Introduction
Esophageal squamous cell carcinoma (ESCC) is one of
the most common malignant tumors and the eighth most frequently
diagnosed cancer in the world (1). It
is estimated that almost 50% of ESCC cases occur in China,
particularly in Hebei, Henan and North China (2,3). The
development of ESCC is a complex process, and numerous
environmental factors may contribute to ESCC, including diet,
infection, poor nutrition status, low intake of fruits and
vegetables, and particularly tobacco smoking and alcohol
consumption (4,5). However, certain individuals who are
exposed to the same risk factors may have different susceptibility
to ESCC, which shows that genetic factors are important in the
development of ESCC (6). Besides, the
differences in genetic factors among patients with ESCC may be
associated with geographic and ethnic characteristics (7).
Tongliao is the most concentrated area of Mongolian
population in the Inner Mongolia Autonomous Region of China, and
even in the whole of China (8). These
Mongolian inhabitants have acquired the habit of consuming diets
with high salt and calorie content, due to the particular
geographic and climate characteristics of this region (9). In recent years, due to the absence of
early ESCC symptoms, traditional screening and treatment methods
have not greatly increased the survival of patients with ESCC
(10).
In Tongliao, there is only a small percentage of
individuals who suffered from esophageal cancer, despite the fact
that all residents share similar environmental risk factors
(11). This suggested that genetic
factors such as genetic polymorphisms may serve a key role in the
pathogenesis of ESCC.
Certain studies have indicated that >50% of
patients with cancer have p53 gene mutations during the occurrence
and development of human cancer (12). In China, certain studies demonstrated
that the genotype Pro/Pro of p53 is a risk factor for ESCC in
Chaoshan in Guangdong, Linzhou in Henan and Yanting in Sichuan, but
in other places such as Hebei, Cixian or Shexian, the genotype
Pro/Pro of p53 is not a risk factor (7). Not only is the occurrence of esophageal
cancer associated with the region where a population lives, but it
is also closely associated with ethnicity, since the Kazakh ethnic
group of Xinjiang in China has a high incidence of ESCC (13).
Studies about the correlation between ESCC in
Mongolian patients and susceptibility genes for ESCC are rarely
reported. The identification of genetic susceptible factors for
ESCC in Mongolian patients will have broad implications in
understanding the pathogenesis of ESCC in the Mongolian population,
and will aid early detection, diagnosis and therapy.
Patients and methods
Subjects
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Inner Mongolia University
for the Nationalities (Tongliao, China). Written informed consent
was obtained from all participants prior to the study via an
institutional patient consent form. The study was conducted in
accordance with the regulations of the Inner Mongolia University
for the Nationalities.
A total of 100 Mongolian patients with surgically
resectable thoracic ESCC who had received curative esophagectomy
and lymph node dissection at the Affiliated Hospital of Inner
Mongolia University for the Nationalities between April 2013 and
June 2014 were enrolled in the present study. According to the
collected information, all patients were permanent residents of
Tongliao, in Southeastern Inner Mongolia. None of the study
participants received neoadjuvant therapy prior to hospital
admission. Patient clinicopathological characteristics are
summarized in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | N (total n=100) |
|---|
| Sex
(male/female) | 91/9 |
| Age/years (mean ±
standard deviation) | 54.32±7.40 |
| Tumor location
(upper/middle/lower) | 9/52/39 |
| TNM
classification |
|
|
T1/T2/T3/T4 | 10/20/43/27 |
|
N0/N1/N2/N3 | 54/31/12/3 |
| Grade
(I/II/III) | 19/19/62 |
| Histopathological
diagnosis |
|
|
PDSCC | 43 |
|
MDSCC | 39 |
|
HDSCC | 18 |
Additionally, 50 healthy Mongolian individuals,
whose physical health had been verified at the Physical Examination
Center of the Affiliated Hospital of Inner Mongolia University for
the Nationalities, were randomly selected as control group. The
control group and ESCC group were all recruited from pastoral areas
of Tongliao city in inner Mongolia. The control subjects were
frequency-matched to the patients by age (±5 years) and sex.
Statistical analysis on the general information of the two groups,
was performed, and results are summarized in Table II.
 | Table II.Comparative analysis of clinical data
in the ESCC and control groups. |
Table II.
