Prostate cancer (PCa) is the most common urological
malignancy and the second leading cause of male cancer-associated
mortality in numerous developed countries (1). The rate of diagnosis differs between
countries due to the difference in coverage of prostate-specific
antigen (PSA) screening (2), but in
populations with and without PSA screening, PCa is the cause of
1–2% of all mortality for men (3).
Its greater prevalence in the West implicates lifestyle and
environmental risk factors (4).
Significant progress has been made in the treatment and
understanding of the underlying biology (5–7). This
includes the approval of several novel effective drugs that prolong
life in men with advanced PCa, and the recognition that the terms
hormone refractory and androgen-independent were misnomers. The
majority of cancers remain hormone-driven despite castration
resistance (8–13). Although the improvements in PCa
detection and multiple treatments have led to a significant
decrease in PCa-associated mortalities in the last three decades,
the majority of men initially diagnosed with early-stage cancer
eventually develop metastatic disease (14). Thus, PCa currently remains challenging
to treat, and the aims of therapy are the improvement of overall
survival (OS) time and quality of life.
Multiple clinical and pathological features, such as
bone scan assay and quantitative imaging parameters (15), and laboratory biochemical indicators,
such as PSA (16), currently guide
treatment decision making in PCa. However, the lack of reliable
prognostic and predictive factors and imperfect tools to precisely
diagnose and evaluate treatment success in the primary and
metastatic setting continues to result in serious overtreatment and
invalid treatment of patients (17).
Novel strategies to improve diagnosis, earlier indications of
treatment response and treatment failure, and improved definition
of patient cohorts that will respond to a specific treatment in
primary and metastatic PCa are therefore urgently required.
Circulating tumor cells (CTCs) are cancer cells that
are present in the blood of patients with solid cancers and are
shed from existing tumor lesions into the bloodstream (18). Numerous laboratory studies and
clinical trials in the past two decades have shown that CTCs may be
used as a biomarker to predict disease progression and survival in
patients with metastatic, advanced (19–22) or
even early-stage PCa (23). High CTC
numbers are associated with aggressive disease, increased
metastasis and decreased time to relapse (22,24,25). In
addition, CTCs isolated from patients with metastatic PCa (mPCa)
may initiate metastasis in a xenograft model (26). A growing number of studies have shown
that CTCs are a source of metastatic cells (27–29); CTCs
have become an integral part of tumor staging criteria, which are
currently focused on several types of tumors, including breast
cancer (30), PCa (29), lung cancer (31) and colorectal cancer (32). Since blood collection is simple,
convenient and minimally invasive, CTCs may be used as a real-time
liquid biopsy for disease progression and survival (19). CTCs also have potential to guide
therapeutic management (33),
indicate tumor sensitivity to therapy, monitor therapy
effectiveness or necessity, even in the absence of detectable
metastases (33), offer insights into
mechanisms of drug resistance (34)
and promote new anti-drug research and development to a certain
degree (35). CTCs may also be
utilized as a surrogate endpoint marker in clinical trials
(36), and may also become a
promising tumor target. Despite the potential, the use of CTCs
faces numerous challenges.
This review describes the current state of research
into CTCs enrichment and detection strategies and clinical
application, the evidence to demonstrate their diagnostic validity
and therapeutic value, and their potential impact for future
clinical trial design and therapeutic decision-making processes in
PCa.
PCa circulating tumor cells (PCTCs) are present in
the bloodstream at a low concentration, ranging between 1–10 cells
per 10 ml in the majority of patients with cancer, which poses a
serious challenge for any analytical system (18). The problem exists of looking for the
proverbial ‘needle in the haystack’. Thus, the detection and
characterization of PCTCs requires highly sensitive and specific
techniques, which consist of a combination of enrichment
(isolation) and detection (identification) strategies, and these
two steps are essential components of the identification process
(23). It was not until recently that
such sensitive molecular techniques were developed.
Several membrane filter devices are available for
CTC enrichment based on the differential cellular size, including
isolation by size of epithelial tumor cells, micro
electro-mechanical system-optics based microfilter, ScreenCell®,
CellSieve™ and CellOptics® (42). Recently, Qin et al (43) used the resettable cell trap mechanism
to separate cells based on their size and deformability using an
adjustable aperture that can be periodically cleared to prevent
clogging. This system was able to capture ≥5 CTCs in 18/22 (82%)
patients with a mean count of 257 in 7.5 ml of whole blood, while
the CellSearch system found ≥5 CTCs in 9/22 (41%) metastatic
castration-resistant PCa (mCRPC) with a mean count of 25 in the 7.5
ml whole blood samples. Filtration by size consents to enrich CTCs
from a wide range of tumors, but occasionally results in loss of
smaller CTCs or clotting of filter pores by leukocytes (40). In addition, emerging CTC capture
strategies typically distinguish these cells based on cultured
cancer cells. However, CTCs were found to have significantly
smaller size, larger nuclear-cytoplasmic ratio and more elongated
shape (44), which may inevitably
degrade the performance of the cell size-dependent capture system.
Another morphology-based enrichment strategy is based on density
gradient centrifugation using Ficoll-hypaque solution (45). Ficoll density gradient-dependent
techniques are easy to operate, even if real losses of tumor cells
have been observed (46).
Immunoselection is the most commonly used approach
for CTC enrichment, which relies on specific CTC markers that are
detected by antibodies (39).
Epithelial markers are expressed on epithelial tumors, but not on
blood cells, and have therefore been used to isolate CTCs from
blood cells (47). EpCAM and members
of the family of cytokeratin (CK)s (CK8, CK18 and CK19) have been
markers for positive selection in PCa (47). Thus far, the most successful approach
belongs to the CellSearch® System (Veridex LLC, Raritan, NJ, USA),
which employs ferromagnetic nanoparticles coupled to EpCAM
antibodies for CTC capture, became the first validated CTC assay
approved by the Food and Drug Administration (FDA) in 2008
(48,49). With this system, CTCs are isolated
using ferrofluid covalently linked to an antibody against the
surface of EpCAM (48). CTCs are
differentiated from leukocytes by labeling the product with
monoclonal antibodies against CKs (CTC markers) and CD45 (leukocyte
marker) (38,48,49). The
labeled sample is analyzed by an automated fluorescence detection
system (CellTracks® Analyzer; Veridex LLC) (48,49).
However, the limitation of the CellSearch System is that
EpCAM-negative tumor cells may not be detected. Downregulation of
EpCAM may occur during the epithelial-mesenchymal transition (EMT),
a process linked to tumor cell invasion and dissemination, and to
the stemness of cancer cells (50).
Mesenchymal-based capture is a strategy that isolates cells based
on osteoblast (OB)-cadherin cell surface expression (51). Using this method, OB-cadherin cellular
events are detectable in men with mPCa and are less common in
healthy volunteers. This method may complement existing
epithelial-based methods and may be particularly useful in patients
with bone metastases (51). For PCa,
PSMA presents a compelling target for immunocapture, as PSMA levels
increase in higher-grade cancers and metastatic disease and are
specific to the prostate epithelium (39). In order to overcome this drawback,
PSMA-based positive immunoselection was developed and may
successfully capture the EMT CTCs (39). Santana et al (52) used monoclonal J591 antibodies and
J415-antibodies that are highly specific for intact extracellular
domains of PSMA on live cells in microfluidic devices for the
capture of LNCaPs. The results showed that J591 outperforms J415
and a mix of the two for PCa capture, and that capture performance
saturates following incubation with antibody concentrations of 10
µg/ml. In addition, negative selection for the antigens CD45
(expressed in leukocytes) and CD61 (expressed in megakaryocytes and
platelets) may markedly reduce and avoid contamination by blood
cells (53). The major advantage of
immune-based separation is that CTCs can be directly visualized and
quantified without requiring cell lysis. Its limitations include
cost and variability, due to the absence of standardized methods
and reagents (53).
