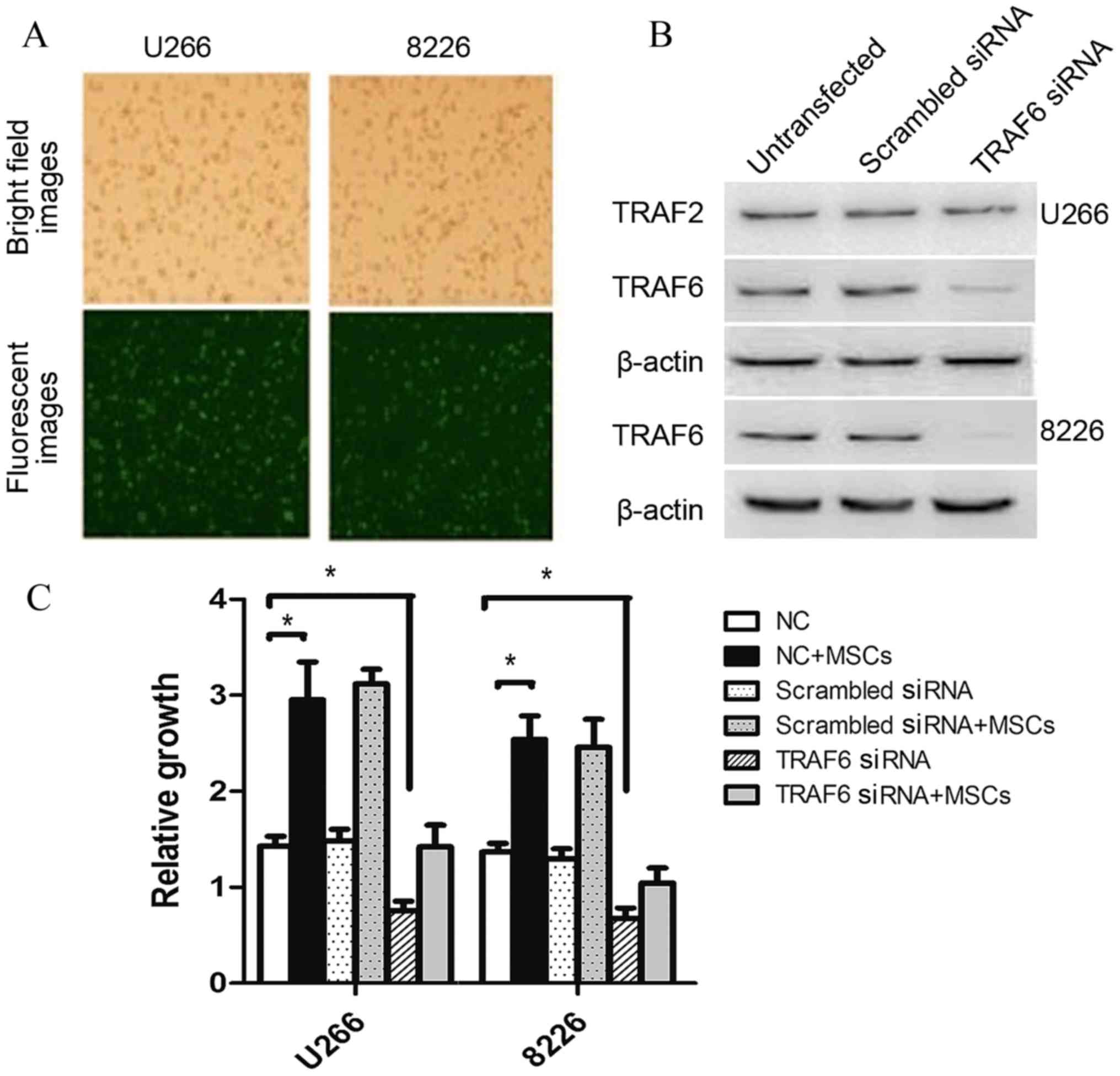
by fluorescent microscopy after 48 h of incubation (Fig. 3A). Following transfection, TRAF6
protein levels decreased after 48 h incubation (Fig. 3B), suggesting effective siTRAF6
transfection in myeloma cells. To determine the viability of
myeloma cells following siTRAF6 transfection, the cells were
recollected, washed and co-cultured with patient stromal cells for
48 h. The siTRAF6-transfected cells exhibited significantly
decreased OD values, compared with control myeloma cells
(P<0.05). HMCL cell growth increased significantly following
co-culture with stromal cells from patients with MM (P<0.05,
whereas no significant growth differences were observed in the
TRAF6-silencing group following co-culture with patient stromal
cells (Fig. 3C).
