Introduction
Gastric carcinoma (GC) is the third most common
cause of cancer mortality worldwide, and >50% of GC cases occur
in Eastern Asia (1,2). Therapy for GC includes surgical
resection, radiation and chemotherapy (3). Surgical resection is the curative
treatment for patients with early stages of disease. However, ~20%
of patients survive 5 years after surgery, and the majority of
patients with advanced GC, which is characterized by poor prognosis
and metastasis, eventually relapse (4). At present, the use of chemotherapy and
combination treatments are alternative therapeutic strategies for
controlling advanced GC (5).
Therefore, it is an urgent requirement to identify new
chemotherapeutic agents for preventing gastric cancer metastasis
and improving the 5-year survival rates of gastric cancer patients
(6,7).
A malignant tumor could be developed from a normal
cell in various mechanisms, including self-sufficiency in growth
signals, insensitivity to antigrowth signals, evasion of apoptosis,
limitless replicative potential, sustained angiogenesis, tissue
invasion and metastasis (8).
Regarding growth signals, the mitogen-activated protein kinase
(MAPK) and phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)
signaling pathways serve crucial roles in controlling fundamental
cellular processes, including growth, proliferation,
differentiation, migration and apoptosis (9). Emerging evidence has suggested that
sustained activation of the MAPK and PI3K signaling pathways is
responsible for anti-apoptosis and carcinogenesis (10). Data from several groups have suggested
that constitutively activated extracellular signal-regulated kinase
(ERK) is involved in the progression of certain types of human
cancer, including carcinomas of the breast (11), colon (12) and prostate (13). Activation of the PI3K signaling
pathway was significantly associated with the tumor development and
progression of human gastric cancer, based on 56 gastric cancer
specimens (14). In addition, An
et al (15) investigated a
total of 290 patients with pT2b gastric cancer and elucidated that
phosphorylated (p-) mammalian target of rapamycin (mTOR) was
expressed in patient-derived gastric cancer samples, and that mTOR
activation was associated with the extent of lymph node metastasis
and poor survival in patients with gastric cancer. Therefore,
inhibition of the PI3K and MAPK signal transduction pathways may
represent a promising strategy in the treatment of the initiation
and progression of gastric cancer.
A body of studies suggest that luteolin
(3′,4′,5,7-tetrahydro-xyflavone), a natural flavonoid compound
highly enriched in a number of medicinal herbals, including
Lonicera japonica, Scutellaria barbata and Ajuga
decumbus (16), possesses diverse
biological activities, including anti-inflammatory (17), antioxidant (18) and antiproliferative effects (19). Furthermore, it has been documented
that luteolin could arrest the cell cycle and induce apoptosis in a
wide variety of cancer cells in vitro, including prostate
cancer cells (20), AGS human gastric
cancer cells (21,22), SMMC7721 liver cancer cells (23), COLO205 human colorectal cancer cells
and HeLa human cervical cancer cells (24). It has also been reported that luteolin
was able to significantly decrease colon cancer incidence and the
number of tumors per rat when administered at the initiation and
post-initiation stages of carcinogenesis (25). To date, various well-controlled
clinical trials have been carried out to evaluate the
chemopreventive potential of luteolin in human subjects (26–30).
However, the molecular events and signal transduction pathways
involved in the mechanism of action of luteolin in gastric cancer
remain to be elucidated.
Therefore, the purpose of the present study was to
investigate the role of the MAPK and PI3K signaling pathways in
regulating luteolin-induced apoptosis in vitro. The present
findings highlight the potential of luteolin as an anti-cancer
therapeutic agent that targets MAPK and PI3K signaling in gastric
cancer cells.
Materials and methods
Reagents and antibodies
Luteolin, MTT and dimethyl sulfoxide were purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). RPMI-1640
medium, fetal bovine serum (FBS), penicillin and streptomycin were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Annexin V-FITC apoptosis detection kit and anti-cytochrome
c antibody (catalogue no. 556433) were purchased from BD
Biosciences (San Jose, CA, USA). Primary antibodies, including
anti-p-PI3K (Y607; catalogue no. YP0765), anti-p-mTOR (S2448;
catalogue no. YP0176) and anti-β-actin (catalogue no. YT0099)
antibodies, were from ImmunoWay Biotechnology Company (Plano, TX,
USA), while anti-p-AKT (Ser473; catalogue no. 4051), anti-p-p38
(Thr180/Tyr182; catalogue no. 9216), anti-p-ERK1/2 (Thr202/Tyr204;
catalogue no. 9106), anti-p-c-Jun N-terminal kinase (JNK)
(Thr183/Tyr185; catalogue no. 4668), anti-B-cell lymphoma (Bcl)-2
(catalogue no. 15071), anti-Bcl-2 associated X protein (Bax)
(catalogue no. 2772), anti-caspase-3 (catalogue no. 9668) and
anti-caspase-9 (catalogue no. 9508) antibodies were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Caspase-3 (3G2,
catalogue no. 9668) is mouse monoclonal antibody that detects
endogenous levels of full length (35 kDa) and the large fragment
(17/19 kDa) of caspase-3 resulting from cleavage at aspartic acid
175. Caspase-9 (C9, catalogue no. 9508) is mouse monoclonal
antibody that detects endogenous levels of the pro form and cleaved
fragments of caspase-9 (47,37 and 35 kDa). The horseradish
peroxidase-conjugated secondary antibodies anti-rabbit
immunoglobulin (Ig)G (catalogue no. sc-2357) and anti-mouse IgG
(catalogue no. sc-516102) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). U0126 (catalogue no. S1102)
and LY294002 (catalogue no. S1105) were purchased from Selleck
Chemicals (Houston, TX, USA). All other chemicals were purchased
from Sangon Biology Engineering Technology Service, Ltd. (Shanghai,
China) and were of analytical grade.
Cancer cell culture
The human gastric cancer cell line BGC-823 was
obtained from the Cell Center at Chinese Academy of Medical
Sciences and Peking Union Medical College (Beijing, China). The
cells were maintained in RPMI-1640 medium supplemented with 10%
FBS, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a 5%
CO2 incubator. The cells were sub-cultured every 2 or 3
days and routinely checked visually under an inverted microscope
(Leica Microsystems GmbH, Wetzlar, Germany) for potential
contamination. No contamination was identified.
Western blot analysis
Cells (1.0×106) were seeded in 10-cm
dishes. When cells were in logarithmic growth phase, they were
treated with luteolin at the 0, 20, 40 and 60 µM for 48 h.
