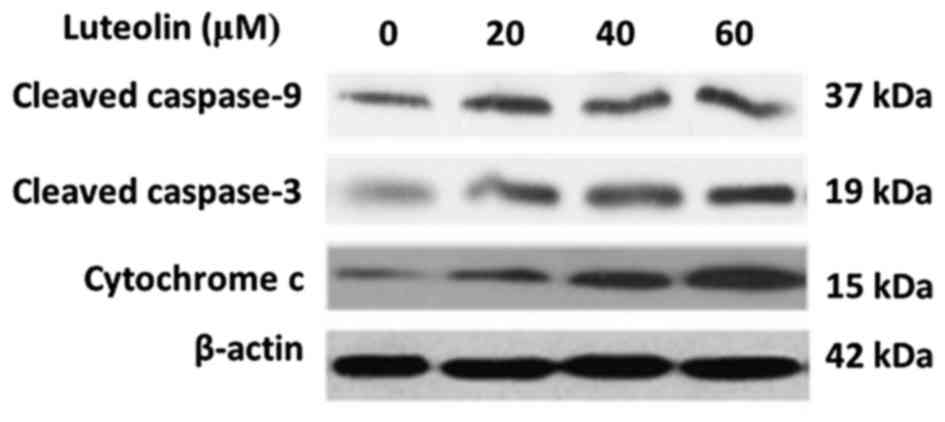
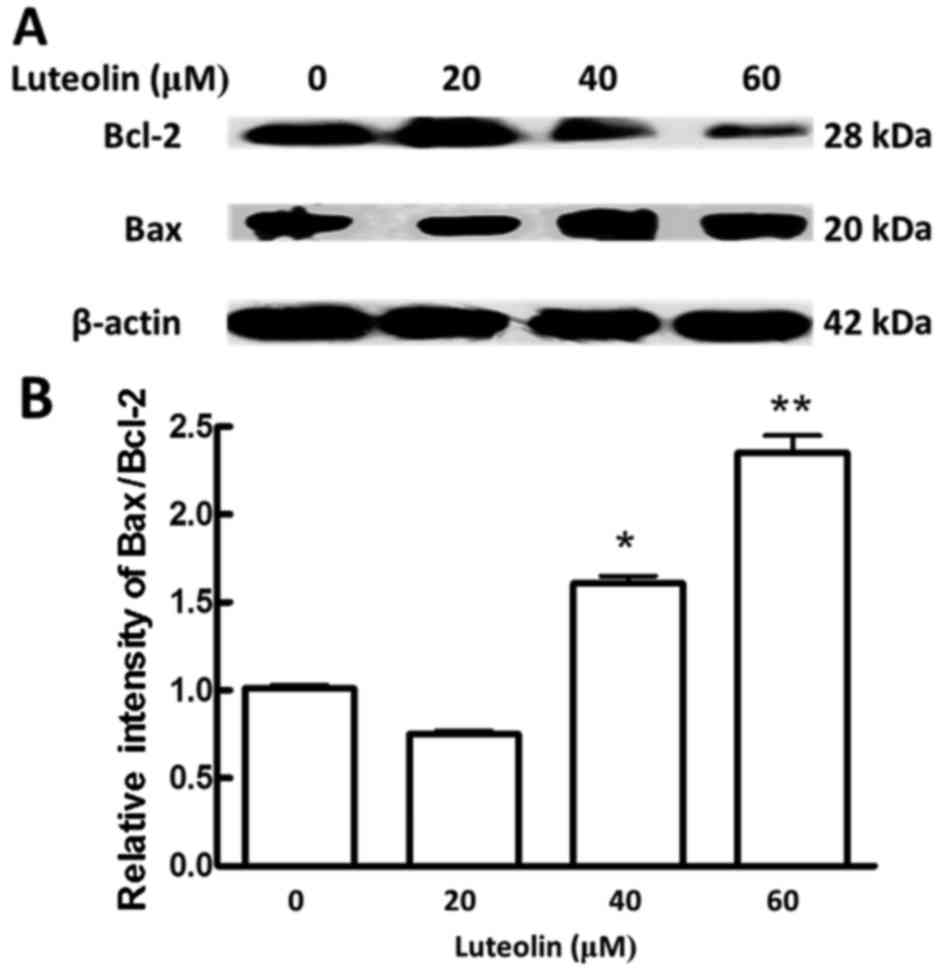
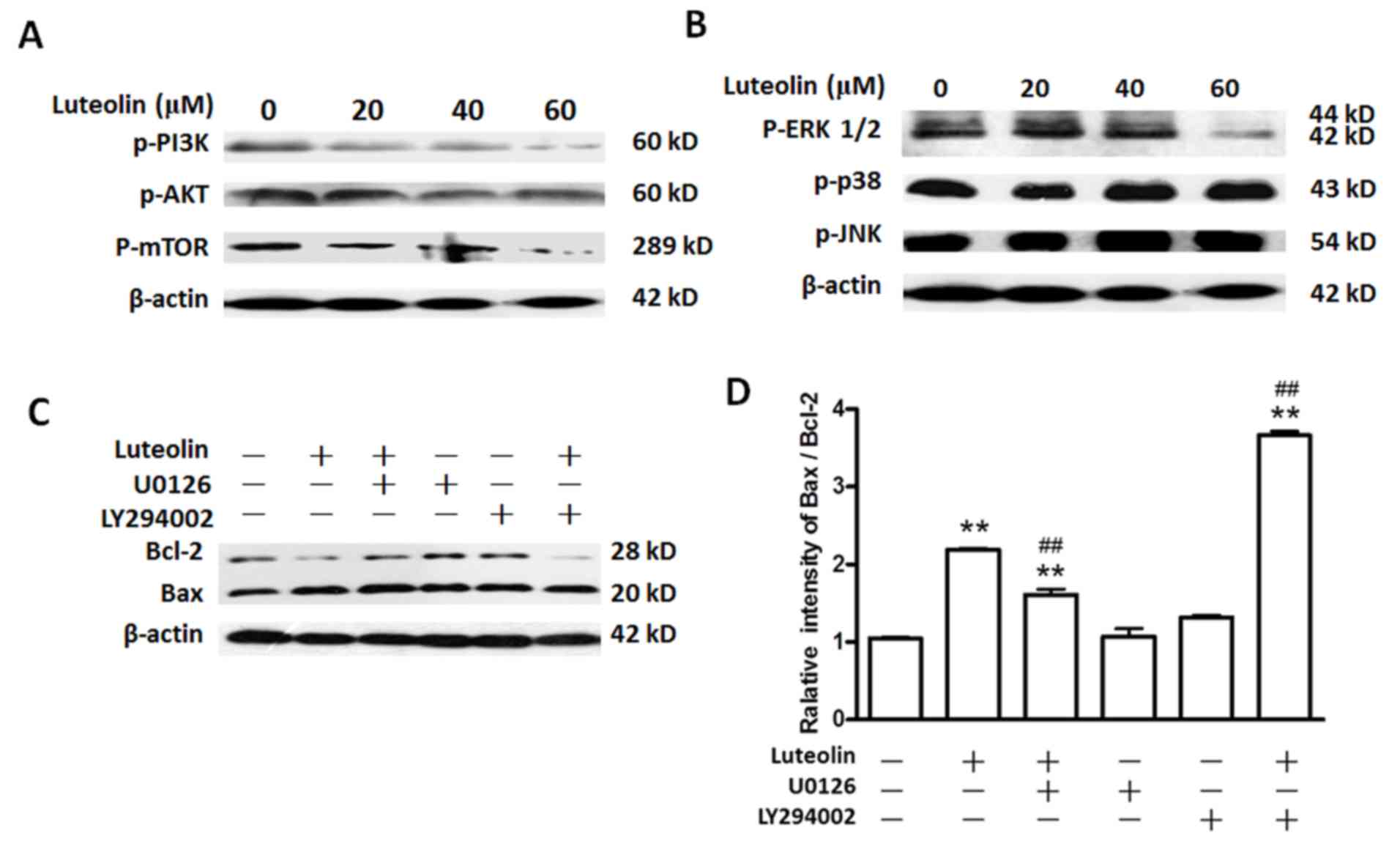
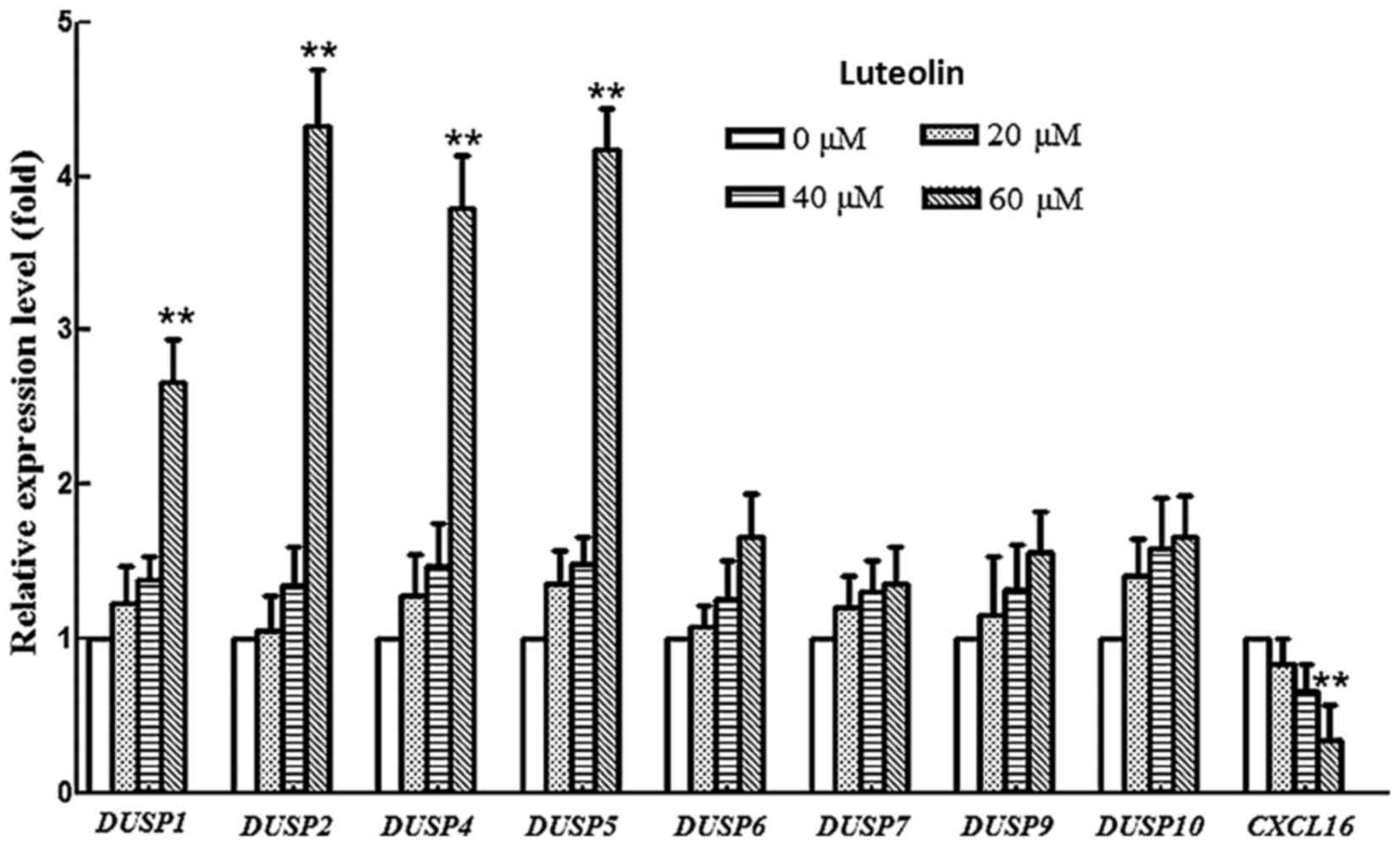
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality
Worldwide: IARC CancerBase No. 11. International Agency for
Research on Cancer; Lyon, France: 2013
|
|
2
|
Rahman R, Asombang AW and Ibdah JA:
Characteristics of gastric cancer in Asia. World J Gastroenterol.