Several other devices based on physical and/or
biological properties of CTCs are available, including a NanoVelcro
Chip assay (54), aptamer-conjugated
graphene oxide membranes (55) and a
microfluidics device that combines the multi-orifice flow
fractionation and the dielectrophoresis/acoustophoresis cell
separation techniques (56,57), among which the microfluidics device
holds promise for CTC enrichment. Ozkumur et al (58) described an inertial focusing-enhanced
microfluidic CTC capture platform, termed CTC-iChip, which is
capable of sorting rare CTCs from whole blood at 107
cells/sec. Most importantly, the iChip is capable of isolating CTCs
using strategies that are either dependent or independent of tumor
membrane epitopes, and thus applicable to virtually all cancers. A
technology that uses magnetic particles bearing tumor cell-specific
EpCAM antibodies, self-assembled in a regular array in a
microfluidic flow cell was reported recently (59), which could capture CTCs in 75% of
patients with mPCa and 80% of patients with metastatic breast
cancer, and showed similar or improved results compared with the
CellSearch device in 10 out of 13 samples. In addition, a single
inlet two-stage acoustophoresis chip could enrich PCa cells from
white blood cells, DU145 spiked into blood were enriched from white
blood cells at a sample flow rate of 100 µl min (−1), providing
86.5±6.7% recovery of the cancer cells with 1.1±0.2% contamination
of white blood cells, and by increasing the acoustic intensity a
recovery of 94.8±2.8% of cancer cells was achieved with 2.2±0.6%
contamination of white blood cells (60). Notably, Sarioglu et al
(61) developed a microchip
technology (the Cluster-Chip) to capture CTC clusters independently
of tumor-specific markers from unprocessed blood. CTC clusters are
isolated through specialized bifurcating traps under low-shear
stress conditions that preserve their integrity, and even two-cell
clusters are captured efficiently (61).
Following enrichment, CTCs require subsequent
identification at the single-cell level and to be separated from
normal blood cells. Detection of CTCs may be performed through
immunology-based techniques or nucleic acid-based techniques.
Immunology-based techniques are the most common of
the strategies and are effective for detection and isolation of
CTCs. Immunological methods utilize labeled antibodies directed
against epithelial or tumor-associated antigens, along with
automated digital microscopy or flow cytometry, to identify and
quantify CTCs (42). Numerous
antigens have been used for this method, including EpCAM and
different subtypes of CKs (62).
However, not all CTCs express these markers, possibly as a
consequence of the EMT process, and likely lead to false-negative
results (62). In addition,
tumor-specific antigens are expressed at an increased level in
cancer cells compared with normal cells. PSA and PSMA are
PCa-associated markers for detecting CTCs, and have been applied in
antibody-based detection and isolation of CTCs (63–65).
EpCAM-based immunological assays are the most common
of the strategies for CTC detection. Among the recently developed
innovative techniques, the CellSearch® system is the most advanced
commercially available technology with combined automated
enrichment and immunostaining (48).
Currently, it is the only technology that has been approved by the
USA FDA for the detection of patients with mPCa (22). The CD45 marker is employed to rule out
white blood cells and increase detection specificity (66). A CTC is often defined as a
CK+/CD45−/DAPI+ intact cell
(66). The nuclear dye DAPI is used
to exclude cell fragments and debris false-positive may occur using
CK as a marker (66). Notably, this
technique allows quantitative measurement of CTCs and it may
provide a more precise discrimination between patients (62).
In addition to the CellSearch® system, a variety of
novel detection technologies have been developed. Myung et
al (67) engineered a novel
multifunctional surface, on the basis of the biomimetic cell
capture, through optimized incorporation of multiple antibodies
directed to cancer cell-specific surface markers, including EpCAM,
human epidermal growth factor receptor-2 and PSA, which may lead to
clinically significant, differential detection of CTCs that are
rare and highly heterogeneous.
Nucleic acid-based techniques have become the most
widely used alternative to immunology-based techniques, which are
considered to be more sensitive than protein-based approaches
(68). Particularly, polymerase chain
reaction (PCR)-based assays evaluate the amount of DNA from CTCs.
However, the drawback of this technique is the inability to
distinguish the DNA free in the blood from apoptotic cells,
creating false-positive results (68). Therefore, the majority of research
groups prefer reverse transcription (RT)-PCR assays to detect
specific mRNA, since only viable CTCs produce mRNA (69–71).
Several specific mRNA markers are used to identify PCTCs in nucleic
acid-based methods (Table II).
To date, the mRNA encoding PSA has been the most
extensively studied in clinical trials (63,69,72).
Mohamadi et al (73) developed
a velocity valley chip to efficiently capture magnetic
nanoparticle-bound CTCs. The approach was successfully validated
using samples collected from patients with PCa: CTCs and PSA mRNA
sequences were detected in all cancer patient samples and not in
the healthy controls. In addition, quantitative RT-PCR analysis of
PSA and PSMA mRNA to detect circulating tumor cells improves
recurrence-free survival nomogram prediction following radical
prostatectomy (72). Furthermore,
androgen receptor (AR), kallikrein-related peptidase (KLK)2, KLK3,
PCa antigen 3 (PCA3), transmembrane protease serine 2 and
ETS-related fusion gene (TMPRSS2-ERG), AR splice variant 7
messenger RNA (AR-V7) and prostate stem cell antigen mRNA are also
utilized to identify PCTCs (63,69,74–76).
However, the major limitations to these techniques are associated
with the mRNA markers utilized, since they may be also present at
low concentrations in normal blood, bone marrow cells and in other
non-tumor cells (77). Therefore, to
seek more specific mRNA markers is the key issue in future
studies.
In addition to the aforementioned types of commonly
used CTC detection techniques, certain novel technologies are also
in rapid development, particularly sensor-based detection
technologies, which show high sensitivity and specificity (78). Sioss et al (78) selected PCA3 RNA as a marker and
developed a nanoresonator chip-based sensor for detection of
prostate circulating tumor cells. Similarly, Ivanov et al
(79) described a chip-based method
using nanostructured microelectrodes and electrochemical readout,
which confirmed the identity of isolated CTCs and successfully
interrogated them for specific biomarkers. Recently, a light
addressable potentiometric sensor was exploited in the label-free
detection of CTCs in PCa, which may be a potential platform for CTC
detection and may provide a powerful tool for downstream analysis
(80).
Overall, in the last two decades, several promising
CTC enrichment and detection methods have been developed. These
strategies should be validated in appropriately sized clinical
trials in order to evaluate their quality, validity and clinical
practicability.
CTCs have an important role in the progression and
metastasis of PCa, and represent tumor characteristics of
individual patients well. Therefore, CTCs may be used as an ideal
biomarker for prognosis, prediction and clinical management of
patients with PCa.