Subsequently, BGC-823 cells were washed twice with PBS (pH 7.4) and
lysed in 100 µl radioimmunoprecipitation assay buffer (AR0102) that
purchased from Boster Biotech (Wuhan, China). The lysed cells were
removed from the culture dish by gentle scraping with a cell
scraper (#3010; Corning Incorporated, USA) and transferred to a
microcentrifuge tube. The samples were centrifuged at 13,000 rpm
for 5 min at 4°C, and the supernatant was then transferred to a new
tube. Total protein concentration was determined using the Pierce
BCA Protein assay kit (Thermo Fisher Scientific, Inc.). Proteins
were separated by 10% SDS-PAGE and electrotransferred to a
polyvinylidene fluoride membrane (0.2 µm; Merck KGaA, Darmstadt,
Germany) using a Trans-Blot SD Semi-Dry Transfer Cell (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Blocking was carried out
for 2 h in TBST [Tris-buffered saline (TBS) containing 1‰ Tween-20,
v/v] with 5% non-fat milk at room temperature. The primary
antibodies (1:1,000) were incubated with the membrane overnight at
4°C. Following three washes in TBST, secondary antibodies (1:2,000)
were added and incubated at room temperature for 2 h. The blots
were washed with TBST three times, and detection was then performed
using Pierce ECL Western Blotting Substrate (Thermo Fisher
Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (qPCR) assay
After BGC-823 cells were exposed to 0, 20, 40 and 60
µM luteolin for 48 h, total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. RNA concentration and purity were
determined based on the measurement of absorbance at 260 and 280
nm. RNase-free DNase I (Takara Bio, Inc., Japan) was used to remove
the DNA contamination. M-MLV Reverse Transcriptase (Fermentas,
Thermo Fisher Scientific, Inc.; Pittsburgh, PA, USA) was used
according to the manufacturer's protocol to treat 2 µg total RNA
for synthesizing first-strand complementary DNA (cDNA). The cDNA
was then subjected to qPCR for evaluation of the relative messenger
RNA (mRNA) levels. Gene-specific amplification was performed using
an StepOne™ (96 wells) Real-Time PCR system (Applied
Biosystems® ABI; Thermo Fisher Scientific, Inc.) with a
20 µl PCR reaction mixture containing 1 µl cDNA (synthesized as
described above), 10 µl 2X Fast SYBR-Green Master Mix (Applied
Biosystems® ABI, USA), forward and reverse primers with
a final concentration of 0.25 µM. The amplification conditions were
95°C for 15 min, followed by 40 cycles of 95°C for 15 sec, 56°C for
20 sec and 72°C for 30 sec. The relative expression levels of the
target genes were normalized to the geometric mean of the internal
control gene, GAPDH. Each gene was performed in a set of
three replicates. No template control was included in all batches.
The Cq (threshold cycles) values were used to calculate the mRNA
levels by the formula 2−∆∆Ct = 2−[∆Ct treatment−∆Ct
control] (31). The primers
used in qPCR analysis were obtained from Sangon Biology Engineering
Technology Service, Ltd. (Shanghai, China), and their sequences are
reported in Table I.
 | Table I.Primers used in quantitative
polymerase chain reaction analysis. |
Table I.
Primers used in quantitative
polymerase chain reaction analysis.
| Gene name | Primer sequence
(5′-3′) |
|---|
| DUSP1 | F:
TTTGAGGGTCACTACCAG |
|
| R:
GAGATGATGCTTCGCC |
| DUSP2 | F:
AGTCACTCGTCAGACC |
|
| R:
TGTTCTTCACCCAGTCAAT |
| DUSP4 | F:
CAAAGGCGGCTATGAG |
|
| R:
GGTTATCTTCCACTGGG |
| DUSP5 | F:
CTGAGTGTTGCGTGGA |
|
| R:
AGTCTATTGCTTCTTGAAAGT |
| DUSP6 | F:
CGAGACCCCAATAGTGC |
|
| R:
AATGGCCTCAGGGAAA |
| DUSP7 | F:
TCATTGACGAAGCCCG |
|
| R:
GCGTATTGAGTGGGAACA |
| DUSP9 | F:
ATCCGCTACATCCTCAA |
|
| R:
AGGTCATAGGCATCGTT |
| DUSP10 | F:
CTGAACATCGGCTACG |
|
| R:
GGTGTAAGGATTCTCGGT |
| CXCL16 | F:
CAGCAAGCCAAGAGGA |
|
| R:
TGACAAAGGCATAGAGCA |
| GAPDH | F:
AAGGTCGGAGTCAACGGATT |
|
| R:
CTCCTGGAAGATGGTGATGG |
Statistical analysis
The data were expressed as the mean ± standard
deviation (n=3). All calculations were performed with SPSS version
16 (SPSS, Inc., Chicago, IL, USA). Statistical significance was
analyzed by one-way analysis of variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
Luteolin induces activation of
caspases
Our group previously reported that luteolin
inhibited BGC-823 cell proliferation and induced apoptosis in a
dose-dependent manner (32). In the
present study, to further examine whether luteolin-induced
apoptosis resulted from activating caspase enzymes, the activities
of initiator caspase (caspase-9) and effector caspase (caspase-3)
(33,34) were measured by western blotting in
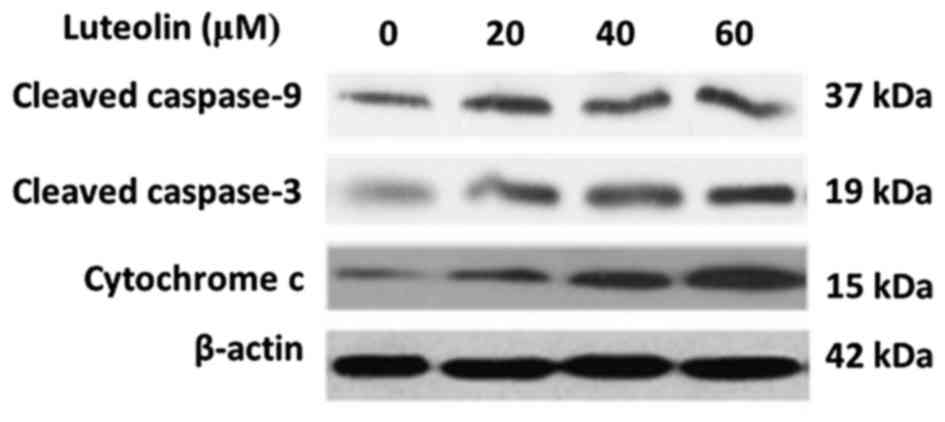
BGC-823 cells. The results revealed that luteolin treatment
increased the expression of cleaved caspase-9 and caspase-3 in a
dose-dependent manner (Fig. 1). Next,
the level of cytochrome c in the cytoplasm was detected
(35–38), as this is a key step in the process of
caspase activation during apoptosis. As shown in Fig. 1, luteolin induced an increase of
cytoplasmatic cytochrome c in a dose-dependent manner. These
results implied that luteolin-induced apoptosis in BGC-823 cells
may occur through the intrinsic pathway.