20:4483–4490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Menges M and Hoehler T: Current strategies
in systemic treatment of gastric cancer and cancer of the
gastroesophageal junction. J Cancer Res Clin Oncol. 135:29–38.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Green D, de Leon S Ponce, Leon-Rodriguez E
and Sosa-Sanchez R: Adenocarcinoma of the stomach: Univariate and
multivariate analysis of factors associated with survival. Am J
Clin Oncol. 25:84–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ajani JA: Evolving chemotherapy for
advanced gastric cancer. Oncologist. 10 Suppl 3:S49–S58. 2005.
View Article : Google Scholar
|
|
6
|
Hejna M, Wöhrer S, Schmidinger M and
Raderer M: Postoperative chemotherapy for gastric cancer.
Oncologist. 11:136–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Costanzo F, Gasperoni S, Manzione L,
Bisagni G, Labianca R, Bravi S, Cortesi E, Carlini P, Bracci R,
Tomao S, et al: Adjuvant chemotherapy in completely resected
gastric cancer: A randomized phase III trial conducted by GOIRC. J
Natl Cancer Inst. 100:388–398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan Y, Wang LF and Wang RF: Role of
cancer-associated fibroblasts in invasion and metastasis of gastric
cancer. World J Gastroenterol. 21:9717–9726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mendoza MC, Er EE and Blenis J: The
Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends
Biochem Sci. 36:320–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trisciuoglio D, Iervolino A, Zupi G and
Del Bufalo D: Involvement of PI3K and MAPK signaling in
bcl-2-induced vascular endothelial growth factor expression in
melanoma cells. Mol Biol Cell. 16:4153–4162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adeyinka A, Nui Y, Cherlet T, Snell L,
Watson PH and Murphy LC: Activated mitogen- activated kinase
expression during human breast tumorigenesis and breast cancer
progression. Clin Cancer Res. 8:1747–1753. 2002.PubMed/NCBI
|
|
12
|
Kress TR, Raabe T and Feller SM: High Erk
activity suppresses expression of the cell cycle inhibitor p27Kip1
in colorectal cancer cells. Cell Commun Signal. 8:12010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uzgare AR, Kaplan PJ and Greenberg NM:
Differential expression and/or activation of P38MAPK, erk1/2, and
jnk during the initiation and progression of prostate cancer.
Prostate. 55:128–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin HL, Yang MH, Wu CW, Chen PM, Yang YP,
Chu YR, Kao CL, Ku HH, Lo JF, Liou JP, et al: 2-Methoxyestradiol
attenuates phosphatidylinositol 3-kinase/Akt pathway- mediated
metastasis of gastric cancer. Int J Cancer. 121:2547–2555. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An JY, Kim KM, Choi MG, Noh JH, Sohn TS,
Bae JM and Kim S: Prognostic role of p-mTOR expression in cancer
tissues and metastatic lymph nodes in pT2b gastric cancer. Int J
Cancer. 126:2904–2913. 2010.PubMed/NCBI
|
|
16
|
Miean KH and Mohamed S: Flavonoid
(myricetin, quercetin, kaempferol, luteolin, and apigenin) content
of edible tropical plants. J Agric Food Chem. 49:3106–3112. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishitani Y, Yamamoto K, Yoshida M, Azuma
T, Kanazawa K, Hashimoto T and Mizuno M: Intestinal
anti-inflammatory activity of luteolin: Role of the aglycone in
NF-κB inactivation in macrophages co-cultured with intestinal
epithelial cells. Biofactors. 39:522–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashokkumar P and Sudhandiran P: Protective
role of luteolin on the status of lipid peroxidation and
antioxidant defense against azoxymethane-induced experimental colon
carcinogenesis. Biomed Pharmacother. 62:590–597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ashokkumar P and Sudhandiran G: Luteolin
inhibits cell proliferation during Azoxymethane-induced