Comparative analysis of clinical data
in the ESCC and control groups.
|
Characteristics | ESCC, n (%) | Control, n (%) | P-value | χ2 |
|---|
| Total no. of
patients | 100 (100) | 50 (100) |
|
|
| Age, years |
|
| 0.073 | 3.211 |
|
≤45 | 1 (1) | 3 (6) |
|
|
|
>45 | 99 (99) | 47 (94) |
|
|
| Sex |
|
| 0.857 | 0.033 |
|
Male | 91 (91) | 39 (78) |
|
|
|
Female | 9 (9) | 11 (22) |
|
|
| Alcohol
consumption |
|
| <0.001 | 15.992 |
| No | 13 (13) | 21 (42) |
|
|
|
Yes | 87 (87) | 29 (58) |
|
|
| Cigarette
smoking |
|
| <0.001 | 11.344 |
| No | 38 (38) | 34 (68) |
|
|
|
Yes | 62 (62) | 16 (32) |
|
|
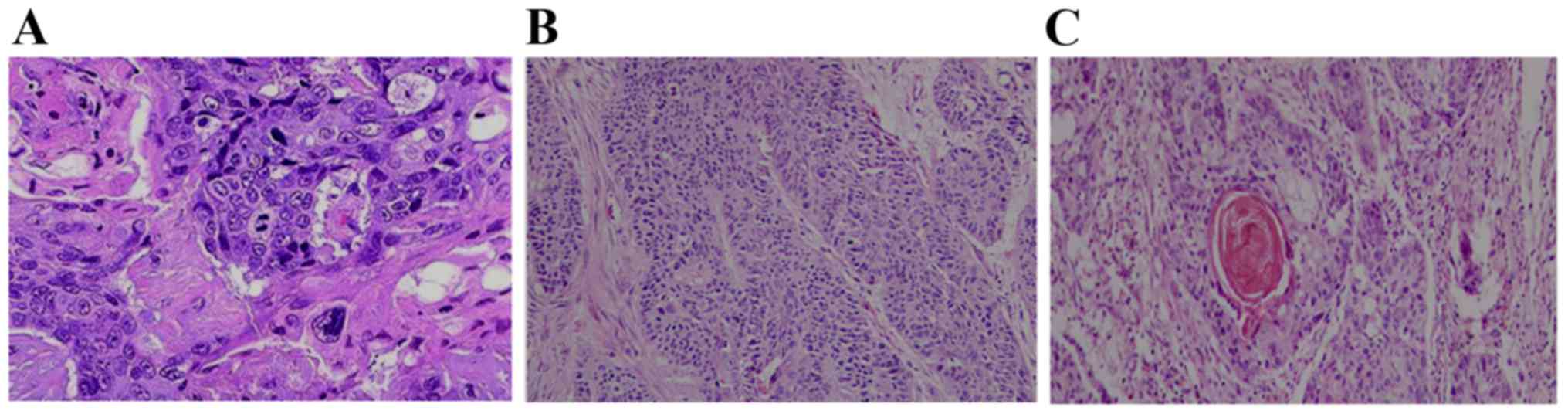
Pathological diagnosis
All ESCC patients had a definite pathological
diagnosis based on the stage of histopathological slice and
differentiation of tumor cells. Accordingly, the patients were
divided into three grades: Poorly, moderately and highly
differentiated SCC. Tissue samples were stained with hematoxylin
and eosin. Three representative histopathological slice images of
different grades are shown in Fig.
1.
Detection method and result
evaluation
Blood samples were collected in EDTA-coated tubes
with the SiMax™ Genomic DNA Extraction kit (SBS Genetech Co., Ltd.,
Beijing, China) to extract the genomic DNA from the peripheral
blood leukocyte pellet of the two groups. Genomic DNA was isolated
and purified from all subjects, and next subjected to β-globin
polymerase chain reaction (PCR) analysis to detect the existence of