Effects of luteolin on the expression
of Bcl-2 family proteins
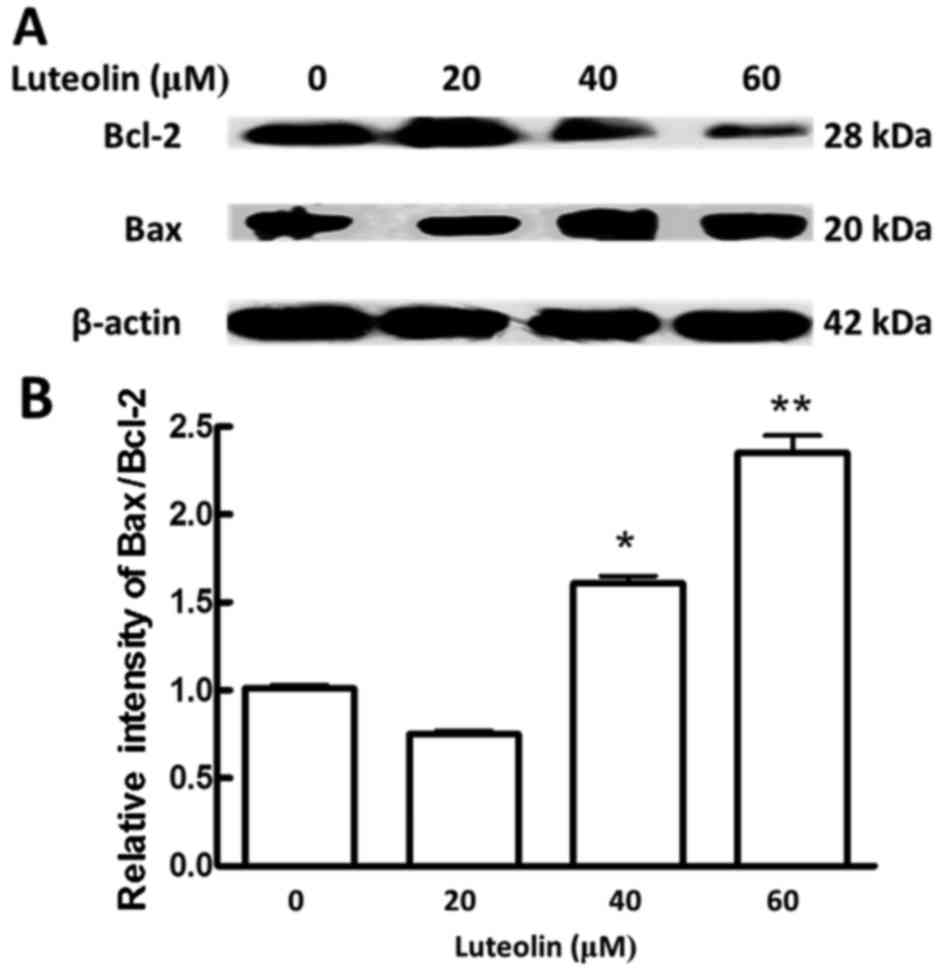
The involvement of the intrinsic pathway in
apoptosis is regulated by proteins of the Bcl-2 family, which
comprises anti-apoptotic (e.g., Bcl-2) and pro-apoptotic (e.g.,
Bax) proteins (39). The ratio of
Bax/Bcl-2 could determine whether the cell undergoes apoptosis
(40,41). In the present study, luteolin
treatment of BGC-823 cells resulted in decreased of Bcl-2 in a
dose-dependent manner (Fig. 2A).
Compared with that in control cells, the ratio of Bax/Bcl-2
significantly increased at the highest concentrations of luteolin
in treated cells (Fig. 2B). These
data further suggested that luteolin induced apoptosis via the
intrinsic pathway in BGC-823 cells.
Luteolin suppresses the PI3K and MAPK
signaling pathways
The key elements of the PI3K and MAPK signaling
pathways were evaluated by western blotting in BGC-823 cells
exposed to luteolin at 0, 20, 40 and 60 µM for 48 h. As shown in
Fig. 3A, luteolin reduced the
expression level of p-PI3K, p-AKT and p-mTOR in a dose-dependent
manner. These results suggested that luteolin treatment suppressed
the PI3K signaling pathway.
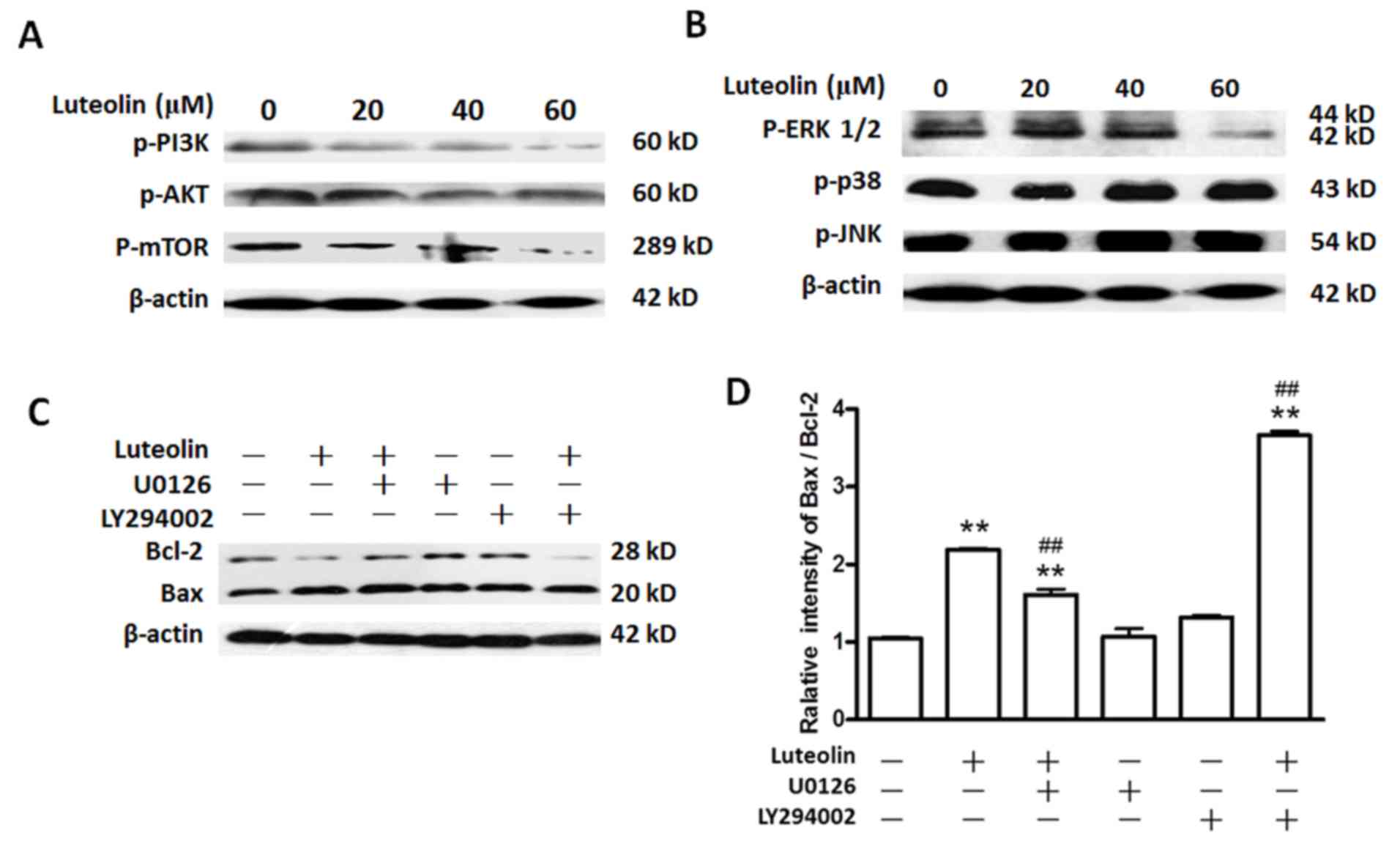 | Figure 3.Luteolin inhibits the activation of
the PI3K and MAPK signaling pathways in BGC-823 cells. Cells were
seeded in 60-mm plates and cultured to 80–90% confluence. The cells
were then treated with various doses of luteolin (20, 40 and 60 µM)
for 48 h. Cells treated with dimethyl sulfoxide alone were used as
the control. (A) Luteolin inhibited the PI3K signaling pathway in
BGC-823 cells. Cells were treated as described above, and the cell
extracts were subjected to immunoblot analysis using anti-p-PI3K,
anti-p-AKT, anti-p-mTOR and anti-β-actin antibodies. (B) Effect of
luteolin on ERK1/2, p38 and JNK pathways. Cells were treated as
described above, and the cell extracts were subjected to
immunoblotting using anti-p-ERK, anti-p-p38, anti-p-JNK and
anti-β-actin antibodies. (C) Effects of luteolin on the Bcl-2 and
Bax in BGC-823 cells. Cells were treated as described above, and
the cell extracts were subjected to immunoblotting using
anti-p-ERK, anti-Bcl-2, anti-Bax and anti-β-actin antibodies. (D)
The relative intensity of Bax/Bcl-2. Values are the mean ± standard
deviation. The data were analyzed by one-way analysis of variance.
**The control group vs. all the other groups (P<0.001);
##luteolin treatment groups vs. luteolin+U0126 and
luteolin+LY294002 treatment groups (P<0.001). PI3K,
phosphatidylinositol-4,5-bisphosphate 3-kinase; mTOR, mammalian