experimental colon carcinogenesis via Wnt/β-catenin pathway. Invest
New Drugs. 29:273–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakurai MA, Ozaki Y, Okuzaki D, Naito Y,
Sasakura T, Okamoto A, Tabara H, Inoue T, Hagiyama M, Ito A, et al:
Gefitinib and luteolin cause growth arrest of human prostate cancer
PC-3 cells via inhibition of cyclin G-associated kinase and
induction of miR-630. PLoS One. 9:e1001242014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu B, Zhang Q, Shen WM and Zhu J:
Anti-proliferative and chemosensitizing effects of luteolin on
human gastric cancer AGS cell line. Mol Cell Biochem. 313:125–132.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang HY, Quan K, Jiang YL, Wu JG and Tang
XW: Effect of Luteolin and its combination with chemotherapeutic
drugs on cytotoxicity of cancer cells. Zhejiang Da Xue Xue Bao Yi
Xue Ban. 39:30–36. 2010.(In Chinese). PubMed/NCBI
|
|
23
|
Ding S, Hu A, Hu Y, Ma J, Weng P and Dai
J: Anti-hepatoma cells function of luteolin through inducing
apoptosis and cell cycle arrest. Tumour Biol. 35:3053–3060. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi RX, Ong CN and Shen HM: Luteolin
sensitizes tumor necrosis factor-alpha-induced apoptosis in human
tumor cells. Oncogene. 23:7712–7721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Manju V and Nalini N: Protective role of
luteolin in 1,2-dimethylhydrazine induced experimental colon
carcinogenesis. Cell Biochem Funct. 25:189–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Samy RP, Gopalakrishnakone P and
Ignacimuthu S: Anti-tumor promoting potential of luteolin against
7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Chem
Biol Interact. 164:1–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amin AR, Kucuk O, Khuri FR and Shin DM:
Perspectives for cancer prevention with natural compounds. J Clin
Oncol. 27:2712–2725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pandurangan AK, Dharmalingam P, Sadagopan
SK Ananda and Ganapasam S: Effect of luteolin on the levels of
glycoproteins during azoxymethane-induced colon carcinogenesis in
mice. Asian Pac J Cancer Prev. 13:1569–1573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sagawa H, Naiki-Ito A, Kato H, Naiki T,
Yamashita Y, Suzuki S, Sato S, Shiomi K, Kato A, Kuno T, et al:
Connexin 32 and luteolin play protective roles in non-alcoholic
steatohepatitis development and its related hepatocarcinogenesis in
rats. Carcinogenesis. 36:1539–1549. 2015.PubMed/NCBI
|
|
30
|
Kasala ER, Bodduluru LN, Barua CC and
Gogoi R: Antioxidant and antitumor efficacy of Luteolin, a dietary
flavone on benzo(a)pyrene-induced experimental lung carcinogenesis.
Biomed Pharmacother. 82:568–577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu Xueying, Li Yanhong, Xiao Xiangwen,
Akber Aisa Haji and Li Xiaobo: Study on inhibition of luteolin on
proliferation of human gastric cancer cell line BGC-823. Mod J
Integr Tradit Chinese Western Med. 3:246–249. 2012.
|
|
33
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pradelli LA, Bénéteau M and Ricci JE:
Mitochondrial control of caspase-dependent and -independent cell
death. Cell Mol Life Sci. 67:1589–1597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Otera H and Mihara K: Mitochondrial
dynamics: Functional link with apoptosis. Int J Cell Biol.
2012:8216762012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar A, Ganini D and Mason RP: Role of
cytochrome c in α-synuclein radical formation: Implications of
α-synuclein in neuronal death in Maneb- and paraquat-induced model
of Parkinson's disease. Mol Neurodegener. 11:702016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lopez-Cruzan M, Sharma R, Tiwari M,
Karbach S, Holstein D, Martin CR, Lechleiter JD and Herman B:
Caspase-2 resides in the mitochondria and mediates apoptosis
directly from the mitochondrial compartment. Cell Death Discov.