Effects of TRAF6 silencing on NF-κB
protein levels in myeloma cells
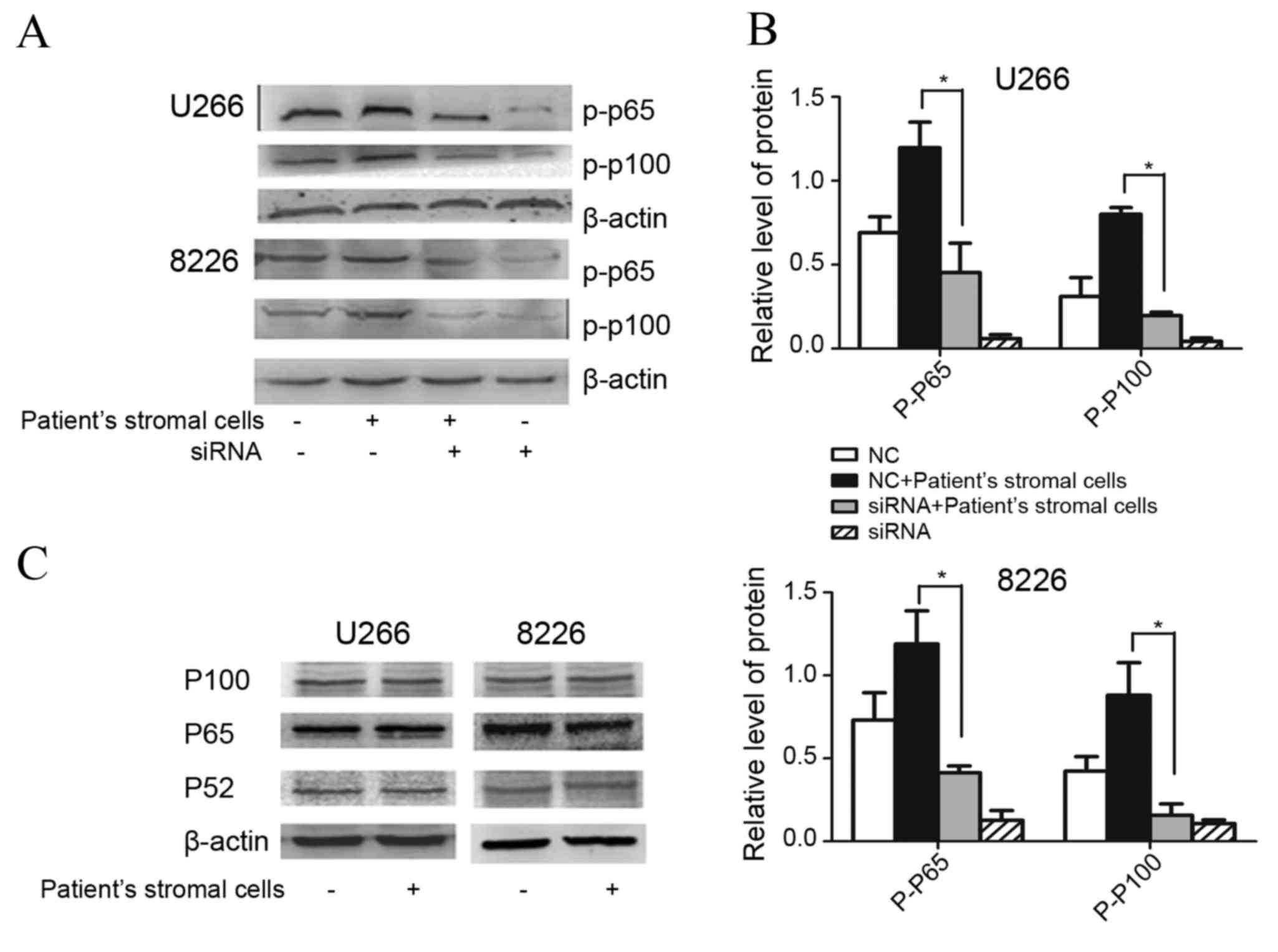
A total of 48 h following siTRAF6 transfection,
HMCLs were co-cultured with MSCs for a further 24 h prior to
collection and the evaluation of p-p65 and p-p100 expression
levels. All three HMCLs exhibited increased expression of p-p65 and
p-p100 in response to co-culture with patient MSCs. By contrast,
decreased expression levels of p-p65 and p-p100 were observed in
TRAF6-silenced myeloma cells, even upon stimulation with patient
stromal cells (Fig. 4A). Following
co-culture with patient stromal cells, the siTRAF6-transfected
cells exhibited significantly decreased expression levels of p-p65
and p-p100 (P<0.05), compared with non-siTRAF6-transfected cells
(Fig. 4B). The levels of total p65,
p100 and p52 expression in myeloma cells were not observed to be
altered following co-culture with MSCs (Fig. 4C).