target of rapamycin; p-, phosphorylated; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; Bcl, B-cell
lymphoma; Bax, Bcl-2 associated X protein. |
With regards to the MAPK signaling pathway, after
BGC-823 cells were treated with luteolin at 0, 20, 40 and 60 µM for
48 h, the results of western blotting indicated that the levels of
p-ERK1/2 were reduced markedly in a dose-dependent manner, while
the levels of p-p38 and p-JNK exhibited no significant change
(P>0.05). These results suggested that luteolin treatment
suppressed the ERK1/2 signaling pathway, but not the JNK or p38
signaling pathways, in BGC-823 cells (Fig. 3B).
To confirm the roles of the ERK1/2 or PI3K signaling
pathways in luteolin-induced apoptosis in BGC-823 cells, the ERK
inhibitor U0126 (42) and the AKT
inhibitor LY294002 (43) were used
for treating the cells in the absence or presence of 60 µM
luteolin. The effects were examined according to the ratio of Bax
to Bcl-2. The results indicated that exposure of BGC-823 cells to
U0126 or LY294002 alone did not significantly alter the Bax/Bcl-2
ratio compared with that of control cells (Fig. 3C and D; P>0.05). The Bax/Bcl-2
ratio in the combination U0126 plus luteolin group increased
compared with that in the control group, while such ratio was lower
than that observed upon luteolin treatment alone (P<0.05). The
Bax/Bcl-2 ratio in the combination LY294002 plus luteolin group
increased compared with that in the control group, being much
higher than that observed following luteolin treatment alone
(P<0.05). Taken together, these results indicated that the ERK
and PI3K signaling pathways were involved in the apoptosis induced
by luteolin in BGC-823 cells. Notably, the Bax/Bcl-2 ratio in the
LY294002 plus luteolin group was markedly higher than that in the
U0126 plus luteolin group (~3.7 vs. 1.6), respectively, indicating
that the PI3K signaling pathway has much stronger effects on
luteolin-induced apoptosis than the MAPK signaling pathway.
Effects of luteolin on the expression
of dual-specificity phosphatase (DUSP) and chemokine (C-X-C motif)
ligand 16 (CXCL16) genes in BGC-823 cells
Dual-specificity phosphatases (DUSPs) are a
heterogeneous group of protein phosphatases that have ability to
dephosphorylate both tyrosine and serine/threonine residues
(44) and serve a critical role in
the inactivation of different isoforms of MAPK (45) DUSPs share common features, including a
cluster of basic amino acids as part of the kinase interactive
motif (KIM) on their amino terminus. The KIM confers substrate
specificity and is the least homologous region demonstrating
individual substrate preferences (46,47).