2(pii): 160052016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rong Y and Distelhorst CW: Bcl-2 protein
family members: Versatile regulators of calcium signaling in cell
survival and apoptosis. Annu Rev Physiol. 70:73–91. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li C, Wu X, Sun R, Zhao P, Liu F and Zhang
C: Croton tiglium extract induces apoptosis via Bax/Bcl-2 pathways
in human lung cancer A549 cells. Asian Pac J Cancer Prev.
17:4893–4898. 2016.PubMed/NCBI
|
|
41
|
Zhang S, Qin F, Yang L, Xian J, Zou Q, Jin
H, Wang L and Zhang L: Nucleophosmin mutations induce
chemosensitivity in THP-1 leukemia cells by suppressing NF-κB
Activity and regulating Bax/Bcl-2 expression. J Cancer.
7:2270–2279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Csibi A, Fendt SM, Li C, Poulogiannis G,
Choo AY, Chapski DJ, Jeong SM, Dempsey JM, Parkhitko A, Morrison T,
et al: The mTORC1 pathway stimulates glutamine metabolism and cell
proliferation by repressing SIRT4. Cell. 153:840–854. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sapey E, Greenwood H, Walton G, Mann E,
Love A, Aaronson N, Insall RH, Stockley RA and Lord JM:
Phosphoinositide 3-kinase inhibition restores neutrophil accuracy
in the elderly: Toward targeted treatments for immunosenescence.
Blood. 123:239–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Patterson KI, Brummer T, O'Brien PM and
Daly RJ: Dual-specificity phosphatases: Critical regulators with
diverse cellular targets. Biochem J. 418:475–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Theodosiou A and Ashworth A: MAP kinase
phosphatases. Genome Biol. 3:REVIEWS3009. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Camps M, Nichols A and Arkinstall S: Dual
specificity phosphatases: A gene family for control of MAP kinase
function. FASEB J. 14:6–16. 2000.PubMed/NCBI
|
|
47
|
Keyse SM: Protein phosphatases and the
regulation of mitogen-activated protein kinase signaling. Curr Opin
Cell Biol. 12:186–192. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chalabi-Dchar M, Cassant-Sourdy S, Duluc
C, Fanjul M, Lulka H, Samain R, Roche C, Breibach F, Delisle MB,
Poupot M, et al: Loss of somatostatin receptor subtype 2 promotes
growth of KRAS-induced pancreatic tumors in mice by activating PI3K
signaling and overexpression of CXCL16. Gastroenterology.
148:1452–1465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chiu FL and Lin JK: Downregulation of
androgen receptor expression by luteolin causes inhibition of cell
proliferation and induction of apoptosis in human prostate cancer
cells and xenografts. Prostate. 68:61–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lim DY, Jeong Y, Tyner AL and Park JH:
Induction of cell cycle arrest and apoptosis in HT-29 human colon
cancer cells by the dietary compound luteolin. Am J Physiol
Gastrointest Liver Physiol. 292:G66–G75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chang L and Karin M: Mammalian MAP kinas
signaling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK Pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Oltersdorf T, Elmore SW, Shoemaker AR,
Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges
J, Hajduk PJ, et al: An inhibitor of Bcl-2 family proteins induces
regression of solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang CY and Tan TH: DUSPs, to MAP kinases
and beyond. Cell Biosci. 2:242012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jeffrey KL, Camps M, Rommel C and Mackay
CR: Targeting dual-specificity phosphatases: Manipulating MAP
kinase signaling and immune responses. Nat Rev Drug Discov.
6:391–403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nunes-Xavier C, Romá-Mateo C, Ríos P,
Tárrega C, Cejudo-Marín R, Tabernero L and Pulido R:
Dual-specificity MAP kinase phosphatases as targets of cancer
treatment. Anticancer Agents Med Chem. 11:109–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Duronio V: The life of a cell: Apoptosis
regulation by the PI3K/PKB pathway. Biochem J. 415:333–344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Guo C, Yang M, Jing L, Wang J, Yu Y, Li Y,
Duan J, Zhou X, Li Y and Sun Z: Amorphous silica nanoparticles
trigger vascular endothelial cell injury through apoptosis and
autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and
PI3K/Akt/mTOR signaling. Int J Nanomedicine. 11:5257–5276. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gardai SJ, Hildeman DA, Frankel SK,
Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL and
Henson PM: Phosphorylation of Bax Ser184 by Akt regulates its
activity and apoptosis in neutrophils. J Biol Chem.