Utilizing multiple blood tests, CTC enumeration and
characterization may provide prognostic and predictive value on
PCa, particularly in metastatic and advanced PCa (47,81–83). As
early as 2005, Moreno et al (84) demonstrated that in patients with
metastatic PCa, CTC enumeration with the cut-off of 5 CTCs was a
predictive factor superior to other clinical variables. They also
demonstrated that increased CTC numbers have been found in patients
with bone metastasis compared with patients with visceral spread,
which suggests a prognostic potential for CTC detecting in
monitoring patients for bone disease in PCa (20–22).
Furthermore, in patients with CRPC, post-treatment CTC counts were
a stronger prognostic factor for survival than a 50% decline in PSA
[receiver operating characteristic (ROC) AUC 0.87 vs. 0.62]
(19,85). Therefore, for patients with CRPC with
no metastases and slow-rising PSA, CTC detection would be
complementary to PSA testing, which may be useful in facilitating
decisions about additional imaging requirements and for early
diagnosis of bone metastases, allowing for earlier intervention
with treatments. In addition, CTC counts appear to be an earlier
and more sensitive predictor for survival and treatment response
compared with current objective response criteria (OR) in patients
with mCRPC (86). Recently,
Marín-Aguilera et al (87)
analyzed the molecular profiling of peripheral blood from 43
patients with mCRPC with known CTC content in order to identify
genes that may be associated with PCa progression. The analysis
revealed a two-gene model (selenium binding protein 1 and matrix
metalloprotein 9) with a high significant prognostic ability
[hazard ratio (HR), 6; 95% confidence interval (CI), 2.61–13.79;
P<0.0001]. Notably, a phase III SWOG-led therapeutic trial
demonstrated that increased CTC telomerase activity was associated
with HR, 1.14 (P= 0.001) for OS in patients with baseline CTC count
≥5 (47% of patients), subsequent to adjusting for other clinical
covariates, including CTC counts and serum PSA at study entry
(88).
However, CTCs were identified in 21% of patients
with PCa prior to radical prostatectomy, which was similar to the
rate of CTC detection in control (non-cancer) patients (89). Consistently, Loh et al
(90) collected samples of peripheral
blood (7.5 ml) drawn from 36 men with newly diagnosed high-risk
non-metastatic PCa, prior to any initiation of therapy, and
analyzed for CTCs using the CellSearch® method. They demonstrated
that patients with high-risk, non-metastatic PCa present
infrequently with a small number of CTCs in peripheral blood.
Furthermore, in these patients with localized PCa, CTC numbers were
not associated with tumor volume, pathological stage and Gleason
score, indicating that CTCs are more likely to originate from
metastasis sites instead of primary lesions (91,92).
Notably, a number of men have low CTCs despite
widespread disease, indicating heterogeneity in CTC phenotype or
detection (93,94). Chen et al (95) suggested that an incremental expression
of EMT-associated genes in CTCs is associated with mCRPC. It was
found that genes that promote mesenchymal transitioning into a more
malignant state, including insulin-like growth factor (IGF)1, IGF2,
epidermal growth factor receptor, forkhead box p3 and transforming
growth factor β3, were commonly observed in these cells. In
addition, Osmulski et al (96)
found that CTCs from patients with CRPC are three times softer,
three times more deformable and seven times more adhesive than
counterparts from patients with castration-sensitive PCa. The
present study indicates that nanomechanical phenotypes of CTCs may
serve as novel and effective biomarkers for mCRPC. More recently,
the detection of small nuclear CTCs was found to be associated with
the presence of visceral metastases and should be formally explored
as a putative blood-borne biomarker to identify patients at risk of
developing this clinical evolution of PCa (24).
Overall, enumeration and characterization of CTCs
has shown a strong potential in the prognosis and prediction of
patients with metastatic and advanced PCa, whereas CTC detection is
unreliable in the early and localized disease state.
An increasing number of systemic therapies with life
extending capacity have become available in mCRPC, including
abiraterone acetate (AA), enzalutamide, sipuleucel-T, docetaxel,
cabazitaxel and radium-223 (97).
More compounds are currently being evaluated in promising pivotal
trials, such as Tasquinimod, ARN-509 and ODM-201 (97). Limitations of the currently available
biomarkers make treatment decisions challenging (97). Considering the increasing complexity
of treatment algorithms in mCRPC, the current demand of research is
to identify and characterize biomarkers with prognostic, predictive
and surrogate quality, allowing for information on clinically
meaningful outcomes and on which therapy to offer patients in
different and complex scenarios. Currently, the selection of a
targeted therapy for an individual patient is based on analysis of
the primary tumor for the expression and/or genomic status of a
specific molecular target (98,99).
However, primary carcinomas, in particular PCa, show a marked
intrapatient heterogeneity in regard to genotypic and phenotypic
characteristics, and tumor cells may transform dynamically during
the extended time period between primary tumor resection and
metastatic relapse due to parallel progression and/or natural
selection of the fittest clones (100). Metastases from different sites are
genetically heterogeneous (29), and
CTCs have the advantage that they may represent the entire spectrum
of distant metastases (101). Thus,
CTC detection and characterization may become a valuable tool to
refine prognosis and serve as a real-time biopsy, and has the
potential to monitor cancer treatment, provide useful predictive
information for the selection of the most appropriate treatment and
guide precision cancer therapies (100).
A growing number of studies have demonstrated that
the dynamic change of CTC count is associated with a significant
curative effect (102,103). Patients with CRPC received AA 1,000
mg daily and continuously, a post-therapy CTC count of <5 per
7.5 ml was prognostic for longer survival relative to a CTC count
of ≥5, which indicated that lower CTC enumeration was positively
associated with AA therapeutic effect (104). Furthermore, the change of CTC counts
was used as an endpoint in patients with mCRPC to determine the
efficacy and tolerability of cabozantinib at lower starting doses
(36,105). For the first time, Dorff et
al (25) detected CTCs in men
with biochemical recurrence PCa to monitor the therapeutic effect
of a combination herbal supplement, Prostate Health Cocktail.
In addition, molecular profile may be characterized
in CTC as a potential predictive value of tumor sensitivity to a
therapeutic modality or not (Table
III), potential clinical uses of CTCs in determining prognosis
and monitoring treatment effects, and as a source of tissue to
identify predictive markers of drug sensitivity to guide treatment
selection. Data from quantitative RT-PCR to detect circulating
prostate-derived PSA and PSMA mRNA pre- and post- radical
prostatectomy improves the accuracy of the Kattan nomogram to
predict biochemical recurrence (72).
In addition, biomarker response to docetaxel treatment was detected
in 20 patients with CRPC (75). In
response to docetaxel treatment, KLK3 levels decreased in 80% (95%
CI, 60–100%), PCA3 in 89% (95% CI, 68–100%) and TMPRSS2-ERG in 86%
(95% CI, 60–100%) of patients. By contrast, the blood samples from
all 32 healthy volunteers were reproducibly negative for all three
markers. A longitudinal study of a single patient with PCa who
received AA treatment demonstrated that CTCs that acquired AR and
MYC gene amplification represented a novel lineage, apparently
resistant to AA, and possibly generated from a single resistant
cell (106). In addition, despite
intrapatient heterogeneity, CTCs from patients with prior exposure
to abiraterone had increased the proliferation marker Ki67
expression, and qualitatively, the majority of AR staining within
CTCs was intranuclear in subcellular localization (107).