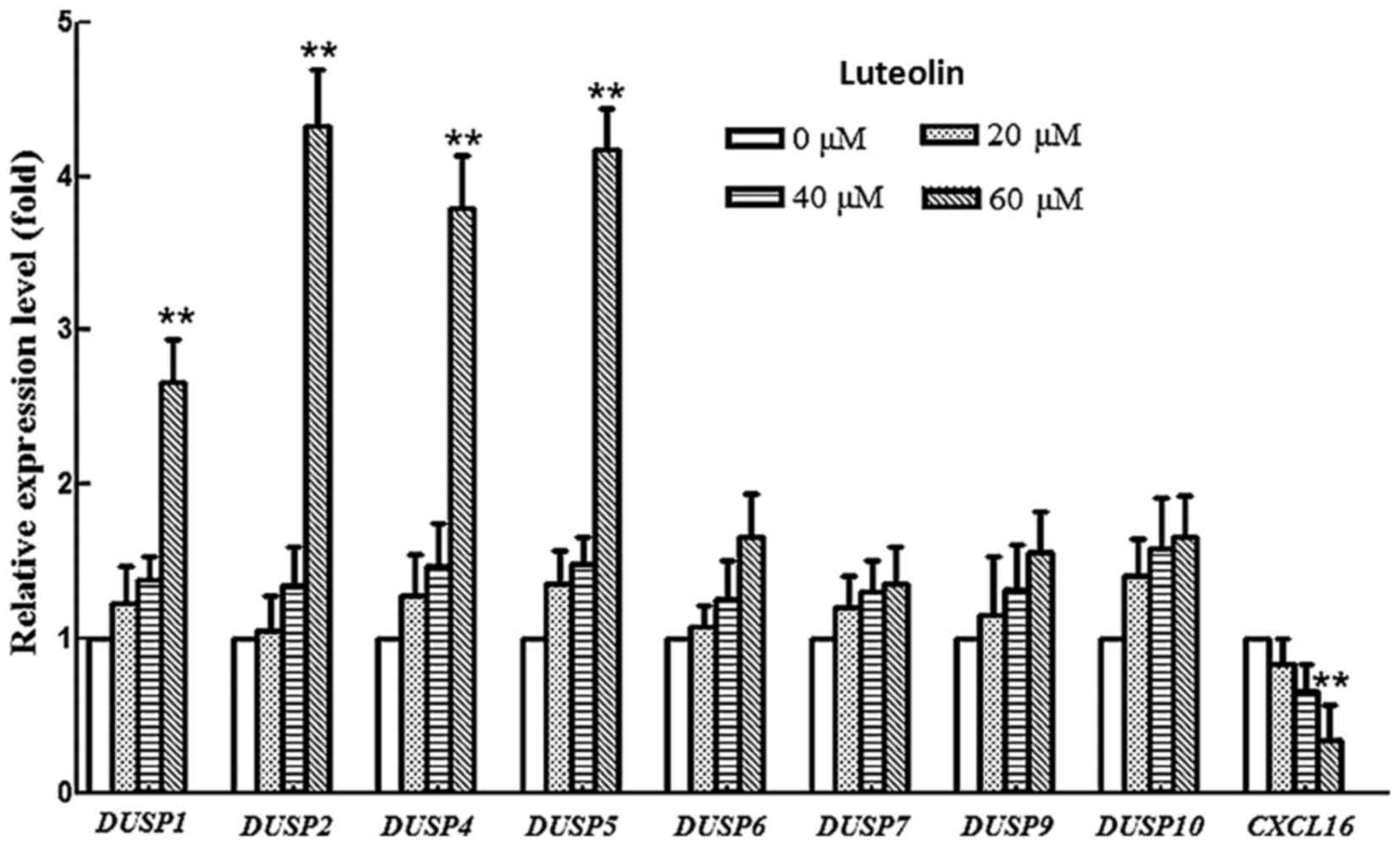
Therefore, the transcription level of DUSP1-DUSP10 was examined.
The mRNA levels of DUSP1, 2, 4 and 5 were upregulated in
luteolin-treated BGC-823 cells compared with those in controls
cells, while the mRNA levels of DUSP6, 7, 9 and 10 did not
change during the treatment (Fig. 4).
DUSP1, 2, 4 and 5 prefer to use the ERK as their substrates.
This result was consistent with the decrease of p-ERK protein in
the western blotting results of the present study, indicating that
luteolin exhibits the potential to regulate the expression of
specific DUSP genes, resulting in an attenuation of the MAPK
signaling pathway.
Chalabi-Dchar et al (48) reported that blocking CXCL16
activity abrogated activation of the PI3K/AKT pathway, implying the
CXCL16 axis may regulate the activity of PI3K pathway.
Therefore, the expression of CXCL16 was monitored at an mRNA
level. The results showed that the CXCL16 mRNA level was
greatly downregulated in a dose manner after treatment with
luteolin, suggesting that luteolin efficiently suppressed the mRNA
level of CXCL16. Hence, the attenuation of PI3K pathway may
be ascribed to the suppression of CXCL16 in BGC-823 cell
lines following luteolin treatment.
Discussion
In the present study, the apoptotic process induced
by luteolin in BGC-823 cells was associated with the activities of
caspases and the expression of Bcl-2 family proteins, which is in
agreement with previous reports regarding the pro-apoptotic effects
of luteolin on other cancer cells, including the gastric cancer
cell line AGS (21,22), human prostate cancer cells (49) and the human colon cancer cell line
HT-29 (50). However, the underlying
mechanism of luteolin-induced apoptosis is not well understood
yet.
MAPKs have been linked to diverse cellular events,
including proliferation, senescence, differentiation, migration and
apoptosis (51). A well-characterized
apoptotic signaling cascade is regulated by MAPKs, including JNK,
ERK and p38 MAPK (51). The ERK1/2
signaling pathway primarily responds to growth and differentiation
factors, and the p38 and JNK signaling pathways primarily responds
to stress conditions (52,53). The present study noticed that the
phosphorylation of ERK1/2 decreased significantly following
luteolin treatment, while that of p38 or JNK did not change in the
course of luteolin treatment, suggesting that p38 and JNK are not
involved in the regulation of apoptosis in the BGC-823 cell line.
It has been reported that ERK1/2 participates in apoptotic
signaling via post-translational regulation of the Bcl-2 family
members, including Bcl-2 interacting mediator of cell death (Bim)
(54). Phosphorylation of Bim by
ERK1/2 results in a change in the Bax/Bcl-2 ratio, which determines
whether cell apoptosis occurs (55).
In the present study, although U0126 treatment alone did not induce
apoptosis in BGC-823 cells, when combined with luteolin, it
increased the Bax/Bcl-2 ratio, suggesting that luteolin-induced
apoptosis was mediated by the ERK1/2 signaling pathway.
A major determinant of the biological outcome of
MAPK signaling is the duration and magnitude of kinase activation,
which can be achieved by serine/threonine phosphatases,
tyrosine-specific phosphatases or dual-specificity phosphatases
(DUSPs) (56,57). DUSPs, whose family consists of 25
members, can specifically dephosphorylate ≥1 MAPKs, and their
substrate specificity is dependent on the cell type and context
(44,46). DUSP1, 2, 4 and 5 are mitogen- and
stress-inducible nuclear DUSPs, which prefer using ERK as a
substrate compared with JNK or p38. DUSP6, 7 and 9 are cytoplasmic
ERK-specific DUSPs, while DUSP8 and 10 are JNK/p38-specific
phosphatases present in both the nucleus and cytoplasm (58). The present study revealed that
DUSP1, 2, 4 and 5 mRNA expression was upregulated, while the
expression of other DUSPs was unchanged, upon luteolin
treatment. These results indicated that highly expressed
DUSP1, 2, 4 and 5 specifically dephosphorylated ERK1/2 as a
substrate, which may explain the decrease in phosphorylation of
ERK1/2. No difference in p-JNK or p-p38 was observed between cells
treated with or without luteolin, which may be due to the unchanged
expression of other DUSPs. To date, no study has reported
that luteolin has the potential to regulate the expression of
DUSP genes on GC. Taken together, increased mRNA levels of
specific DUSPs in luteolin-treated BGC-823 cell lines
resulted in decreased p-ERK1/2 in the present study, and the
suppression of p-ERK1/2 could increase the ratio of Bax to Bcl-2,
eventually triggering apoptosis.