Discussion
A previous study demonstrated that increased
expression levels of TRAF6 are present in myeloma cell lines and
primary myeloma cells, and that TRAF6 siRNAs are able to decrease
the downstream activity of NF-κB (19). This suggests that TRAF6 may be a novel
target for the treatment of MM.
It has been established that TRAF6 is important in
the osteoclasts of patients with MM (10); however, the role of TRAF6 in myeloma
cells has yet to be elucidated. As TRAF6 is a signaling adapter
molecule and patients with MM frequently overexpress various
cytokines (16), it was hypothesized
in the present study that TRAF6 expression may be associated with
the tumor microenvironment (20) and
promote cell proliferation upon upstream signal transduction.
The present study revealed that MM cell lines and
CD138+ cells from patients with MM exhibited enhanced
TRAF6 expression levels, and TRAF6 expression in the majority of
patients with MM was higher, compared with that observed in MM cell
lines. In the absence of cytokine stimulation, U266 and RPMI-8226
cell lines expressed TRAF6 autonomously. In addition, the presence
of higher TRAF6 expression levels in primary MM cells suggests that
TRAF6 expression in MM cells may be regulated by the
microenvironment and may be associated with the patient
condition.
It has been established that the levels of
biochemical markers, including β2M, albumin and LDH, are correlated
with the pathogenesis of MM (21,22). β2M
levels are an independent prognostic factor, albumin levels are
associated with tumor mass and physical status, LDH is one of the
markers associated with disease stage and Hb levels are an
important criterion for Durie-Salmon staging (23). In the present study, TRAF6 expression
was not associated with the blood levels of LDH and Hb, but was
positively correlated with blood β2M levels and negatively
correlated with blood albumin levels. The ISS for MM evaluates the
levels of β2M and albumin (24);
therefore, TRAF6 levels may correlate with patient prognosis. Based
on the observation that TRAF6 expression is correlated with certain
myeloma markers, it is hypothesized in the current study that TRAF6
may be a cell-survival factor that promotes the proliferation of
myeloma cells, and its expression may controlled by upstream
signaling occurring outside of myeloma cells.
To evaluate this hypothesis, the present study
co-cultured MM cell lines with stromal cells from patients with
myeloma, revealing TRAF6 expression was induced. Subsequently,
siRNAs targeting the TRAF6 C-terminus were transfected into the
myeloma cell lines and cell proliferation was evaluated. In
concordance with previous studies, the stromal cells were able to
stimulate the proliferation of myeloma cells (25,26). In
addition, cells transfected with TRAF6 siRNA exhibited growth
inhibition, as well as partial resistance to the growth-stimulatory
effects of stromal cells. The effects of the NF-κB family in
myeloma cell proliferation have previously been established
(27), during which the canonical
p50/p65/inhibitor of κB signaling pathway and the non-canonical
p52/NF-κB-inducing kinase signaling pathway (including p100 of
which p52 is a derivative) are activated to regulate the expression
levels of certain downstream genes. In the present study, the
expression of p-p65 and p-p100 was induced in response to patient
stromal cells, indicating that the NF-κB family members may be
activated by MSCs. In myeloma cells transfected with TRAF6 siRNA,
the expression of p-p65 and p-p100 was downregulated, further
supporting the hypothesis that myeloma stromal cells may secrete
numerous cytokines that are able to regulate the transcriptional
activity of NF-κB. The observation that TRAF6 siRNA is able to
inhibit the proliferation of myeloma cells suggests that TRAF6 has
an important role in signal transduction, possibly contributing to
the proliferation of myeloma cells. Additionally, according to the
aforementioned results, certain cytokines that are secreted from
MSCs may enhance TRAF6 expression levels in myeloma cells and
affect downstream targets in the NF-κB family.
Previous studies have demonstrated that CD40L,
interleukin (IL)-1 (1), IL-17
(28) and RANKL (29) are able to bind to TRAF6 receptors, a
number of which originate from stromal cells. Investigating the
upstream signaling factors involved in this process may enable
improved understanding of the TRAF6 signaling pathway in myeloma
cells. As the TRAF6 signaling pathway is important in various other
cell types, in addition to the microenvironment of myeloma cells
(30), TRAF6 may be a novel target
for the treatment of MM.