Several studies have demonstrated that detection of
AR-V7 in circulating tumor cells from patients with mCRPC may be
associated with resistance to enzalutamide and abiraterone
(76,108). However, in AR-V7-positive men,
taxanes appear to be more efficacious than enzalutamide or
abiraterone therapy, whereas in AR-V7-negative men, taxanes and
enzalutamide or abiraterone may have comparable efficacy (108). In addition, Onstenk et al
(109) recently demonstrated that
the response to cabazitaxel appeared to be independent of the AR-V7
status of CTCs from patients with mCRPC. Consequently, cabazitaxel
may be a valid treatment option for patients with AR-V7-positive
CTCs. In addition, patients with PCa harbored AR point mutations (a
p.T878A point mutation and a p.H875Y mutation), which was another
common AR modification mediating resistance to androgen deprivation
therapy (34).
Furthermore, CTCs may be used as a viable
pharmacodynamic marker to provide additional information on the
safety profile and efficacy of agents. A phase I study determined
the maximum tolerated dose of AEZS-108 in men with taxane- and
CRPC, which was partly based on AEZS-108 internalization in CTCs
using its autofluorescence (35).
In conclusion, CTC detection and characterization
have shown potential to monitor cancer treatment and guide
precision cancer therapies; however, the molecular profile of CTCs
requires additional study and exploration, and more rigorous large
clinical trials are required to confirm its clinical utility.
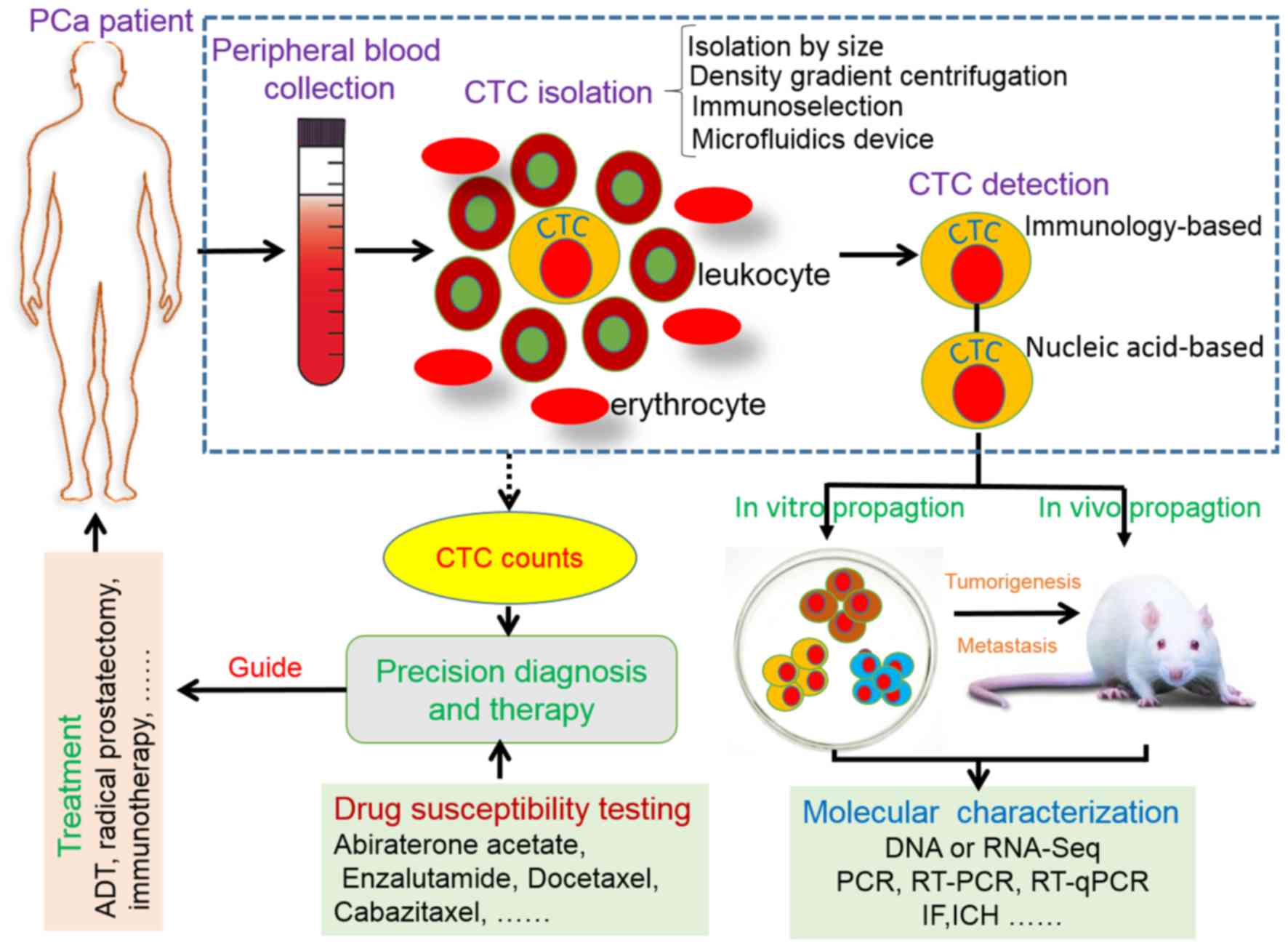
The present study mainly described current advances
in CTC enrichment and detection strategies, and reviewed how CTCs
could move toward precision diagnosis and therapy in PCa (Fig. 1). Recently, Miyamoto et al
reported a landmark study on RNA-sequencing (RNA-Seq) of single
prostate CTCs (112). This study
established single-cell RNA-Seq profiles of 77 intact CTCs isolated
from 13 patients (mean, 6 CTCs per patient), by using microfluidic
enrichment. It was found that single-cell analysis of prostate CTCs
revealed heterogeneity in signaling pathways, most importantly,
ectopic expression of Wnt5a in PCa cells could attenuate the
antiproliferative effect of AR inhibition, whereas its suppression
in drug-resistant cells restored partial sensitivity (112). Beyond that, circulating tumor cells
may be used as potential therapeutic targets for PCa treatment;
killing and eliminating circulating tumor cells has become a novel
and effective strategy for PCa therapy (113,114),
and surrogate predictors of survival could shorten drug trials for
PCa treatment (115). In conclusion,
there is great promise in utilizing CTCs as a platform for
precision diagnosis and therapy in PCa. Although the future is
bright, there is also a huge challenge. More sensitive and
effective devices for CTC enrichment and detection are urgently
required to be developed. More importantly, CTC assays require
validation in clinical trials to achieve clinical validity and
clinical utility.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prostate cancer: Send away the PSA?
Lancet. 380:3072012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marugame T and Katanoda K: International
comparisons of cumulative risk of breast and prostate cancer, from
cancer incidence in five continents Vol. VIII. Jpn J Clin Oncol.
36:399–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee J, Demissie K, Lu SE and Rhoads GG:
Cancer incidence among Korean-American immigrants in the United
States and native Koreans in South Korea. Cancer Control. 14:78–85.