human DNA in the sample. Those β-globin-PCR negative samples were
excluded from the analysis. β-globin primers were designed to
verify the quality and quantity of the DNA template. PCR primers
were also synthesized according to the p53 gene sequence published
on GenBank (http://www.ncbi.nlm.nih.gov/WebSub/?tool=genbank)
(Fig. 2) by Beijing Genomics
Institute (Beijing, China), and were designed by Primer 5.0
software (Beijing Genomics Institute, Beijing, China). p53 gene was
amplified by PCR, and the procedure included an initial melting
step of 5 min at 94°C, followed by 35 cycles of denaturation at
94°C for 60 sec, annealing at 60°C for 60 sec and extension at 72°C
for 60 sec, and preservation at 4°C. The PCR mixture (50 µl)
contained 2 ng template DNA, 0.5 µl Taq enzyme (5 U/µl) (Treasure
Bioengineering Co., Ltd., Dalian, China), 2.5 µl 10X PCR Buffer
(Mg2+) (Hamilton's product, Bonaduz, Switzerland), 1.5
µl (0.2 µmol/l) forward primer, 1.5 µl reverse primer, 4 µl (200
umol/l) deoxynucleotide triphosphates and ≤25 µl double distilled
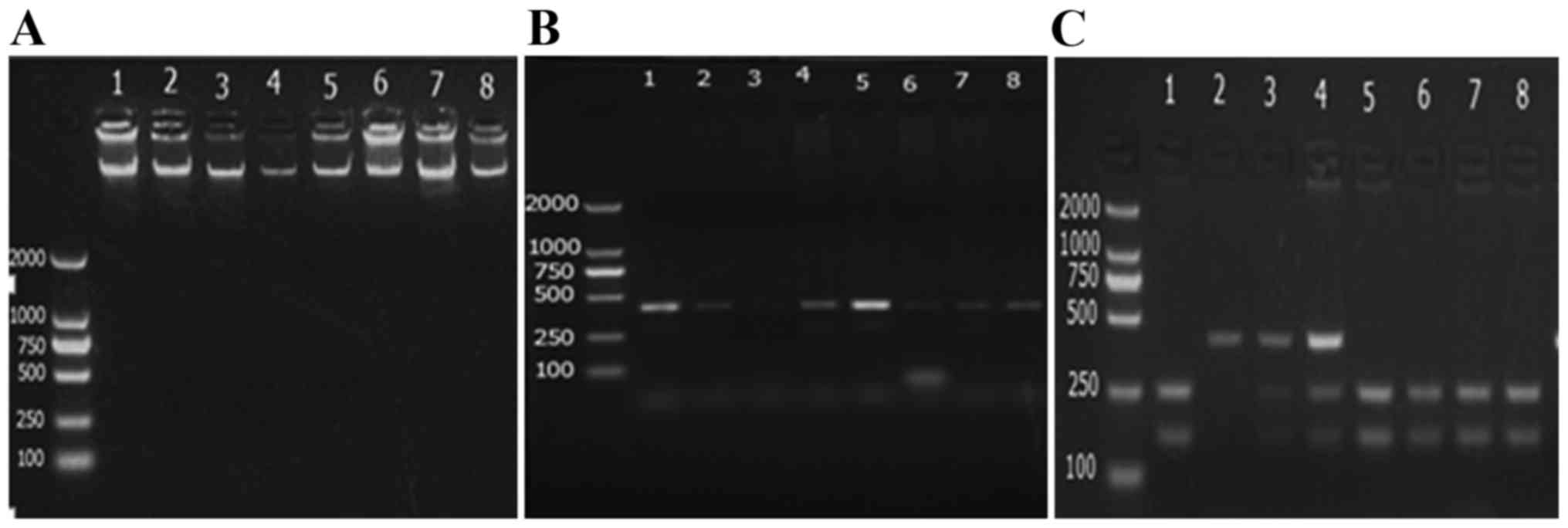
(dd) H2O. The PCR product was detected through
electrophoresis (3% agarose gel) performed at 90 V/cm for 40 min
and after the electrophoresis, the results were observed and
photographed under the ultraviolet light of the gel imaging system,
followed by evaluation with the UV Transmission Analyzer (Shanghai
Spectrum Instrument Co. Ltd, Shanghai, China).
