Ovarian cancer is the most life-threatening type of
gynecological cancer in females. According to Cancer Statistics
(1), there were an estimated 22,440
novel cases of ovarian cancer in 2017. In addition, the number of
ovarian cancer-associated mortalities was 14,080, which was the
fifth most common cause of cancer-associated mortality in American
females (1). Epithelial ovarian
cancer (EOC) is the most common histological type of ovarian
cancer, with ~75% cases of ovarian cancer diagnosed at an advanced
stage (FIGO stages III–IV) and the overall survival rate of EOC
ranging between 15 and 30% for the last 20 years (2). The exact cause of ovarian cancer remains
unclear; however, a number of associated risk factors have been
identified. Ovulation-associated factors including pregnancy
frequency, breastfeeding, early menarche, late menopause and the
use of the oral contraceptive pill were all associated with EOC. In
addition, hereditary factors served an important function in the
development of ovarian cancer, with females who had a family
history of ovarian cancer, personal history of breast cancer or
alteration in breast cancer early onset (BRCA)1 or BRCA2 genes
contributed to an increased risk of EOC. Furthermore, inflammation
was a risk factor of EOC, and females who have experienced pelvic
inflammatory disease (PID), endometriosis or frequent exposure to
talc and asbestos were identified to exhibit an increased risk of
ovarian cancer (3). Lin et al
identified that females with an episode of clinically apparent PID
exhibited a 1.9-fold increase in the development of EOC, whereas
females who had experienced ≥5 episodes of PID exhibited a 2.5-fold
increased risk. In addition, patients with PID aged ≤35 years were
at an increased risk of developing ovarian cancer compared with the
control population during 1–3 years of follow-up (4). In early 1995, a case-control study,
including 450 females with ovarian cancer and 564 controls,
revealed that 23.1% of cases and 18.1% of controls had PID and,
adjusted for age, smoking, country of birth, parity, duration of
oral contraceptive use and abortion, the odds ratio was 1.53 [95%
confidence interval (CI), 1.10–2.13; P=0.012] (5). Furthermore, females with recurrent PID
presented an increased risk (odds ratio, 1.88; 95% CI, 1.13–3.12;
P=0.014), which suggested that PID may increase the risk of
developing ovarian cancer (5). In a
Chinese study conducted in 1989, PID was associated with an
increased risk of inducing ovarian cancer (odds ratio, 3.0; 95% CI,
0.30–30.2) (6).
Malignancies are hypothesized to be partially
initiated by microbial infections and >15% of malignancies may
be attributed to infections (7).
According to the International Agency for Research on Cancer
(IARC), 6 viruses and 1 bacterium are certified as causes of
cancer, which are human papillomavirus (HPV), hepatitis B virus
(HBV), hepatitis C virus (HCV), Epstein-Barr virus (EBV), human
T-lymphotropic virus 1 and Kaposi's sarcoma-associated herpes
virus, and Helicobacter pylori, respectively. Persistent
infections induce chronic inflammation which may cause successive
inflammatory reactions, thus dysregulated innate or adaptive immune
responses may be pro-tumorigenic (8).
In the present review, studies of microorganisms that may induce
ovarian carcinogenesis are summarized and the underlying molecular
mechanisms that link chronic inflammation to ovarian cancer are
examined.
HPV is one of the most common types of sexually
transmitted virus in the world. In the USA, the overall HPV
prevalence was 26.8% in females aged between 14 and 59 years;
however, in females aged between 20 and 24 years, the HPV
prevalence rate was ≤44.8% (49). In
China, a pooled analysis of 17 population-based studies, including
30,207 females, identified the HPV prevalence to be 17.7% (50). Furthermore, a province-wide study was
conducted in Ningbo province which identified 185 of 1,373 females
aged between 22 and 64 years (13.5%) as HPV-positive and the
prevalence rates were 13.8, 8.8 and 7.9% in Shenzhen, Xinjiang and
Chaozhou, respectively (51–54). The papillomavirus family is
heterogeneous and highly species-specific. Over 200 HPV genotypes
have been identified and these are subdivided into three broad
categories, depending on their oncogenic potential. High-risk HPV
types include HPV-16, HPV-18, HPV-31, HPV-33, HPV-35, HPV-45,
HPV-51, HPV-52, HPV-56, HPV-58, HPV-59, HPV-68 andHPV-73. HPV-16
and HPV-18 are the most common types of HPV identified in
malignancies (55). In addition, HPV
was demonstrated to be positive in a number of types of malignancy.