2007.PubMed/NCBI
|
|
5
|
Sottnik JL, Dai J, Zhang H, Campbell B and
Keller ET: Tumor-induced pressure in the bone microenvironment
causes osteocytes to promote the growth of prostate cancer bone
metastases. Cancer Res. 75:2151–2158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan L, Peng G, Sahgal N, Fazli L, Gleave
M, Zhang Y, Hussain A and Qi J: Regulation of c-Myc expression by
the histone demethylase JMJD1A is essential for prostate cancer
cell growth and survival. Oncogene. 35:2441–2452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee S, Luong R, Johnson DT, Cunha GR,
Rivina L, Gonzalgo ML and Sun Z: Androgen signaling is a
confounding factor for β-catenin-mediated prostate tumorigenesis.
Oncogene. 35:702–714. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al: Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: A randomised open-label trial. Lancet. 376:1147–1154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fizazi K, Carducci M, Smith M, Damião R,
Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, et al:
Denosumab versus zoledronic acid for treatment of bone metastases
in men with castration-resistant prostate cancer: A randomised,
double-blind study. Lancet. 377:813–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Laderach DJ, Gentilini LD, Giribaldi L,
Delgado VC, Nugnes L, Croci DO, Al Nakouzi N, Sacca P, Casas G,
Mazza O, et al: A unique galectin signature in human prostate
cancer progression suggests galectin-1 as a key target for
treatment of advanced disease. Cancer Res. 73:86–96. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trewartha D and Carter K: Advances in
prostate cancer treatment. Nat Rev Drug Discov. 12:823–824. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Violette PD, Agoritsas T, Alexander P,
Riikonen J, Santti H, Agarwal A, Bhatnagar N, Dahm P, Montori V,
Guyatt GH and Tikkinen KA: Decision aids for localized prostate
cancer treatment choice: Systematic review and meta-analysis. CA
Cancer J Clin. 65:239–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Locke JA, Guns ES, Lubik AA, Adomat HH,
Hendy SC, Wood CA, Ettinger SL, Gleave ME and Nelson CC: Androgen
levels increase by intratumoral de novo steroidogenesis during
progression of castration-resistant prostate cancer. Cancer Res.
68:6407–6415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gundem G, Van Loo P, Kremeyer B,
Alexandrov LB, Tubio JM, Papaemmanuil E, Brewer DS, Kallio HM,
Högnäs G, Annala M, et al: The evolutionary history of lethal
metastatic prostate cancer. Nature. 520:353–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee KC, Sud S, Meyer CR, Moffat BA,
Chenevert TL, Rehemtulla A, Pienta KJ and Ross BD: An imaging
biomarker of early treatment response in prostate cancer that has
metastasized to the bone. Cancer Res. 67:3524–3528. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stephan C, Cammann H, Meyer HA, Lein M and
Jung K: PSA and new biomarkers within multivariate models to
improve early detection of prostate cancer. Cancer Lett. 249:18–29.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barbieri CE, Baca SC, Lawrence MS,
Demichelis F, Blattner M, Theurillat JP, White TA, Stojanov P, Van
Allen E, Stransky N, et al: Exome sequencing identifies recurrent
SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet.
44:685–689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression and survival in metastatic breast cancer. N Engl J Med.
351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scher HI, Jia X, de Bono JS, Fleisher M,
Pienta KJ, Raghavan D and Heller G: Circulating tumour cells as
prognostic markers in progressive, castration-resistant prostate
cancer: a reanalysis of IMMC38 trial data. Lancet Oncol.
10:233–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Olmos D, Arkenau HT, Ang JE, Ledaki I,
Attard G, Carden CP, Reid AH, A'Hern R, Fong PC, Oomen NB, et al:
Circulating tumour cell (CTC) counts as intermediate end points in
castration-resistant prostate cancer (CRPC): A single-centre
experience. Ann Oncol. 20:27–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Danila DC, Heller G, Gignac GA,
Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson
S, Fleisher M and Scher HI: Circulating tumor cell number and
prognosis in progressive castration-resistant prostate cancer. Clin
Cancer Res. 13:7053–7058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagrath S, Sequist LV, Maheswaran S, Bell
DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky
A, et al: Isolation of rare circulating tumour cells in cancer
patients by microchip technology. Nature. 450:1235–1239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen JF, Ho H, Lichterman J, Lu YT, Zhang
Y, Garcia MA, Chen SF, Liang AJ, Hodara E, Zhau HE, et al:
Subclassification of prostate cancer circulating tumor cells by
nuclear size reveals very small nuclear circulating tumor cells in
patients with visceral metastases. Cancer. 121:3240–3251. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dorff TB, Groshen S, Tsao-Wei DD, Xiong S,
Gross ME, Vogelzang N, Quinn DI and Pinski JK: A Phase II trial of
a combination herbal supplement for men with biochemically
recurrent prostate cancer. Prostate Cancer Prostatic Dis.
17:359–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rossi E, Rugge M, Facchinetti A, Pizzi M,
Nardo G, Barbieri V, Manicone M, De Faveri S, Scaini M Chiara,
Basso U, et al: Retaining the long-survive capacity of circulating
tumor cells (CTCs) followed by xeno-transplantation: Not only from
metastatic cancer of the breast but also of prostate cancer
patients. Oncoscience. 1:49–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paris PL, Kobayashi Y, Zhao Q, Zeng W,
Sridharan S, Fan T, Adler HL, Yera ER, Zarrabi MH, Zucker S, et al:
Functional phenotyping and genotyping of circulating tumor cells
from patients with castration resistant prostate cancer. Cancer
Lett. 277:164–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Helzer KT, Barnes HE, Day L, Harvey J,
Billings PR and Forsyth A: Circulating tumor cells are
transcriptionally similar to the primary tumor in a murine prostate
model. Cancer Res. 69:7860–7866. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lohr JG, Adalsteinsson VA, Cibulskis K,
Choudhury AD, Rosenberg M, Cruz-Gordillo P, Francis JM, Zhang CZ,
Shalek AK, Satija R, et al: Whole-exome sequencing of circulating
tumor cells provides a window into metastatic prostate cancer. Nat
Biotechnol. 32:479–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu M, Bardia A, Aceto N, Bersani F, Madden
MW, Donaldson MC, Desai R, Zhu H, Comaills V, Zheng Z, et al:
Cancer therapy. Ex vivo culture of circulating breast tumor cells
for individualized testing of drug susceptibility. Science.
345:216–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ni X, Zhuo M, Su Z, Duan J, Gao Y, Wang Z,
Zong C, Bai H, Chapman AR, Zhao J, et al: Reproducible copy number
variation patterns among single circulating tumor cells of lung
cancer patients. Proc Natl Acad Sci USA. 110:pp. 21083–21088. 2013;
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Steinert G, Schölch S, Niemietz T, Iwata
N, García SA, Behrens B, Voigt A, Kloor M, Benner A, Bork U, et al:
Immune escape and survival mechanisms in circulating tumor cells of
colorectal cancer. Cancer Res. 74:1694–1704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Anantharaman A and Friedlander TW: A
stepping stone toward personalized oncology: Genomic analysis of
circulating tumor cells to guide management of metastatic
castration-resistant prostate cancer. Eur Urol. 68:946–948. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Steinestel J, Luedeke M, Arndt A,
Schnoeller TJ, Lennerz JK, Wurm C, Maier C, Cronauer MV, Steinestel
K and Schrader AJ: Detecting predictive androgen receptor
modifications in circulating prostate cancer cells. Oncotarget. Apr
23–2015.(Epub ahead of print).