279:21085–21095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yao R and Cooper GM: Requirement for
phosphatidylinositol-3 kinase in the prevention of apoptosis by
nerve growth factor. Science. 267:2003–2006. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Scheid MP, Lauener RW and Duronio V: Role
of phosphatidylinositol 3-OH-kinase activity in the inhibition of
apoptosis in haemopoietic cells: Phosphatidylinositol 3-OH-kinase
inhibitors reveal a difference in signalling between interleukin-3
and granulocyte-macrophage colony stimulating factor. Biochem J.
312:159–162. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pande M, Bondy ML, Do KA, Sahin AA, Ying
J, Mills GB, Thompson PA and Brewster AM: Association between
germline single nucleotide polymorphisms in the PI3K-AKT-mTOR
pathway, obesity, and breast cancer disease-free survival. Breast
Cancer Res Treat. 147:381–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Luster AD: Chemokines-chemotactic
cytokines that mediate inflammation. N Engl J Med. 338:436–445.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Struyf S, Proost P and Van Damme J:
Regulation of the immune response by the interaction of chemokines
and proteases. Adv Immunol. 81:1–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Guerreiro R, Santos-Costa Q and
Azevedo-Pereira JM: The chemokines and their receptors:
Characteristics and physiological functions. Acta Med Port. 24
Suppl 4:S967–S976. 2011.(In Portuguese).
|
|
68
|
Xing YN, Xu XY, Nie XC, Yang X, Yu M, Xu
HM, Liu YP, Takano Y and Zheng HC: Role and clinicopath- ologic
significance of CXC chemokine ligand 16 and chemokine (C-X-C motif)
receptor 6 expression in gastric carcinomas. Hum Pathol.
43:2299–2307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang J, Lu Y, Wang J, Koch AE, Zhang J and
Taichman RS: CXCR6 induces prostate cancer progression by the
AKT/Mammalian target of rapamycin signaling pathway. Cancer Res.
68:10367–10376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chandrasekar B, Bysani S and Mummidi S:
CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, I kappa
B kinase, and nuclear factor-kappa B and induces cell-cell adhesion
and aortic smooth muscle cell proliferation. J Biol Chem.
279:3188–3196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Deng L, Chen N, Li Y, Zheng H and Lei Q:
CXCR6/CXCL16 functions as a regulator in metastasis and progression
of cancer. Biochim Biophys Acta. 1806:42–49. 2010.PubMed/NCBI
|
|
72
|
Singh R, Kapur N, Mir H, Singh N, Lillard
JW Jr and Singh S: CXCR6-CXCL16 axis promotes prostate cancer by
mediating cytoskeleton rearrangement via Ezrin activation and αvβ3
integrin clustering. Oncotarget. 7:7343–7353. 2016.PubMed/NCBI
|
|
73
|
Hu ZB, Chen Y, Gong YX, Gao M, Zhang Y,
Wang GH, Tang RN, Liu H, Liu BC and Ma KL: Activation of the
CXCL16/CXCR6 pathway by inflammation contributes to atherosclerosis
in patients with End-stage renal disease. Int J Med Sci.
13:858–867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hald SM, Kiselev Y, Al-Saad S, Richardsen
E, Johannessen C, Eilertsen M, Kilvaer TK, Al-Shibli K, Andersen S,
Busund LT, et al: Erratum to: Prognostic impact of CXCL16 and CXCR6
in non-small cell lung cancer: Combined high CXCL16 expression in
tumor stroma and cancer cells yields improved survival. BMC Cancer.
16:9162016. View Article : Google Scholar : PubMed/NCBI
|