The interactions of the myeloma microenvironment,
including osteoclasts, with myeloma cells and TRAF6 are essential
to the functions of osteoclasts. Therefore, an investigation of
TRAF6 and its upstream signaling pathway may aid the elucidation of
novel therapeutic targets for myeloma, improving the available
treatment strategies for myeloma.
Whilst the present study demonstrated that patient
stromal cells are able to induce TRAF6 expression in myeloma cells,
the cytokines and mechanisms underlying this process remain to be
elucidated and must be investigated in future studies. In
conclusion, the present study revealed that MSCs from patients with
MM are able to stimulate the growth of myeloma cells via the TRAF6
signaling pathway, and that TRAF6 expression levels are correlated
with patient prognosis. It is hypothesized that further
investigation of the TRAF6 signal transduction pathway may improve
understanding of the pathogenesis of MM and lead to the
identification of novel therapeutic targets upstream of TRAF6.
Acknowledgements
The authors would like to thank the patients,
clinical staff and their colleagues at The Affiliated Hospital of
Nantong University (Nantong, China) for their assistance throughout
the current study. This study was supported by the Young Scientists
Fund of the National Natural Science Foundation of China (grant no.
81201857) and the Natural Science Foundation of Jiangsu Province,
China (grant no. BK2011388).
References
|
1
|
Wu H and Arron JR: TRAF6, a molecular
bridge spanning adaptive immunity, innate immunity and
osteoimmunology. Bioessays. 25:1096–1105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhong L, Cao F and You Q: Effect of TRAF6
on the biological behavior of human lung adenocarcinoma cell.
Tumour Biol. 34:231–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaudhry SI, Hooper S, Nye E, Williamson
P, Harrington K and Sahai E: Autocrine IL-1β-TRAF6 signalling
promotes squamous cell carcinoma invasion through paracrine TNFα
signalling to carcinoma-associated fibroblasts. Oncogene.
32:747–758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun YS, Ye ZY, Qian ZY, Xu XD and Hu JF:
Expression of TRAF6 and ubiquitin mRNA in skeletal muscle of
gastric cancer patients. J Exp Clin Cancer Res. 31:812012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang J, Rhyasen G, Bolanos L, Rasch C,
Varney M, Wunderlich M, Goyama S, Jansen G, Cloos J, Rigolino C, et
al: Cytotoxic effects of bortezomib in myelodysplastic
syndrome/acute myeloid leukemia depend on autophagy-mediated
lysosomal degradation of TRAF6 and repression of PSMA1. Blood.
120:858–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang G, Gao Y, Li L, Jin G, Cai Z, Chao JI
and Lin HK: K63-linked ubiquitination in kinase activation and
cancer. Front Oncol. 2:52012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abe M: Targeting the interplay between
myeloma cells and the bone marrow microenvironment in myeloma. Int
J Hematol. 94:334–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Armstrong AP, Tometsko ME, Glaccum M,
Sutherland CL, Cosman D and Dougall WC: A RANK/TRAF6-dependent
signal transduction pathway is essential for osteoclast
cytoskeletal organization and resorptive function. J Biol Chem.
277:44347–44356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sezer O, Heider U, Zavrski I, Kühne CA and
Hofbauer LC: RANK ligand and osteoprotegerin in myeloma bone
disease. Blood. 101:2094–2098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hongming H and Jian H: Bortezomib inhibits
maturation and function of osteoclasts from PBMCs of patients with
multiple myeloma by downregulating TRAF6. Leuk Res. 33:115–122.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng Y, Cai Z, Wang S, Zhang X, Qian J,
Hong S, Li H, Wang M, Yang J and Yi Q: Macrophages are an abundant
component of myeloma microenvironment and protect myeloma cells
from chemotherapy drug-induced apoptosis. Blood. 114:3625–3628.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tucci M, Stucci S, Strippoli S, Dammacco F
and Silvestris F: Dendritic cells and malignant plasma cells: An
alliance in multiple myeloma tumor progression? Oncologist.