|
|
35
|
Liu SV, Tsao-Wei DD, Xiong S, Groshen S,
Dorff TB, Quinn DI, Tai YC, Engel J, Hawes D, Schally AV and Pinski
JK: Phase I, dose-escalation study of the targeted cytotoxic LHRH
analog AEZS-108 in patients with castration- and taxane-resistant
prostate cancer. Clin Cancer Res. 20:6277–6283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee RJ, Saylor PJ, Michaelson MD,
Rothenberg SM, Smas ME, Miyamoto DT, Gurski CA, Xie W, Maheswaran
S, Haber DA, et al: A dose-ranging study of cabozantinib in men
with castration-resistant prostate cancer and bone metastases. Clin
Cancer Res. 19:3088–3094. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Coumans FA, van Dalum G, Beck M and
Terstappen LW: Filter characteristics influencing circulating tumor
cell enrichment from whole blood. PLoS One. 8:e617702013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Allard WJ, Matera J, Miller MC, Repollet
M, Connelly MC, Rao C, Tibbe AG, Uhr JW and Terstappen LW: Tumor
cells circulate in the peripheral blood of all major carcinomas but
not in healthy subjects or patients with nonmalignant diseases.
Clin Cancer Res. 10:6897–6904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gleghorn JP, Pratt ED, Denning D, Liu H,
Bander NH, Tagawa ST, Nanus DM, Giannakakou PA and Kirby BJ:
Capture of circulating tumor cells from whole blood of prostate
cancer patients using geometrically enhanced differential
immunocapture (GEDI) and a prostate-specific antibody. Lab Chip.
10:27–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu L, Mao X, Imrali A, Syed F, Mutsvangwa
K, Berney D, Cathcart P, Hines J, Shamash J and Lu YJ: Optimization
and evaluation of a novel size based circulating tumor cell
isolation system. PLoS One. 10:e01380322015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Joshi P, Jacobs B, Derakhshan A, Moore LR,
Elson P, Triozzi PL, Borden E and Zborowski M: Enrichment of
circulating melanoma cells (CMCs) using negative selection from
patients with metastatic melanoma. Oncotarget. 5:2450–2461. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Toss A, Mu Z, Fernandez S and
Cristofanilli M: CTC enumeration and characterization: Moving
toward personalized medicine. Ann Transl Med. 2:108.
2014.PubMed/NCBI
|
|
43
|
Qin X, Park S, Duffy SP, Matthews K, Ang
RR, Todenhöfer T, Abdi H, Azad A, Bazov J, Chi KN, et al: Size and
deformability based separation of circulating tumor cells from
castrate resistant prostate cancer patients using resettable cell
traps. Lab Chip. 15:2278–2286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sunyoung P, Ang RR, Duffy SP, Bazov J, Chi
KN, Black PC and Ma H: Morphological differences between
circulating tumor cells from prostate cancer patients and cultured
prostate cancer cells. PLoS One. 9:e852642014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rosenberg R, Gertler R, Friederichs J,
Fuehrer K, Dahm M, Phelps R, Thorban S, Nekarda H and Siewert JR:
Comparison of two density gradient centrifugation systems for the
enrichment of disseminated tumor cells in blood. Cytometry.
49:150–158. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Woelfle U, Breit E, Zafrakas K, Otte M,
Schubert F, Müller V, Izbicki JR, Löning T and Pantel K:
Bi-specific immunomagnetic enrichment of micrometastatic tumour
cell clusters from bone marrow of cancer patients. J Immunol
Methods. 300:136–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Coumans FA, Doggen CJ, Attard G, de Bono
JS and Terstappen LW: All circulating EpCAM+CK+CD45-objects predict
overall survival in castration-resistant prostate cancer. Ann
Oncol. 21:1851–1857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Riethdorf S, Fritsche H, Müller V, Rau T,
Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F, et al:
Detection of circulating tumor cells in peripheral blood of
patients with metastatic breast cancer: A validation study of the
CellSearch system. Clin Cancer Res. 13:920–928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dotan E, Cohen SJ, Alpaugh KR and Meropol
NJ: Circulating tumor cells: Evolving evidence and future
challenges. Oncologist. 14:1070–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pavese JM and Bergan RC: Circulating tumor
cells exhibit a biologically aggressive cancer phenotype
accompanied by selective resistance to chemotherapy. Cancer Lett.
352:179–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bitting RL, Boominathan R, Rao C, Kemeny
G, Foulk B, Garcia-Blanco MA, Connelly M and Armstrong AJ:
Development of a method to isolate circulating tumor cells using
mesenchymal-based capture. Methods. 64:129–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Santana SM, Liu H, Bander NH, Gleghorn JP
and Kirby BJ: Immunocapture of prostate cancer cells by use of
anti-PSMA antibodies in microdevices. Biomed Microdevices.
14:401–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Witzig TE, Bossy B, Kimlinger T, Roche PC,
Ingle JN, Grant C, Donohue J, Suman VJ, Harrington D, Torre-Bueno J
and Bauer KD: Detection of circulating cytokeratin-positive cells
in the blood of breast cancer patients using immunomagnetic
enrichment and digital microscopy. Clin Cancer Res. 8:1085–1091.
2002.PubMed/NCBI
|
|
54
|
Lu YT, Zhao L, Shen Q, Garcia MA, Wu D,
Hou S, Song M, Xu X, Ouyang WH, Ouyang WW, et al: NanoVelcro Chip
for CTC enumeration in prostate cancer patients. Methods.
64:144–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nellore BP Viraka, Kanchanapally R,
Pramanik A, Sinha SS, Chavva SR, Hamme A II and Ray PC:
Aptamer-conjugated graphene oxide membranes for highly efficient
capture and accurate identification of multiple types of
circulating tumor cells. Bioconjug Chem. 26:235–242. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Antfolk M, Magnusson C, Augustsson P,
Lilja H and Laurell T: Acoustofluidic, label-free separation and
simultaneous concentration of rare tumor cells from white blood
cells. Anal Chem. 87:9322–9328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Huang SB, Wu MH, Lin YH, Hsieh CH, Yang
CL, Lin HC, Tseng CP and Lee GB: High-purity and label-free
isolation of circulating tumor cells (CTCs) in a microfluidic
platform by using optically-induced-dielectrophoretic (ODEP) force.
Lab on A Chip. 13:1371–1383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ozkumur E, Shah AM, Ciciliano JC, Emmink
BL, Miyamoto DT, Brachtel E, Yu M, Chen PI, Morgan B, Trautwein J,
et al: Inertial focusing for tumor antigen-dependent and
-independent sorting of rare circulating tumor cells. Sci Transl
Med. 5:179ra472013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Autebert J, Coudert B, Champ J, Saias L,
Guneri ET, Lebofsky R, Bidard FC, Pierga JY, Farace F, Descroix S,
et al: High purity microfluidic sorting and analysis of circulating
tumor cells: Towards routine mutation detection. Lab Chip.
15:2090–2101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Antfolk M, Antfolk C, Lilja H, Laurell T
and Augustsson P: A single inlet two-stage acoustophoresis chip
enabling tumor cell enrichment from white blood cells. Lab Chip.