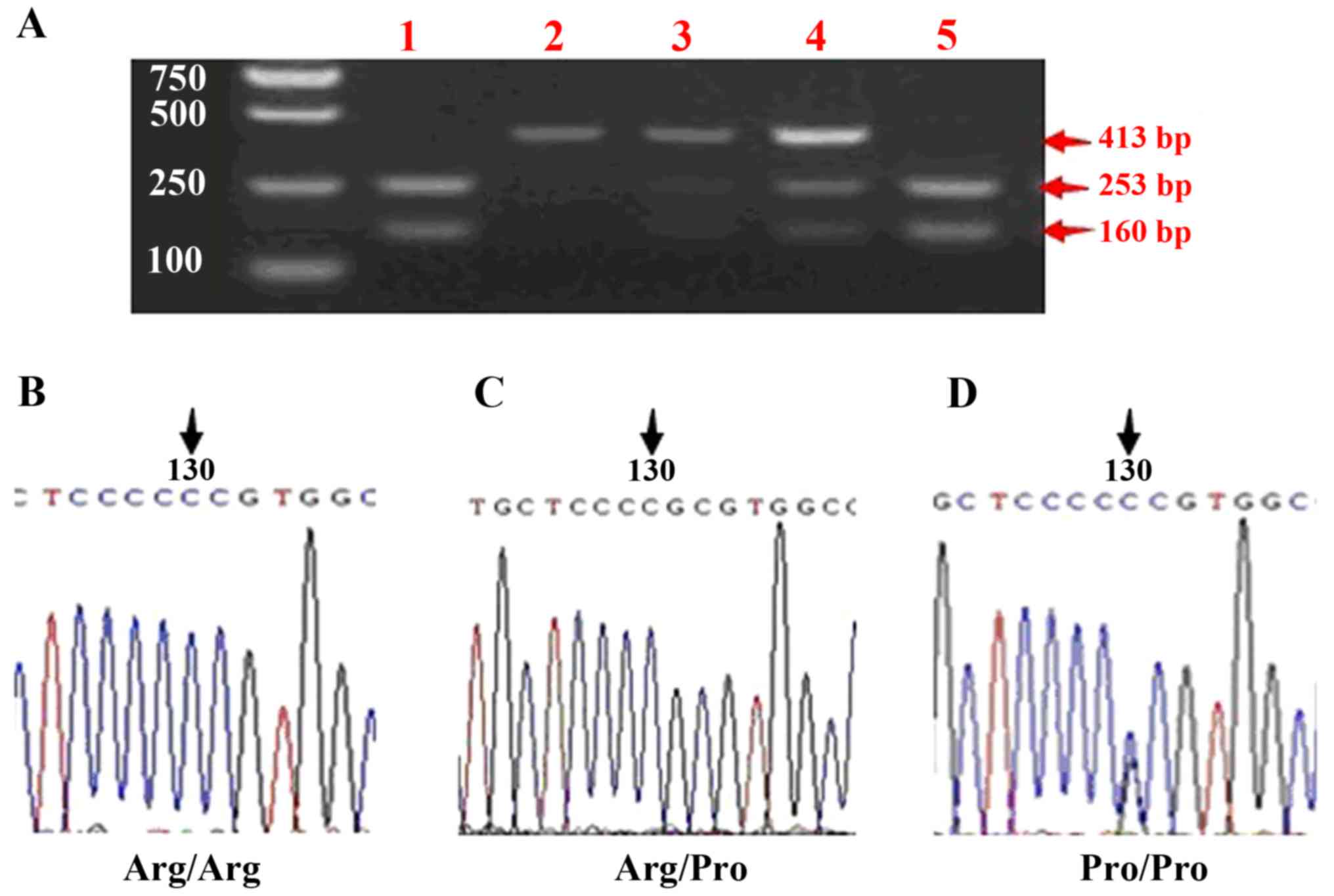
AccII restriction enzyme was used to digest
the p53-amplified product of PCR at 37°C. The enzymatic digestion
reaction (20 µl) contained 10 µl PCR-amplified product, 1 µl
AccII (10 U/µl), 1 µl 10X buffer and 8 µl sterilized
ddH20. The digested product (8 µl) was dissolved in 1X
Tris base, acetic acid and EDTA solution, and then electrophoresed
in a 3% agarose gel for 40 min at 90 V. The results were observed
and photographed under ultraviolet light in a gel imaging system
(Figs. 3 and 4).
In order to test the reliability of the assay, 30
samples with identical mutations were randomly selected to be
re-tested. Sequence analysis was performed with the BigDye
Terminator Cycle Sequencing kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and the ABI PRISM 3730 DNA Analyzer (Applied
Biosystems; Thermo Fisher Scientific, Inc.) (Fig. 4).
Statistical analysis
Pearson's χ2 test was used to examine the
differences in age, sex, cigarette smoking, alcohol consumption,
allele polymorphism and genotype distribution between case and
control subjects. Hardy-Weinberg goodness-of-fit test was used to
analyze the different genotype distribution equilibrium for the
same polymorphic site. The statistical software SPSS 15 (SPSS,
Inc., Chicago, IL, USA) was used to analyze the data. Phase 2.1
software (http://www.stat.washington.edu/stephens/home.html) was
used to analyze the haplotype, and the conditional logistic
regression model was used to analyze the correlation between gene
polymorphisms and haplotypes and susceptibility to ESCC. The
association between mutant genotype or allele polymorphism and
esophageal cancer risk was calculated by odds ratio (OR) with 95%
confidence interval (CI). P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of the study
subjects
Based on the information of clinical records
(Table II), the distribution of the
two groups regarding factors such as sex, age, cigarette smoking
and alcohol consumption was analyzed. The results revealed that the
age distribution of the two groups was adequately matched
(χ2=3.211, P=0.073). According to the sex distribution,
no significant difference was observed in patients with ESCC vs.
controls (χ2=0.033, P=0.857). However, both the
distribution of alcohol consumption and cigarette smoking had a
significant difference in ESCC vs. control groups
(χ2=15.992, P<0.001 and χ2=11.344,
P=0.001, respectively), which indicated that alcohol drinking and
cigarette smoking were high-risk factors for ESCC in the Mongolian
population.