According to Tang et al (56),
HPV was detected in 96.6% of cervical carcinomas, in 14.1% of head
and neck squamous cell carcinomas, and in bladder urothelial
carcinoma and lung squamous cell carcinoma. Whether HPV infection,
C. trachomatis or M. genitalium are associated with
ovarian cancer is debated. A previous meta-analysis, summarizing 24
primary studies from 11 countries on 3 continents, contained
information on HPV and ovarian cancer and included 880 subjects
(57). This study identified an
association between HPV infection and ovarian cancer, as HPV
prevalence in patients with ovarian cancer was demonstrated to be
17.5%. In Saudi patients, an increased proportion of HPV-16 and
HPV-18 was observed in 42.9 and 26.2% ovarian cancer tissues,
respectively (58). In addition,
previous studies identified that increased-risk HPV DNA may be
determined in ovarian serous carcinomas and present in females at a
high risk of developing EOC (including females with ≥2 first-degree
relatives with ovarian or breast cancer or females with mutations
in BRCA1 or BRCA2 genes) (59,60).
However, a study from Iraq identified that HPV-16 existed in only
9.67% of malignant ovarian epithelial tumors, which suggested that
HPV infection served a relatively minor function in the
pathogenesis of ovarian cancer (61).
HPV replicates and assembles exclusively in the
nucleus, and initially establishes infection in undifferentiated
and actively proliferating cells in the basal layer of epithelium
cells. During carcinogenic progression, the HPV genome typically
integrates into a host cell chromosome, and the viral oncoproteins
E6 and E7 induce cell immortalization and transformation (62). E6 and E7 inactivate two cellular tumor
suppressor proteins: p53 and retinoblastoma protein (63). HPV-16 E7 increased the retention of
γ-H2A histone family, member X (a marker for cellular response to
DNA damage) and decreased sublethal DNA damage repair in head and
neck cancer cells. The results of this study suggested that E7
expression markedly delayed radiation-induced DNA damage repair in
head and neck normal epithelial cells, and head and neck cancer
cells (64). In addition, HPV E6/E7
were identified to increase the intracellular expression of the
oncogenic microRNA 17–92 cluster and decrease the expression of the
anti-proliferative p21 gene in HPV-positive cancer cells (65). According to Gregoire et al
(66), introduction of HPV-16 E6/E7
genes into human ovarian surface epithelial (HOSE) cells may extend
the lifespan and induce malignant transformation of these
cells.
Inflammation is a biological response to disrupted
tissue homeostasis and possesses four essential factors including
inducers, sensors, inflammatory mediators and target tissues
(78). Microbial infection is one of
the most common types of inducer which promotes inflammatory
responses; other inducers include autoimmune diseases and agnogenic
inflammatory diseases (79).
Inflammatory sensors include macrophages, dendritic cells, mast
cells, T cells, B cells, fibrocytes and endothelial cells.
Inflammation is mediated by immune cells as an immediate defense in
response to infection or injury by noxious stimuli. Innate immune
cells, including neutrophils, mast cells and macrophages, exhibit
receptors that signal the activation and production of an array of
biologically active proteins and defense molecules, in response to
detrimental substances and damaged or altered self-molecules
(80). Inflammatory mediators
including inflammatory cytokines, chemokines, growth factors,
reactive oxygen and nitrogen species and cytokines, secreted by
inflammatory cells, cause genomic alterations in the epithelium and
subsequent cancer initiation. Chronic infections are responsible
for 15% of malignancies worldwide (81,82). For
example, GM-CSF/IFN-γ-and GM-CSF/IL-3/IFN-γ-deficient mice were
administered with acute and chronic inflammatory reactions in a
number of organs, particularly in the lungs, soft tissues, lymph
nodes, ovaries, adrenal glands and the liver. A previous study
indicated that the cause of cancer formation in these mice was
bacterial, presumably due to the creation of a persistent
inflammatory response (83). There
have been notable studies which identified that factors associated
with immune responses may alter the pathogenesis and initiation of
ovarian cancer, through genetic and protein analysis (84). According to Curiel et al
(85), regulatory T (Treg) cells
served a substantial role in ovarian cancers, and blocking Treg
cell migration or function may aid ovarian cancer therapy.