15:2102–2109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sarioglu AF, Aceto N, Kojic N, Donaldson
MC, Zeinali M, Hamza B, Engstrom A, Zhu H, Sundaresan TK, Miyamoto
DT, et al: A microfluidic device for label-free, physical capture
of circulating tumor cell clusters. Nat Methods. 12:685–691. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hou JM, Krebs M, Ward T, Morris K, Sloane
R, Blackhall F and Dive C: Circulating tumor cells, enumeration and
beyond. Cancers (Basel). 2:1236–1250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Stott SL, Lee RJ, Nagrath S, Yu M,
Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z,
et al: Isolation and characterization of circulating tumor cells
from patients with localized and metastatic prostate cancer. Sci
Transl Med. 2:25ra232010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kirby BJ, Jodari M, Loftus MS, Gakhar G,
Pratt ED, Chanel-Vos C, Gleghorn JP, Santana SM, Liu H, Smith JP,
et al: Functional characterization of circulating tumor cells with
a prostate-cancer-specific microfluidic device. PLoS One.
7:e359762012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Friedlander TW, Ngo VT, Dong H,
Premasekharan G, Weinberg V, Doty S, Zhao Q, Gilbert EG, Ryan CJ,
Chen WT and Paris PL: Detection and characterization of invasive
circulating tumor cells derived from men with metastatic
castration-resistant prostate cancer. Int J Cancer. 134:2284–2293.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tseng JY, Yang CY, Liang SC, Liu RS, Jiang
JK and Lin CH: Dynamic changes in numbers and properties of
circulating tumor cells and their potential applications. Cancers
(Basel). 6:2369–2386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Myung JH, Gajjar KA, Chen J, Molokie RE
and Hong S: Differential detection of tumor cells using a
combination of cell rolling, multivalent binding, and multiple
antibodies. Anal Chem. 86:6088–6094. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gerges N, Rak J and Jabado N: New
technologies for the detection of circulating tumour cells. Br Med
Bull. 94:49–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Helo P, Cronin AM, Danila DC, Wenske S,
Gonzalez-Espinoza R, Anand A, Koscuiszka M, Väänänen RM, Pettersson
K, Chun FK, et al: Circulating prostate tumor cells detected by
reverse transcription-PCR in men with localized or
castration-refractory prostate cancer: Concordance with CellSearch
assay and association with bone metastases and with survival. Clin
Chem. 55:765–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gervasoni A, Muñoz RM Monasterio, Wengler
GS, Rizzi A, Zaniboni A and Parolini O: Molecular signature
detection of circulating tumor cells using a panel of selected
genes. Cancer Lett. 263:267–279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Stott SL, Hsu CH, Tsukrov DI, Yu M,
Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK,
et al: Isolation of circulating tumor cells using a
microvortex-generating herringbone-chip. Proc Natl Acad Sci USA.
107:pp. 18392–18397. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yates DR, Rouprêt M, Drouin SJ, Comperat
E, Ricci S, Lacave R, Sèbe P, Cancel-Tassin G, Bitker MO and
Cussenot O: Quantitative RT-PCR analysis of PSA and
prostate-specific membrane antigen mRNA to detect circulating tumor
cells improves recurrence-free survival nomogram prediction after
radical prostatectomy. Prostate. 72:1382–1388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mohamadi RM, Ivanov I, Stojcic J, Nam RK,
Sargent EH and Kelley SO: Sample-to-answer isolation and mRNA
profiling of circulating tumor cells. Anal Chem. 87:6258–6264.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jiang Y, Palma JF, Agus DB, Wang Y and
Gross ME: Detection of androgen receptor mutations in circulating
tumor cells in castration-resistant prostate cancer. Clin Chem.
56:1492–1495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dijkstra S, Leyten GH, Jannink SA, de Jong
H, Mulders PF, van Oort IM and Schalken JA: KLK3, PCA3 and
TMPRSS2-ERG expression in the peripheral blood mononuclear cell
fraction from castration-resistant prostate cancer patients and
response to docetaxel treatment. Prostate. 74:1222–1230. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Antonarakis ES, Lu C, Wang H, Luber B,
Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, et
al: AR-V7 and resistance to enzalutamide and abiraterone in
prostate cancer. N Engl J Med. 371:1028–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Panteleakou Z, Lembessis P, Sourla A,
Pissimissis N, Polyzos A, Deliveliotis C and Koutsilieris M:
Detection of circulating tumor cells in prostate cancer patients:
Methodological pitfalls and clinical relevance. Mol Med.
15:101–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sioss JA, Bhiladvala RB, Pan W, Li M,
Patrick S, Xin P, Dean SL, Keating CD, Mayer TS and Clawson GA:
Nanoresonator chip-based RNA sensor strategy for detection of
circulating tumor cells: Response using PCA3 as a prostate cancer
marker. Nanomedicine. 8:1017–1025. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ivanov I, Stojcic J, Stanimirovic A,
Sargent E, Nam RK and Kelley SO: Chip-based nanostructured sensors
enable accurate identification and classification of circulating
tumor cells in prostate cancer patient blood samples. Anal Chem.
85:398–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gu Y, Ju C, Li Y, Shang Z, Wu Y, Jia Y and
Niu Y: Detection of circulating tumor cells in prostate cancer
based on carboxylated graphene oxide modified light addressable
potentiometric sensor. Biosens Bioelectron. 66:24–31. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kerr BA, Miocinovic R, Smith AK, West XZ,
Watts KE, Alzayed AW, Klink JC, Mir MC, Sturey T, Hansel DE, et al:
CD117+ cells in the circulation are predictive of advanced prostate
cancer. Oncotarget. 6:1889–1897. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang H, Yang M, Xu J, Zou B, Zhou Q, Bian
J and Wang X: Survivin mRNA-circulating tumor cells are associated
with prostate cancer metastasis. Tumour Biol. 37:723–727. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Danila DC, Anand A, Schultz N, Heller G,
Wan M, Sung CC, Dai C, Khanin R, Fleisher M, Lilja H and Scher HI:
Analytic and clinical validation of a prostate cancer-enhanced
messenger RNA detection assay in whole blood as a prognostic
biomarker for survival. Eur Urol. 65:1191–1197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Moreno JG, Miller MC, Gross S, Allard WJ,
Gomella LG and Terstappen LW: Circulating tumor cells predict
survival in patients with metastatic prostate cancer. Urology.
65:713–718. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Scher HI, Jia X, de Bono JS, Fleisher M,
Pienta KJ, Raghavan D and Heller G: Circulating tumour cells as
prognostic markers in progressive, castration-resistant prostate
cancer: A reanalysis of IMMC38 trial data. Lancet Oncol.
10:233–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Thalgott M, Heck MM, Eiber M, Souvatzoglou
M, Hatzichristodoulou G, Kehl V, Krause BJ, Rack B, Retz M,
Gschwend JE, et al: Circulating tumor cells versus objective
response assessment predicting survival in metastatic
castration-resistant prostate cancer patients treated with
docetaxel chemotherapy. J Cancer Res Clin Oncol. 141:1457–1464.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Marín-Aguilera M, Reig Ò, Lozano JJ,
Jiménez N, García-Recio S, Erill N, Gaba L, Tagliapietra A, Ortega
V, Carrera G, et al: Molecular profiling of peripheral blood is
associated with circulating tumor cells content and poor survival
in metastatic castration-resistant prostate cancer. Oncotarget.