Tumor protein p53 (TP53) Pro72 allele
is enriched in patients with ESCC but not in the control group
The distribution of p53 genotypes is different in
the two groups. In the ESCC group, 25.0, 41.7 and 33.3% of patients
had Pro/Pro, Pro/Arg and Arg/Arg genotypes, respectively, compared
with 14.0, 30.0 and 56.0% of subjects, respectively, in the control
group, which indicated that the distribution of genotypes was
statistically significant (χ2=7.49, P<0.05) and that
the p53 genotype is associated with ESCC in Mongolian patients. The
genotype Arg/Pro was the most abundant in the ESCC group, while in
the control group, the most abundant genotype was Arg/Arg. This
demonstrates that Arg/Pro is involved in the occurrence of ESCC in
the Mongolian population. The frequency of Arg and Pro alleles in
the two groups was analyzed by χ2 test, which revealed
χ2=6.165, P=0.013, indicating that the two alleles
served different roles in the occurrence of ESCC in Mongolian
patients (Table III).
 | Table III.Comparative analysis of p53 Pro72Arg
polymorphism status in ESCC and healthy control samples. |
Table III.
Comparative analysis of p53 Pro72Arg
polymorphism status in ESCC and healthy control samples.
|
| Genotype
distribution, n (%)a |
|
| Allele
distribution, % |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Group | Arg/Arg | Arg/Pro | Pro/Pro | χ2 |
P-valueb | Arg | Pro | χ2 | P-value |
|---|
| ESCC | 33c (33.3)d | 42 (41.7) | 25 (25) | 7.49 | 0.024 | 54.0d | 46.0 | 6.165 | 0.013 |
| Control | 28 (56.0%) | 15 (30.0) | 7 (14.0) |
|
| 71.0 | 29.0 |
|
|
TP53 Pro72 allele increases the risk
of ESCC in Mongolian patients
In order to unravel the role of allele mutations in
p53 codon 72 on ESCC in Mongolian patients, an unconditional
logistic regression model was used to estimate the association
between genotypes and the risk of ESCC. A risk analysis was
conducted in which genotypes such as Pro/Pro and Arg/Pro were
regarded as the exposure factors, and Arg/Arg was regarded as the
non-exposure factor (Table IV). By
comparing both types of factors, the allele mutations of p53 codon
72 appeared to be associated with an increased risk of developing
ESCC (OR=1.659, 95% CI=1.112–2.474), compared with the risk
exhibited by the Arg/Arg genotype.
 | Table IV.Risk analysis of p53 gene
polymorphism and esophageal cancer in Mongolia. |
Table IV.
Risk analysis of p53 gene
polymorphism and esophageal cancer in Mongolia.
| p53 codon 72
genotypes | ESCC, n (%) | Control, n (%) | χ2 | P-value | OR (95% Cl) |
|---|
| Arg/Arg | 33 (33.0) | 28 (56.0) | 7.308 | 0.007 | 1.659
(1.112–2.474) |
| Arg/Pro +
Pro/Pro | 67 (67.0) | 22 (44.0) |
|
|
|
Discussion
To the best of our knowledge, the present study is
the first report showing the association between TP53 codon 72
polymorphisms and the risk of ESCC in a Mongolian population. It
was concluded that TP53 codon 72 polymorphisms were associated with
an increased risk of developing ESCC through the present
hospital-based case-control analysis on a Mongolian population.
Besides, it was demonstrated that cigarette smoking and alcohol
consumption could markedly increase the risk of ESCC.
It is known that passive smoking is a major risk
factor for lung cancer, but a recent study conducted on passive
smoking demonstrated that it was also significantly associated with
increased risk of ESCC and breast cancer in women (14). The present study also observed that
cigarette smoking and alcohol consumption are risk factors for
ESCC, which is in agreement with previous studies (15–17). A
possible explanation for these findings is that alcohol drinking
and cigarette smoking cause irreversible genetic damage, or inhibit
the repair mechanisms of genetic damage (2,18).