The PI3K pathway relies on an array of intracellular
events that have been intensively studied in previous years
(59). The PI3K signaling pathway may
influence apoptosis regulation, including the regulation of the
Bcl-2 family proteins (10,60). One of the important members of the
Bcl-2 family of proteins is Bax, which can be phosphorylated at the
inhibition site Ser184 near the C-terminus by AKT, leading to
suppression of the apoptotic activity mediated by Bax (61). Thus, the activity of PI3K in cancer
cells protects them from undergoing apoptosis; conversely,
inhibiting the activity of PI3K can induce apoptosis in cancer
cells (62,63). In the present study, it was
demonstrated that luteolin may inhibit p-PI3K, p-AKT and p-mTOR in
the gastric cancer cell line BGC-823, suggesting that the PI3K
signaling pathway may be involved in luteolin-induced apoptosis.
Further experiments verified that combined treatment with LY294002
and luteolin had an enhanced effect on the induction of apoptosis,
as evidenced by a significant increase in the Bax/Bcl-2 ratio,
indicating that luteolin-induced apoptosis mainly occurred through
the PI3K signaling pathway.
The activation of PI3K family members is a universal
event in response to cytokines, growth factors and hormones
(59,64). Chemokines, a superfamily of
chemotactic cytokines consisting of nearly 50 cytokine members and
20 chemokine receptors (65,66), are classified into four major families
based on the relative position of their cysteine residues near the
NH2 terminus: CC, CXC, C and CX3C (67). Xing et al reported that
aberrant expression of CXCL16 and CXCR6 may be involved in gastric
carcinogenesis, and that the expression and serum concentration of
CXCL16 could indicate the aggressiveness and prognosis of GCs
(68). Furthermore, a previous study
elucidated that PI3K/AKT/mTOR signaling may be involved in the
CXCL16/CXCR6 biological axis (69).
In that study, the phosphorylation of AKT was reduced with
decreased CXCR6 expression, and mTOR was activated by CXCL16's
stimulation of CXCR6 (69). Since the
PI3K signaling pathway is involved in the activation of the
CXCR6/CXCL16 axis (70,71), a number of therapy options may target
blocking this axis or exploit other antibodies against this
signaling pathway in order to prevent metastasis. Based on such
strategy, various antibodies have been designed to specifically
block the CXCL16/CXCR6 axis; however, the positive outcomes are
poor (72–74). The present study demonstrated that
luteolin efficiently inhibited CXCL16 mRNA expression. In
the present study, based on the suppression of PI3K/AKT/mTOR
signaling, it was hypothesized that luteolin may inhibit the
expression of CXCL16, resulting in the suppression of the
signaling PI3K pathway. To the best of our knowledge, the present
study is the first to report that chemicals have the potential to
regulate the CXCL16/CXCR6 axis, which may be beneficial in the
development of a more effective anti-metastasis therapeutic
strategy for gastric cancer. The present results also provide the
first evidence suggesting that luteolin treatment may also alter
the tumor microenvironment.
In summary, the present study has demonstrated that
luteolin could induce apoptosis in the BGC-823 cell line through
the intrinsic pathway. Luteolin upregulated the mRNA levels of
specific DUSPs, which suppressed the protein phosphorylation
of ERK1/2. In addition, luteolin attenuated the mRNA levels of
CXCL16, leading to the suppression of the PI3K signaling
pathway, and both the ERK1/2 and the PI3K signaling pathways were
closely associated with the regulation of apoptosis in the gastric
cancer cell line BGC-823. The present results provide useful
information for considering luteolin as an attractive
chemotherapeutic agent against gastric cancer.
Acknowledgements
The authors thank Professor Michael Guiver, National
Research Council Canada, Ottawa (Ontario, Canada) for his critical
review of the manuscript. The present study was supported by the
Joint Funds of the National Natural Science Foundation of China
(grant no. U1203203) and the West Light Foundation of the Chinese
Academy of Sciences (grant no. 2016-QNXZ-B-5).
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality
Worldwide: IARC CancerBase No. 11. International Agency for
Research on Cancer; Lyon, France: 2013
|
|
2
|
Rahman R, Asombang AW and Ibdah JA:
Characteristics of gastric cancer in Asia. World J Gastroenterol.
20:4483–4490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Menges M and Hoehler T: Current strategies
in systemic treatment of gastric cancer and cancer of the
gastroesophageal junction. J Cancer Res Clin Oncol. 135:29–38.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Green D, de Leon S Ponce, Leon-Rodriguez E
and Sosa-Sanchez R: Adenocarcinoma of the stomach: Univariate and
multivariate analysis of factors associated with survival. Am J
Clin Oncol. 25:84–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ajani JA: Evolving chemotherapy for
advanced gastric cancer. Oncologist. 10 Suppl 3:S49–S58. 2005.
View Article : Google Scholar
|
|
6
|
Hejna M, Wöhrer S, Schmidinger M and
Raderer M: Postoperative chemotherapy for gastric cancer.
Oncologist. 11:136–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Costanzo F, Gasperoni S, Manzione L,
Bisagni G, Labianca R, Bravi S, Cortesi E, Carlini P, Bracci R,
Tomao S, et al: Adjuvant chemotherapy in completely resected
gastric cancer: A randomized phase III trial conducted by GOIRC. J
Natl Cancer Inst. 100:388–398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan Y, Wang LF and Wang RF: Role of
cancer-associated fibroblasts in invasion and metastasis of gastric
cancer. World J Gastroenterol. 21:9717–9726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mendoza MC, Er EE and Blenis J: The
Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends
Biochem Sci. 36:320–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trisciuoglio D, Iervolino A, Zupi G and
Del Bufalo D: Involvement of PI3K and MAPK signaling in
bcl-2-induced vascular endothelial growth factor expression in
melanoma cells. Mol Biol Cell. 16:4153–4162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adeyinka A, Nui Y, Cherlet T, Snell L,
Watson PH and Murphy LC: Activated mitogen- activated kinase
expression during human breast tumorigenesis and breast cancer
progression. Clin Cancer Res. 8:1747–1753. 2002.PubMed/NCBI
|
|
12
|
Kress TR, Raabe T and Feller SM: High Erk
activity suppresses expression of the cell cycle inhibitor p27Kip1
in colorectal cancer cells. Cell Commun Signal. 8:12010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uzgare AR, Kaplan PJ and Greenberg NM:
Differential expression and/or activation of P38MAPK, erk1/2, and
jnk during the initiation and progression of prostate cancer.