Furthermore, Treg cells were associated with an increased risk of
mortality and decreased survival time. C-C motif chemokine 22,
produced by the tumor microenvironment, was suggested to be a
mediator of Treg cells and tumors (85). According to Block et al
(86), pro-inflammatory factor
NF-κB-associated single-nucleotide polymorphisms were associated
with the overall survival time of patients with ovarian cancer. A
case-control study, including 7,776 cases and 11,843 controls,
revealed that regular use of non-steroidal anti-inflammatory drugs,
including aspirin, decreased the risk of ovarian cancer (87).
Inflammation-induced angiogenesis is associated with
a number of pathophysiological processes including tumor viability,
wound healing and ovulation. The process of angiogenesis is
regulated by angiogenic cytokines and growth factors secreted by
inflammatory cells. Inflammation and hypoxia are two primary types
of angiogenesis inducers. Vascular endothelial growth factor
(VEGF), induced by chronic inflammation, serves functions in tumor
angiogenesis, viability and metastasis, and targeting VEGF to
inhibit angiogenesis may prevent cancer progression (88,89). The
NF-κB pathway, critical for pro-inflammatory gene expression, is
considered to exhibit a function in angiogenesis of tumor and
inflamed tissues, and the NF-κB-inducing kinase may be a
therapeutic target in chronic inflammatory diseases and tumor
neoangiogenesis (90). Hypoxia, when
tissues lack oxygen, induces angiogenesis and inflammation, and
hypoxia-inducible factor (HIF)-1 activation is well-known as an
adaptive strategy to hypoxia and consists of two subunits: HIF-1α
and HIF-1β. HIF-1 activates transcription of genes encoding
angiogenic growth factors, including VEGF, angiopoietin (ANGPT)1,
ANGPT2 and platelet-derived growth factor, which are secreted by
hypoxic cells and stimulate epithelial cells, resulting in
angiogenesis (91). Angiogenesis has
been identified as a necessary process for oncogenesis and
subsequent tumor growth. Exosomes, extracted from high-grade
ovarian cancer cells, induce angiogenesis, and activating
transcription factor 2 and metastasis-associated 1 may serve a key
function in exosomal enhancement of tumor development (92). In addition, angiogenesis is associated
with the formation of malignant ascites in ovarian cancer (93). Anti-angiogenesis therapy is regarded
as a novel effective therapeutic strategy for ovarian cancer and a
number of clinical trials have validated angiogenesis as a target
in ovarian cancer, through the addition of VEGF pathway inhibitors,
including the monoclonal anti-VEGF antibody bevacizumab. A previous
meta-analysis, including 12 studies, demonstrated that the
incorporation of anti-angiogenesis therapy was markedly associated
with an improved clinical outcome (94,95).
Microbial infection which induces angiogenesis serves a vital
function in tumorigenesis. According to Li et al (96), lung cancer cells which overexpress
HPV-16 E6 and E7 oncoproteins markedly stimulate capillary tube
formation of human umbilical vein endothelial cells in vitro
and increase tumor angiogenesis, HIF-1α and VEGF proteins in
vitro and in vivo. HCMV infection is present in >90%
of glioblastoma multiforme (GBM) and HCMV viral protein pp71
induced the production of stem cell factor (SCF), an important
pro-angiogenic factor in GBM. Furthermore, the secretion of SCF
stimulated by pp71 requires the activation of the NF-κB signaling
pathway (97).