Besides, cigarette smokers or alcohol drinkers with p53 Arg/Arg or
Arg/Pro could have a higher risk of infection by human papilloma
virus (HPV) 16, which would increase their ESCC risk (19).
p53 gene is a tumor-suppressor gene that is
associated with cell cycle regulation, DNA repair, cell
differentiation, apoptosis and other important biological functions
(20). The 72nd codon of the p53 gene
in exon 4 codes for arginine (Arg72R) or proline (Pr72P) (21). Previous studies have shown that the
p53 Pro72P subtype is more easily located in the nucleus, while the
p53 Arg72R subtype is more easily located in the mitochondria under
stress (22). Therefore, it is
possible that the p53 Pro72p subtype may more effectively induce
gene expression of nuclear DNA compared with the p53 Arg72R subtype
(23). The polymorphism of the 72
codon of the p53 gene can affect the induction of cell apoptosis
and the repair of damaged DNA, which are functions closely
associated with the maintenance of the integrity of the nuclear
envelope (23,24). In addition, the polymorphism of the 72
codon of the p53 gene may affect the accumulation of mitochondrial
DNA mutations by combining with DNA polymerase γ (19). Therefore, the polymorphism of the p53
gene on codon 72 can affect the normal functions of the p53 gene,
and may be involved in the prognosis of patients with ESCC.
To the best of our knowledge, the majority of
previous Chinese studies have shown that there is an association
between the polymorphism of p53 Arg72Pro in Chinese patients with
ESCC, and the heterozygous genotype (Arg/Pro) and the mutant
homozygous genotype (Pro/Pro) can significantly increase the
incidence of esophageal cancer risk (25). The present study demonstrated that the
allele Pro may increase the risk of ESCC in Mongolian patients by
1.659-fold compared with that of the allele Arg (Table IV). However, the conclusion may be
different for different countries and nationalities. For example,
the homozygous Pro/Pro polymorphism can increase the risk of
esophageal cancer in the population of Korea (26). Notably, another study from USA
reported that no association existed between p53 Pro72Arg and ESCC
(27,28). In Japan, a previous report revealed
that alteration of p53 tumor-suppressor protein was suspected to be
the key molecular event in multifocal carcinogenesis (29). In addition, the authors observed
markedly high levels of p53 protein accumulation in early ESCC in
Japanese alcoholic men (21).
According to recent studies, the susceptible
genotype of p53 72 codon appears to be different for different
tumors. Buyru's study revealed that there was a significant
correlation between the genotype Arg/Arg on p53 72 codon and female
breast cancer in the population of Turkey (29). Previous studies (30–32)
indicated that the Arg/Arg genotype could increase the risk of
HPV-associated tumors. Buller et al (33) demonstrated that the Arg/Pro genotype
and borderline ovarian cancer had a close association. Previous
studies on Northern Chinese populations revealed that the
homozygous genotype Pro/Pro was an independent risk for ESCC, and
it could increase the risk of ESCC by 2-fold compared with that of
the Arg/Arg genotype (34–37). In the present study, this risk was
determined to be 1.659-fold instead (Table IV).
The present results revealed a significant
association between the polymorphism of TP53 codon 72 and the risk
of developing ESCC risk in Mongolian patients. These results may be
useful for esophageal cancer prevention. In order to unravel the
biological progression of ESCC in Mongolian patients, the
association of other gene polymorphisms with the risk of ESCC must
be explored to display a potential multiplicative gene-gene
interaction.
In conclusion, the present study revealed that
alcohol drinking and cigarette smoking could increase the risk of
ESCC in Mongolian patients, and suggested the need to advocate for
a healthy lifestyle among the Mongolian population. Our results
support previous conclusions suggesting different behavior of the
p53 Pro72Arg alleles in different cancer types and ethnicities in
ESCC. These results may provide clinical support for survival and
chemo/radiotherapies of ESCC, although further studies to analyze
the effect of p53 codon 72 genotype polymorphism on patients are
required. Animal models may help to unravel the function of
mutations in the codon 72 of the p53 gene in the process of ESCC
development.
Acknowledgements
The present study was supported in part by the
National Natural Science Foundation of China (grant no. 81450036
awarded to Y.W. and grant no. 81260196), Scientific Research
Projects in Universities of Inner Mongolia Autonomous Region (grant
no. NJZZ14271) and Natural Science Foundation of Inner Mongolia
Autonomous Region (grant no. 2013MS1176). The authors thank the
Department of General Surgery of the Affiliated Hospital of Inner
Mongolia University for Nationalities, the staff members of the
Oncology Hospital of Khorchin and Mrs Guoli Liu (Animal Laboratory
of Affiliated Hospital of Inner Mongolia University for the
Nationalities) for their help in sample collection. The authors
also thank Dr Haihua Bai and Mr Wenyan Huo (Centre for Cellular and
Molecular Biology of Inner Mongolia University for the
Nationalities) for the collection of DNA samples from healthy
individuals. In addition, the authors thank Beijing Genomics
Institute for their help in validating the present results by
providing gene sequencing services.