Prostate. 55:128–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin HL, Yang MH, Wu CW, Chen PM, Yang YP,
Chu YR, Kao CL, Ku HH, Lo JF, Liou JP, et al: 2-Methoxyestradiol
attenuates phosphatidylinositol 3-kinase/Akt pathway- mediated
metastasis of gastric cancer. Int J Cancer. 121:2547–2555. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An JY, Kim KM, Choi MG, Noh JH, Sohn TS,
Bae JM and Kim S: Prognostic role of p-mTOR expression in cancer
tissues and metastatic lymph nodes in pT2b gastric cancer. Int J
Cancer. 126:2904–2913. 2010.PubMed/NCBI
|
|
16
|
Miean KH and Mohamed S: Flavonoid
(myricetin, quercetin, kaempferol, luteolin, and apigenin) content
of edible tropical plants. J Agric Food Chem. 49:3106–3112. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishitani Y, Yamamoto K, Yoshida M, Azuma
T, Kanazawa K, Hashimoto T and Mizuno M: Intestinal
anti-inflammatory activity of luteolin: Role of the aglycone in
NF-κB inactivation in macrophages co-cultured with intestinal
epithelial cells. Biofactors. 39:522–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashokkumar P and Sudhandiran P: Protective
role of luteolin on the status of lipid peroxidation and
antioxidant defense against azoxymethane-induced experimental colon
carcinogenesis. Biomed Pharmacother. 62:590–597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ashokkumar P and Sudhandiran G: Luteolin
inhibits cell proliferation during Azoxymethane-induced
experimental colon carcinogenesis via Wnt/β-catenin pathway. Invest
New Drugs. 29:273–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakurai MA, Ozaki Y, Okuzaki D, Naito Y,
Sasakura T, Okamoto A, Tabara H, Inoue T, Hagiyama M, Ito A, et al:
Gefitinib and luteolin cause growth arrest of human prostate cancer
PC-3 cells via inhibition of cyclin G-associated kinase and
induction of miR-630. PLoS One. 9:e1001242014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu B, Zhang Q, Shen WM and Zhu J:
Anti-proliferative and chemosensitizing effects of luteolin on
human gastric cancer AGS cell line. Mol Cell Biochem. 313:125–132.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang HY, Quan K, Jiang YL, Wu JG and Tang
XW: Effect of Luteolin and its combination with chemotherapeutic
drugs on cytotoxicity of cancer cells. Zhejiang Da Xue Xue Bao Yi
Xue Ban. 39:30–36. 2010.(In Chinese). PubMed/NCBI
|
|
23
|
Ding S, Hu A, Hu Y, Ma J, Weng P and Dai
J: Anti-hepatoma cells function of luteolin through inducing
apoptosis and cell cycle arrest. Tumour Biol. 35:3053–3060. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi RX, Ong CN and Shen HM: Luteolin
sensitizes tumor necrosis factor-alpha-induced apoptosis in human
tumor cells. Oncogene. 23:7712–7721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Manju V and Nalini N: Protective role of
luteolin in 1,2-dimethylhydrazine induced experimental colon
carcinogenesis. Cell Biochem Funct. 25:189–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Samy RP, Gopalakrishnakone P and
Ignacimuthu S: Anti-tumor promoting potential of luteolin against
7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Chem
Biol Interact. 164:1–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amin AR, Kucuk O, Khuri FR and Shin DM:
Perspectives for cancer prevention with natural compounds. J Clin
Oncol. 27:2712–2725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pandurangan AK, Dharmalingam P, Sadagopan
SK Ananda and Ganapasam S: Effect of luteolin on the levels of
glycoproteins during azoxymethane-induced colon carcinogenesis in
mice. Asian Pac J Cancer Prev. 13:1569–1573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sagawa H, Naiki-Ito A, Kato H, Naiki T,
Yamashita Y, Suzuki S, Sato S, Shiomi K, Kato A, Kuno T, et al:
Connexin 32 and luteolin play protective roles in non-alcoholic
steatohepatitis development and its related hepatocarcinogenesis in
rats. Carcinogenesis. 36:1539–1549. 2015.PubMed/NCBI
|
|
30
|
Kasala ER, Bodduluru LN, Barua CC and
Gogoi R: Antioxidant and antitumor efficacy of Luteolin, a dietary
flavone on benzo(a)pyrene-induced experimental lung carcinogenesis.
Biomed Pharmacother. 82:568–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu Xueying, Li Yanhong, Xiao Xiangwen,
Akber Aisa Haji and Li Xiaobo: Study on inhibition of luteolin on
proliferation of human gastric cancer cell line BGC-823. Mod J
Integr Tradit Chinese Western Med. 3:246–249. 2012.
|
|
33
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pradelli LA, Bénéteau M and Ricci JE:
Mitochondrial control of caspase-dependent and -independent cell
death. Cell Mol Life Sci. 67:1589–1597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Otera H and Mihara K: Mitochondrial
dynamics: Functional link with apoptosis. Int J Cell Biol.
2012:8216762012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar A, Ganini D and Mason RP: Role of
cytochrome c in α-synuclein radical formation: Implications of
α-synuclein in neuronal death in Maneb- and paraquat-induced model
of Parkinson's disease. Mol Neurodegener. 11:702016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lopez-Cruzan M, Sharma R, Tiwari M,
Karbach S, Holstein D, Martin CR, Lechleiter JD and Herman B:
Caspase-2 resides in the mitochondria and mediates apoptosis
directly from the mitochondrial compartment. Cell Death Discov.
2(pii): 160052016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rong Y and Distelhorst CW: Bcl-2 protein
family members: Versatile regulators of calcium signaling in cell
survival and apoptosis. Annu Rev Physiol. 70:73–91. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li C, Wu X, Sun R, Zhao P, Liu F and Zhang
C: Croton tiglium extract induces apoptosis via Bax/Bcl-2 pathways
in human lung cancer A549 cells. Asian Pac J Cancer Prev.
17:4893–4898. 2016.PubMed/NCBI
|
|
41
|
Zhang S, Qin F, Yang L, Xian J, Zou Q, Jin
H, Wang L and Zhang L: Nucleophosmin mutations induce
chemosensitivity in THP-1 leukemia cells by suppressing NF-κB
Activity and regulating Bax/Bcl-2 expression. J Cancer.
7:2270–2279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Csibi A, Fendt SM, Li C, Poulogiannis G,
Choo AY, Chapski DJ, Jeong SM, Dempsey JM, Parkhitko A, Morrison T,
et al: The mTORC1 pathway stimulates glutamine metabolism and cell
proliferation by repressing SIRT4. Cell. 153:840–854. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sapey E, Greenwood H, Walton G, Mann E,
Love A, Aaronson N, Insall RH, Stockley RA and Lord JM:
Phosphoinositide 3-kinase inhibition restores neutrophil accuracy
in the elderly: Toward targeted treatments for immunosenescence.