Epithelial-mesenchymal transition (EMT) is a
biological process where epithelial cells lose their planar and
apical-basal polarity and cell-cell adhesion, and gain migratory
and invasive properties to become mesenchymal stem cells. EMT was
first recognized as a feature of embryogenesis and it additionally
occurs in wound healing, organ fibrosis, cancer progression and
cancer metastasis (98). EMT is
characterized primarily by the loss of epithelial (E-)cadherin and
a number of transcription factors have been identified to repress
E-cadherin. Zinc finger protein SNAI1 (Snail), zinc finger
E-box-binding homeobox (ZEB), E2A immunoglobulin-enhancer binding
factor (E47) and Krüppel-like factor 8 bound to and repressed the
activity of the E-cadherin promoter; whereas Twist-related protein
1 (Twist) and FOXC2 were common factors that repressed E-cadherin
transcription indirectly. Furthermore, a previous study
demonstrated that EMT was utilized by cancer cells to enhance
aggressiveness by acquiring chemoresistance and stem-cell-like
properties and escaping from host immunity (99). Snail was upregulated in ovarian cancer
and was identified to be positively associated with the expression
of fibronectin and neuralcadherin, but was negatively associated
with the expression of E-cadherin and β-catenin (100). Oncogene high-mobility group AT-Hook
2 was revealed to be a EMT-associated gene that was overexpressed
in OSE cell lines and assisted in the understanding of the
tumorigenesis of ovarian serous carcinoma (101). In addition, EMT is associated with
ovarian cancer which is resistant to conventional chemotherapy. A
previous study identified EMT genes, including Snail, zinc finger
protein SNAI2 (Slug), Twist2 and Zeb2, upregulated in
cisplatin-resistant ovarian cancer cell lines; however, following
knockdown of the Snail and Slug genes, the EMT phenotype was
reversed and drug sensitivity was restored (102). Inflammatory factors tumor necrosis
factor-α, transforming growth factor (TGF)-β1 and IL-6 induced EMT
in inflammatory breast cancer cells, through the NF-κB and STAT3
signaling pathways (103). Microbes
including Helicobacter, Mycoplasma hyorhinis, Citrobacter
rodentium, EBV and HCV were reported to induce EMT (104–108).
The components and products of bacteria are considered to serve
functions in the induction of EMT. Li et al (109) identified that lipopolysaccharide
(LPS) promoted invasion and metastasis of liver hepatocellular
carcinoma HepG2 cells and downregulated the expression of
E-cadherin, suggesting that TLR4 may involve the process of EMT.
Furthermore, LPS was demonstrated to decrease E-cadherin expression
in intrahepatic biliary epithelial cells (HIBEpiCs) and increased
the mesenchymal markers S100 calcium-binding protein A1 and sterile
α motif. In addition, it was hypothesized that LPS induced EMT of
HIBEpiCs, through the TGF-β1/Smad2/3 signaling pathway (110). Flagellin and muramyl dipeptides are
two bacterial products that maybe associated with EMT. A previous
study revealed that flagellin induced EMT by activating NF-κB and
mitogen-activated protein kinase (MAPK), but failed to increase the
level of Snail in A549 adenocarcinomic human alveolar basal
epithelial cells and BEAS-2B human bronchial epithelial cells
(111). Muramyl dipeptides were
considered to activate nucleotide-binding oligomerization
domain-like receptors (NOD) and subsequently recruit
receptor-interacting serine/threonine kinase 2, a kinase required
for NOD-mediated NF-κB and MAPK activation (112). A previous study revealed that EBV
induced EMT of human corneal epithelial cells through activation of
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and
extracellular-signal-regulated kinase (ERK) signaling pathways
(113). The EBV latent membrane
protein 1 (LMP1) and 2A (LMP2A) are involved in EMT; it was first
identified that LMP1 induced EMT via Twist in nasopharyngeal
carcinoma tissues. Furthermore, LMP1 in lung epithelium predisposed
cells to undergo EMT by enhancing signaling through the ERK
signaling pathway and the interaction with TGF-β1 may also
stimulate EMT (114,115). In addition, HCV induces EMT: HCV
core protein repressed E-cadherin expression by upregulating
E12/E47 to induce EMT. In cholangiocarcinoma, HCV has been shown to
induce EMT, promoting carcinoma progression through a mechanism
dependent on the lysyloxidase-like 2 signaling pathway (116).
CSCs are defined as cells that possess the capacity
of self-renewal, cancer viability, metastasis, recurrence and they
are not sensitive to radio- and chemotherapy. CSCs exist in a
number of types of cancer, including ovarian, breast, colon,
prostate and leukemia. Bapat et al (117) were the first to isolate ovarian CSCs
from ascites of patients with ovarian cancer, 19 of which were
spontaneously immortalized and two cell lines were characterized
with CSCs that presented the specific markers of stem cells
including Nestin, octamer-binding transcription factor 4 and Nanog.