16:1040–1048. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Zhu X, Li N, Chen T, Yang M, Yao M,
Liu X, Jin B, Wang X and Cao X: CMRF-35-like molecule 3
preferentially promotes TLR9-triggered proinflammatory cytokine
production in macrophages by enhancing TNF receptor-associated
factor 6 ubiquitination. J Immunol. 187:4881–4889. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kobayashi T, Walsh PT, Walsh MC, Speirs
KM, Chiffoleau E, King CG, Hancock WW, Caamano JH, Hunter CA, Scott
P, et al: TRAF6 is a critical factor for dendritic cell maturation
and development. Immunity. 19:353–363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen H, Li M, Campbell RA, Burkhardt K,
Zhu D, Li SG, Lee HJ, Wang C, Zeng Z, Gordon MS, et al:
Interference with nuclear factor kappa B and c-Jun NH2-terminal
kinase signaling by TRAF6C small interfering RNA inhibits myeloma
cell proliferation and enhances apoptosis. Oncogene. 25:6520–6527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng MM, Zhang Z, Bemis K, Belch AR,
Pilarski LM, Shively JE and Kirshner J: The systemic cytokine
environment is permanently altered in multiple myeloma. PLoS One.
8:e585042013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greipp PR, San Miguel J, Dune BG, Crowley
JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H,
Kyle RA, et al: International staging system for multiple myeloma.
J Clin Oncol. 23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao M, Xie ZQ, Han YJ, An G, Meng HX,
Huang J, Li CH, Zou DH and Qiu LG: Effect of mesenchymal stem cells
on multiple myeloma cells growth and inhibition of bortezomib
induced cell apoptosis. Zhonghua Xue Ye Xue Za Zhi. 31:680–683.
2010.(In Chinese). PubMed/NCBI
|
|
19
|
Huang HM, Wang XF, Liu XX, Xu RR, Shi W,
Ding RS and Jiang SH: Effects of down-regulated TRAF6 gene
expression on the proliferation and apoptosis in multiple myeloma
cells. Zhonghua Xue Ye Xue Za Zhi. 34:941–945. 2013.(In Chinese).
PubMed/NCBI
|
|
20
|
Liu H, Tamashiro S, Baritaki S, Penichet
M, Yu Y, Chen H, Berenson J and Bonavida B: TRAF6 activation in
multiple myeloma: A potential therapeutic target. Clin Lymphoma
Myeloma Leuk. 12:155–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaneko M, Kanda Y, Oshima K, Nannya Y,
Suguro M, Yamamoto R, Chizuka A, Hamaki T, Matsuyama T, Takezako N,
et al: Simple prognostic model for patients with multiple myeloma:
A single-cencer study in Japan. Ann Hematol. 81:33–36. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dimopoulos MA, Barlogie B, Smith TL and
Alexanian R: High serum lactate dehydrogenase level as a marker for
drug resistance and short survival in multiple myeloma. Ann Intern
Med. 115:931–935. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagura E: Prognositic factors in multiple
myeloma. Nihon Rinsho. 65:2351–2356. 2007.(In Japanese). PubMed/NCBI
|
|
24
|
Rajkumar SV and Kyle RA: Multiple myeloma:
Diagnosis and treatment. Mayo Clin Proc. 80:pp. 1371–1382. 2005;
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gunn WG, Conley A, Deininger L, Olson SD,
Prockop DJ and Gregory CA: A crosstalk between myeloma cells and
marrow stromal cells stimulates production of DKK1 and
interleukin-6: A potential role in the development of lytic bone
disease and tumor progression in multiple myeloma. Stem Cells.
24:986–991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu G, Liu K, Anderson J, Patrene K,
Lentzsch S, Roodman GD and Ouyang H: Expression of XBP1s in bone
marrow stromal cells is critical for myeloma cell growth and
osteocast formation. Blood. 119:4205–4214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Annunziata CM, Davis RE, Demchenko Y,
Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W,
et al: Frequent engagement of the classical and alternative
NF-kappaB pathways by diverse genetic abnormalities in multiple
myeloma. Cancer Cell. 12:115–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rong Z, Cheng L, Ren Y, Li Z, Li Y, Li X,
Li H, Fu XY and Chang Z: Interleukin-17F signaling requires
ubiquitination of interleukin-17 receptor via TRAF6. Cell Signal.
19:1514–1520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Theoleyre S, Wittrant Y, Tat SK, Fortun Y,
Redini F and Heymann D: The molecular triad OPG/RANK/RANKL:
Involvement in the orchestration of pathophysiological bone
remodeling. Cytokine Growth Factor Rev. 15:457–475. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu H, Tamashiro S, Baritaki S, Penichet
M, Yu Y, Chen H, Berenson J and Bonavida B: TRAF6 activation in
multiple myeloma: A potential therapeutic target. Clin Lymphoma
Myeloma Leuk. 12:155–163. 2012. View Article : Google Scholar : PubMed/NCBI
|