References
|
1
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang J, Liu B, Li W, Xiong H, Qiu H, Fu Q,
Chen B, Hu G and Yuan X: Association of p53 and MDM2 polymorphisms
with risk of human papillomavirus (HPV)-related esophageal squamous
cell carcinoma (ESCC). Cancer Epidemiol. 37:629–633. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Islami F, Boffetta P, Ren JS, Pedoeim L,
Khatib D and Kamangar F: High-temperature beverages and foods and
esophageal cancer risk-a systematic review. Int J Cancer.
125:491–524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamangar F, Chow WH, Abnet CC and Dawsey
SM: Environmental causes of esophageal cancer. Gastroenterol Clin
North Am. 38:27–57, vii. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang HZ, Jin GF and Shen HB:
Epidemiologic differences in esophageal cancer between Asian and
Western populations. Chin J Cancer. 31:281–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gong Jinshan, Ao Shuyun and Ao Qimuge
Deng: Related research of Mongolia national diet and esophageal
cancer in Inner Mongolia area of Tongliao. Chinese Medical Guide.
1–451. 2013.
|
|
9
|
Davidoff AM, Iglehart JD and Marks JR:
Immune response to p53 is dependent upon p53/HSP70 complexes in
breast cancers. Proc Natl Acad Sci USA. 89:pp. 3439–3442. 1992;
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vogiatzi P, Vindigni C, Roviello F,
Renieri A and Giordano A: Deciphering the underlying genetic and
epigenetic events leading to gastric carcinogenesis. J Cell
Physiol. 211:287–295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y: Clinical study of reflux esophagitis
in Mongolian. Inner Mongol J Med. 02:208–209. 2014.
|
|
12
|
Shimada H, Nabeya Y, Okazumi S, Matsubara
H, Funami Y, Shiratori T, Hayashi H, Takeda A and Ochiai T:
Prognostic significance of serum p53 antibody in patients with
esophageal squamous cell carcinoma. Surgery. 132:41–47. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Li X, Gao F, et al: Analysis of
the incidence of malignant tumors in Hami area from 2010 to 2012.
Xinjiang Med. 10:1501–1504. 2015.
|
|
14
|
Wu M, Zhao JK, Hu XS, Wang PH, Qin Y, Lu
YC, Yang J, Liu AM, Wu DL, Zhang ZF, et al: Association of smoking,
alcohol drinking and dietary factors with esophageal cancer in
high- and low-risk areas of Jiangsu Province, China. World J
Gastroenterol. 12:1686–1693. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakata K, Hoshiyama Y, Morioka S,
Hashimoto T, Takeshita T and Tamakoshi A; JACC Study Group, :
Smoking, alcohol drinking and esophageal cancer: Findings from the
JACC Study. J Epidemiol. 15 Suppl 2:S212–S219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang CX, Wang HY, Wang ZM, Du HZ, Tao DM,
Mu XY, Chen HG, Lei Y, Matsuo K and Tajima K: Risk factors for
esophageal cancer: A case-control study in South-western China.
Asian Pac J Cancer Prev. 6:48–53. 2005.PubMed/NCBI
|
|
17
|
Castelli E, Hrelia P, Maffei F, Fimognari
C, Foschi FG, Caputo F, Cantelli-Forti G, Stefanini GF and
Gasbarrini G: Indicators of genetic damage in alcoholics:
Reversibility after alcohol abstinence. Hepatogastroenterology.
46:1664–1668. 1999.PubMed/NCBI
|
|
18
|
Zhao Y, Wang F, Shan S, Zhao Y, Qiu X, Li
X, Jiao F, Wang J and Du Y: Genetic polymorphism of p53, but not
GSTP1, is association with susceptibility to esophageal cancer
risk-a meta-analysis. Int J Med Sci. 7:300–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Altilia S, Santoro A, Malagoli D,
Lanzarini C, Álvarez JA Ballesteros, Galazzo G, Porter DC, Crocco
P, Rose G, Passarino G, et al: TP53 codon 72 polymorphism affects
accumulation of mtDNA damage in human cells. Aging (Albany NY).
4:28–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen H, Yang X and Wang Z: Association
between p53 Arg72Pro polymorphism and recurrent pregnancy loss: An
updated systematic review and meta-analysis. Reprod Biomed Online.