6:10604–10616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Goldkorn A, Ely B, Tangen CM, Tai YC, Xu
T, Li H, Twardowski P, Veldhuizen PJ, Agarwal N, Carducci MA, et
al: Circulating tumor cell telomerase activity as a prognostic
marker for overall survival in SWOG 0421: A phase III metastatic
castration resistant prostate cancer trial. Int J Cancer.
136:1856–1862. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Thalgott M, Rack B, Maurer T, Souvatzoglou
M, Eiber M, Kreß V, Heck MM, Andergassen U, Nawroth R, Gschwend JE
and Retz M: Detection of circulating tumor cells in different
stages of prostate cancer. J Cancer Res Clin Oncol. 139:755–763.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Loh J, Jovanovic L, Lehman M, Capp A,
Pryor D, Harris M, Nelson C and Martin J: Circulating tumor cell
detection in high-risk non-metastatic prostate cancer. J Cancer Res
Clin Oncol. 140:2157–2162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Davis JW, Nakanishi H, Kumar VS,
Bhadkamkar VA, McCormack R, Fritsche HA, Handy B, Gornet T and
Babaian RJ: Circulating tumor cells in peripheral blood samples
from patients with increased serum prostate specific antigen:
Initial results in early prostate cancer. J Urol. 179:2187–2191.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kolostova K, Broul M, Schraml J, Cegan M,
Matkowski R, Fiutowski M and Bobek V: Circulating tumor cells in
localized prostate cancer: Isolation, cultivation in vitro and
relationship to T-stage and Gleason score. Anticancer Res.
34:3641–3646. 2014.PubMed/NCBI
|
|
93
|
Bitting RL, Healy P, Halabi S, George DJ,
Goodin M and Armstrong AJ: Clinical phenotypes associated with
circulating tumor cell enumeration in metastatic
castration-resistant prostate cancer. Urol Oncol. 33:110.e1–9.
2015. View Article : Google Scholar
|
|
94
|
Schoenborn JR, Nelson P and Fang M:
Genomic profiling defines subtypes of prostate cancer with the
potential for therapeutic stratification. Clin Cancer Res.
19:4058–4066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen CL, Mahalingam D, Osmulski P, Jadhav
RR, Wang CM, Leach RJ, Chang TC, Weitman SD, Kumar AP, Sun L, et
al: Single-cell analysis of circulating tumor cells identifies
cumulative expression patterns of EMT-related genes in metastatic
prostate cancer. Prostate. 73:813–826. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Osmulski P, Mahalingam D, Gaczynska ME,
Liu J, Huang S, Horning AM, Wang CM, Thompson IM, Huang TH and Chen
CL: Nanomechanical biomarkers of single circulating tumor cells for
detection of castration resistant prostate cancer. Prostate.
74:1297–1307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Boegemann M, Schrader AJ, Krabbe LM and
Herrmann E: Present, emerging and possible future biomarkers in
castration resistant prostate cancer (CRPC). Curr Cancer Drug
Targets. 15:243–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Alexandrov LB, Nik-Zainal S, Wedge DC,
Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A,
Børresen-Dale AL, et al: Signatures of mutational processes in
human cancer. Nature. 500:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Stratton MR: Exploring the genomes of
cancer cells: Progress and promise. Science. 331:1553–1558. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Pantel K, Brakenhoff RH and Brandt B:
Detection, clinical relevance and specific biological properties of
disseminating tumour cells. Nat Rev Cancer. 8:329–340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Alix-Panabières C, Schwarzenbach H and
Pantel K: Circulating tumor cells and circulating tumor DNA. Annu
Rev Med. 63:199–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang J, McGuire TR, Britton HC, Schwarz
JK, Loberiza FR Jr, Meza JL and Talmadge JE: Lenalidomide and
cyclophosphamide immunoregulation in patients with metastatic,
castration-resistant prostate cancer. Clin Exp Metastasis.
32:111–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wilbaux M, Tod M, De Bono J, Lorente D,
Mateo J, Freyer G, You B and Hénin E: A joint model for the
kinetics of CTC count and PSA concentration during treatment in
metastatic castration-resistant prostate cancer. CPT
Pharmacometrics Syst Pharmacol. 4:277–285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Danila DC, Anand A, Sung CC, Heller G,
Leversha MA, Cao L, Lilja H, Molina A, Sawyers CL, Fleisher M and
Scher HI: TMPRSS2-ERG status in circulating tumor cells as a
predictive biomarker of sensitivity in castration-resistant
prostate cancer patients treated with abiraterone acetate. Eur
Urol. 60:897–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Smith MR, Sweeney CJ, Corn PG, Rathkopf
DE, Smith DC, Hussain M, George DJ, Higano CS, Harzstark AL, Sartor
AO, et al: Cabozantinib in chemotherapy-pretreated metastatic
castration-resistant prostate cancer: Results of a phase II
nonrandomized expansion study. J Clin Oncol. 32:3391–3399. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Dago AE, Stepansky A, Carlsson A, Luttgen
M, Kendall J, Baslan T, Kolatkar A, Wigler M, Bethel K, Gross ME,
et al: Rapid phenotypic and genomic change in response to
therapeutic pressure in prostate cancer inferred by high content
analysis of single circulating tumor cells. PLoS One.
9:e1017772014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Reyes EE, VanderWeele DJ, Isikbay M,
Duggan R, Campanile A, Stadler WM, Griend DJ Vander and Szmulewitz
RZ: Quantitative characterization of androgen receptor protein
expression and cellular localization in circulating tumor cells
from patients with metastatic castration-resistant prostate cancer.
J Transl Med. 12:3132014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Antonarakis ES, Lu C, Luber B, Wang H,
Chen Y, Nakazawa M, Nadal R, Paller CJ, Denmeade SR, Carducci MA,
et al: Androgen receptor splice variant 7 and efficacy of taxane
chemotherapy in patients with metastatic castration-resistant
prostate cancer. JAMA Oncol. 1:582–591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Onstenk W, Sieuwerts AM, Kraan J, Van M,
Nieuweboer AJ, Mathijssen RH, Hamberg P, Meulenbeld HJ, De Laere B,
Dirix LY, et al: Efficacy of cabazitaxel in castration-resistant
prostate cancer is independent of the presence of AR-V7 in
circulating tumor cells. Eur Urol. 68:939–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bichsel CA, Gobaa S, Kobel S, Secondini C,
Thalmann GN, Cecchini MG and Lutolf MP: Diagnostic microchip to
assay 3D colony-growth potential of captured circulating tumor
cells. Lab Chip. 12:2313–2316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Gao D, Vela I, Sboner A, Iaquinta PJ,
Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora
VK, et al: Organoid cultures derived from patients with advanced
prostate cancer. Cell. 159:176–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Miyamoto DT, Zheng Y, Wittner BS, Lee RJ,
Zhu H, Broderick KT, Desai R, Fox DB, Brannigan BW, Trautwein J, et
al: RNA-Seq of single prostate CTCs implicates noncanonical Wnt
signaling in antiandrogen resistance. Science. 349:1351–1356. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mitchell MJ, Wayne E, Rana K, Schaffer CB
and King MR: TRAIL-coated leukocytes that kill cancer cells in the
circulation. Proc Natl Acad Sci USA. 111:pp. 930–935. 2014;
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kim G and Gaitas A: Extracorporeal
photo-immunotherapy for circulating tumor cells. PLoS One.
10:e01272192015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Prostate cancer: Send away the PSA? Cancer
Discov. 5:570–571. 2015.
|