Blood. 123:239–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Patterson KI, Brummer T, O'Brien PM and
Daly RJ: Dual-specificity phosphatases: Critical regulators with
diverse cellular targets. Biochem J. 418:475–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Theodosiou A and Ashworth A: MAP kinase
phosphatases. Genome Biol. 3:REVIEWS3009. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Camps M, Nichols A and Arkinstall S: Dual
specificity phosphatases: A gene family for control of MAP kinase
function. FASEB J. 14:6–16. 2000.PubMed/NCBI
|
|
47
|
Keyse SM: Protein phosphatases and the
regulation of mitogen-activated protein kinase signaling. Curr Opin
Cell Biol. 12:186–192. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chalabi-Dchar M, Cassant-Sourdy S, Duluc
C, Fanjul M, Lulka H, Samain R, Roche C, Breibach F, Delisle MB,
Poupot M, et al: Loss of somatostatin receptor subtype 2 promotes
growth of KRAS-induced pancreatic tumors in mice by activating PI3K
signaling and overexpression of CXCL16. Gastroenterology.
148:1452–1465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chiu FL and Lin JK: Downregulation of
androgen receptor expression by luteolin causes inhibition of cell
proliferation and induction of apoptosis in human prostate cancer
cells and xenografts. Prostate. 68:61–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lim DY, Jeong Y, Tyner AL and Park JH:
Induction of cell cycle arrest and apoptosis in HT-29 human colon
cancer cells by the dietary compound luteolin. Am J Physiol
Gastrointest Liver Physiol. 292:G66–G75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chang L and Karin M: Mammalian MAP kinas
signaling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK Pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Oltersdorf T, Elmore SW, Shoemaker AR,
Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges
J, Hajduk PJ, et al: An inhibitor of Bcl-2 family proteins induces
regression of solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang CY and Tan TH: DUSPs, to MAP kinases
and beyond. Cell Biosci. 2:242012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jeffrey KL, Camps M, Rommel C and Mackay
CR: Targeting dual-specificity phosphatases: Manipulating MAP
kinase signaling and immune responses. Nat Rev Drug Discov.
6:391–403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nunes-Xavier C, Romá-Mateo C, Ríos P,
Tárrega C, Cejudo-Marín R, Tabernero L and Pulido R:
Dual-specificity MAP kinase phosphatases as targets of cancer
treatment. Anticancer Agents Med Chem. 11:109–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Duronio V: The life of a cell: Apoptosis
regulation by the PI3K/PKB pathway. Biochem J. 415:333–344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Guo C, Yang M, Jing L, Wang J, Yu Y, Li Y,
Duan J, Zhou X, Li Y and Sun Z: Amorphous silica nanoparticles
trigger vascular endothelial cell injury through apoptosis and
autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and
PI3K/Akt/mTOR signaling. Int J Nanomedicine. 11:5257–5276. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gardai SJ, Hildeman DA, Frankel SK,
Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL and
Henson PM: Phosphorylation of Bax Ser184 by Akt regulates its
activity and apoptosis in neutrophils. J Biol Chem.
279:21085–21095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yao R and Cooper GM: Requirement for
phosphatidylinositol-3 kinase in the prevention of apoptosis by
nerve growth factor. Science. 267:2003–2006. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Scheid MP, Lauener RW and Duronio V: Role
of phosphatidylinositol 3-OH-kinase activity in the inhibition of
apoptosis in haemopoietic cells: Phosphatidylinositol 3-OH-kinase
inhibitors reveal a difference in signalling between interleukin-3
and granulocyte-macrophage colony stimulating factor. Biochem J.
312:159–162. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pande M, Bondy ML, Do KA, Sahin AA, Ying
J, Mills GB, Thompson PA and Brewster AM: Association between
germline single nucleotide polymorphisms in the PI3K-AKT-mTOR
pathway, obesity, and breast cancer disease-free survival. Breast
Cancer Res Treat. 147:381–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Luster AD: Chemokines-chemotactic
cytokines that mediate inflammation. N Engl J Med. 338:436–445.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Struyf S, Proost P and Van Damme J:
Regulation of the immune response by the interaction of chemokines
and proteases. Adv Immunol. 81:1–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Guerreiro R, Santos-Costa Q and
Azevedo-Pereira JM: The chemokines and their receptors:
Characteristics and physiological functions. Acta Med Port. 24
Suppl 4:S967–S976. 2011.(In Portuguese).
|
|
68
|
Xing YN, Xu XY, Nie XC, Yang X, Yu M, Xu
HM, Liu YP, Takano Y and Zheng HC: Role and clinicopath- ologic
significance of CXC chemokine ligand 16 and chemokine (C-X-C motif)
receptor 6 expression in gastric carcinomas. Hum Pathol.
43:2299–2307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang J, Lu Y, Wang J, Koch AE, Zhang J and
Taichman RS: CXCR6 induces prostate cancer progression by the
AKT/Mammalian target of rapamycin signaling pathway. Cancer Res.
68:10367–10376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chandrasekar B, Bysani S and Mummidi S:
CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, I kappa
B kinase, and nuclear factor-kappa B and induces cell-cell adhesion
and aortic smooth muscle cell proliferation. J Biol Chem.
279:3188–3196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Deng L, Chen N, Li Y, Zheng H and Lei Q:
CXCR6/CXCL16 functions as a regulator in metastasis and progression
of cancer. Biochim Biophys Acta. 1806:42–49. 2010.PubMed/NCBI
|
|
72
|
Singh R, Kapur N, Mir H, Singh N, Lillard
JW Jr and Singh S: CXCR6-CXCL16 axis promotes prostate cancer by
mediating cytoskeleton rearrangement via Ezrin activation and αvβ3
integrin clustering. Oncotarget. 7:7343–7353. 2016.PubMed/NCBI
|
|
73
|
Hu ZB, Chen Y, Gong YX, Gao M, Zhang Y,
Wang GH, Tang RN, Liu H, Liu BC and Ma KL: Activation of the
CXCL16/CXCR6 pathway by inflammation contributes to atherosclerosis
in patients with End-stage renal disease. Int J Med Sci.
13:858–867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hald SM, Kiselev Y, Al-Saad S, Richardsen
E, Johannessen C, Eilertsen M, Kilvaer TK, Al-Shibli K, Andersen S,
Busund LT, et al: Erratum to: Prognostic impact of CXCL16 and CXCR6
in non-small cell lung cancer: Combined high CXCL16 expression in
tumor stroma and cancer cells yields improved survival. BMC Cancer.
16:9162016. View Article : Google Scholar : PubMed/NCBI
|