31:149–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Papadakis EN, Dokianakis DN and Spandidos
DA: p53 codon 72 polymorphism as a risk factor in the development
of breast cancer. Mol Cell Biol Res Commun. 3:389–392. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas M, Kalita A, Labrecque S, Pim D,
Banks L and Matlashewski G: Two polymorphic variants of wild-type
p53 differ biochemically and biologically. Mol Cell Biol.
19:1092–1100. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pereira L, Carvalho MR, Fonseca CG, Lima
SS, Cerqueira EM, Jorge W and Castro MC: Influence of Arg72Pro
polymorphisms of TP53 on the response of buccal cells to
radiotherapy. Genet Mol Res. 10:3552–3558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vogelstein B and Kinzler KW: p53 function
and dysfunction. Cell. 70:523–526. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Piao JM, Kim HN, Song HR, Kweon SS, Choi
JS, Yoon JY, Chung IJ, Kim SH and Shin MH: p53 codon 72
polymorphism and the risk of esophageal cancer: A Korean
case-control study. Dis Esophagus. 24:596–600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamajima N, Matsuo K, Suzuki T, Nakamura
T, Matsuura A, Hatooka S, Shinoda M, Kodera Y, Yamamura Y, Hirai T,
et al: No associations of p73 G4C14-to-A4T14 at exon 2 and p53
Arg72Pro polymorphisms with the risk of digestive tract cancers in
Japanese. Cancer Lett. 181:81–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu G, Cescon DW, Zhai R, Zhou W, Kulke
MH, Ma C, Xu W, Su L, Asomaning K, Heist RS, et al: p53 Arg72Pro,
MDM2 T309G and CCND1 G870A polymorphisms are not associated with
susceptibility to esophageal adenocarcinoma. Dis Esophagus.
23:36–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aida J, Yokoyama A, Shimomura N, Nakamura
K, Ishikawa N, Terai M, Poon S, Matsuura M, Fujiwara M, Sawabe M,
et al: Telomere shortening in the esophagus of Japanese alcoholics:
Relationships with chromoendoscopic findings, ALDH2 and ADH1B
genotypes and smoking history. PLoS One. 8:e638602013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Buyru N, Altinisik J, Demokan S and Dalay
N: p53 genotypes and haplotypes associated with risk of breast
cancer. Cancer Detect Prev. 31:207–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zehbe I, Voglino G, Wilander E, Genta F
and Tommasino M: Codon 72 polymorphism of p53 and its association
with cervical cancer. Lancet. 354:218–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jee SH, Lee JE and Park JS: Polymorphism
of codon 72 of p53 and environmental factors in the development of
cervical cancer. Int J Gynaecol Obstet. 80:69–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Buller RE, Sood A, Fullenkamp C, Sorosky
J, Powills K and Anderson B: The influence of the p53 codon 72
polymorphism on ovarian carcinogenesis and prognosis. Cancer Gene
Ther. 4:239–245. 1997.PubMed/NCBI
|
|
33
|
Buller RE, Shahin MS, Holmes RW, Hatterman
M, Kirby PA and Sood AK: p53 Mutations and microsatellite
instability in ovarian cancer: Yin and yang. Am J Obstet Gynecol.
184:891–903. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cai L, Mu LN, Lu H, Lu QY, You NC, Yu SZ,
Le AD, Zhao J, Zhou XF, Marshall J, et al: Dietary selenium intake
and genetic polymorphisms of the GSTP1 and p53 genes on the risk of
esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers
Prev. 15:294–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Xing D, He Z and Lin D: p53 gene
codon 72 polymorphism and susceptibility to esophageal squamous
cell carcinoma in a Chinese population. Zhonghua Yi Xue Yi Chuan
Xue Za Zhi. 19:10–13. 2002.(In Chinese). PubMed/NCBI
|
|
36
|
Hong Y, Miao X, Zhang X, Ding F, Luo A,
Guo Y, Tan W, Liu Z and Lin D: The role of P53 and MDM2
polymorphisms in the risk of esophageal squamous cell carcinoma.
Cancer Res. 65:9582–9587. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ge H, Cao YY, Chen LQ, Wang YM, Chen ZF,
Wen DG, Zhang XF, Guo W, Wang N, Li Y and Zhang JH: PTEN
polymorphisms and the risk of esophageal carcinoma and gastric
cardiac carcinoma in a high incidence region of China. Dis
Esophagus. 21:409–415. 2008. View Article : Google Scholar : PubMed/NCBI
|