Additional biomarkers of ovarian CSCs include aldehyde
dehydrogenases, cluster of differentiation (CD)44, CD133, CD24,
epithelial cell adhesion molecule, CD117, lymphocyte antigen 6
complex, locus A and leucine-rich repeat-containing
G-protein-coupled receptor 5 (118).
CSCs interact with, and are regulated by, a number of signaling
pathways and cytokines inthe tumor microenvironment. Inflammatory
cytokines, including IL-1, IL-6 and IL-8, activate the STAT3/NF-κB
signaling pathway in tumor and stromal cells, which stimulates
cytokine production and self-renewal of CSCs. The positive-feedback
loops contribute to the interactions between chronic inflammation
and cancer (119). NF-κB is a
primary source of pro-inflammatory cytokines and a previous study
demonstrated that NF-κB inhibitors may induce cell death in ovarian
CSCs, which prevented cancer recurrence and chemoresistance
(120). In addition, TLRs that
respond to PAMPs served an important function in EOC stem cells and
TLR2, TLR4, TLR5 and TLR9 recognize primarily bacterial products,
whereas TLR3 and TLR8 recognize viral components. A previous study
identified the TLR2-myeloid differentiation primary response gene
88 (MyD88)-NF-κB signaling pathway as being able to promote tumor
repair and enhance self-renewal in
CD44+/MyD88+ EOC stem cells (121). IL-17, secreted by T helper 17 cells
and macrophages in the tumor microenvironment, binds to IL-17
receptor, overexpressed in ovarian CD133+ cancer
stem-like cells, and subsequently increased the tumorigenic
potential and self-renewal, through the NF-κB and p38 MAPK
signaling pathway in vitro and in vivo (122). Cell viability of ovarian cancer
cells was markedly increased following co-culture with
carcinoma-associated mesenchymal stem cells (CA-MSCs), which
existed in the tumor microenvironment and protected ovarian cancer
cells from carboplatin-induced viability inhibition and apoptosis.
Furthermore, phosphorylation of Akt and X-linked inhibitor of
apoptosis protein served an important function in stimulating
CA-MSC-secreted factors which protect ovarian cancers from
carboplatin-induced apoptosis (123).
The tumor microenvironment is composed of distinct
cellular and structural factors, including the vasculature,
immune-associated cells, fibroblasts and extracellular matrix.
Cancer-associated fibroblasts (CAFs) exhibit a function in
tumor-stroma crosstalk. Fibroblasts are involved in tissue repair,
the inflammatory response, human tumorigenesis and metastasis. EBV
infection was suggested to induce myofibroblast activation in
scleroderma (SSc) fibroblasts and the profibroblast-associated
factors, including TGF-β1, endothelin 1, SMA as well as TGF
β-regulated genes such as early growth response 1, plasminogen
activator inhibitor-1, cartilage oligomeric matrix protein and
basement membrane-zone genes coding for collagen IV, were
up-regulated in SSc fibroblasts (124). LPS of bacteria directly induces lung
fibroblast viability by activating TLR4 signaling, and TLR4-induced
activation of the PI3K-Aktsignaling pathway and downregulation of
phosphatase and tensin homolog served a function in the process
(125). In ovarian cancer, CAFs
upregulated the expression of the pro-inflammatory factors IL-6,
cyclooxygenase-2 and the chemokine (C-X-C motif) ligand 1.
Furthermore, NF-κB expression was enhanced by CAFs, which suggested
that pro-tumorigenic signaling in the microenvironment of ovarian
tumors via the NF-κB signaling pathway was mediated partly by CAFs
(126). TGF-β is a
fibrosis-associated cytokine that serves a significant function in
cancer invasion and metastasis. Ovarian cancer cells exhibited
increased motility when co-cultured with fibroblasts in the
presence of exogenous of TGF-β1 and TGF-β2, suggesting that TGF-β
modulated molecular crosstalk between ovarian cancer cells and CAFs
in ovarian cancer microenvironment (127). In addition, CAFs may facilitate the
invasiveness of originally non-invasive cancer cells, through
protease-activated receptor-dependent Ca2+ signals and
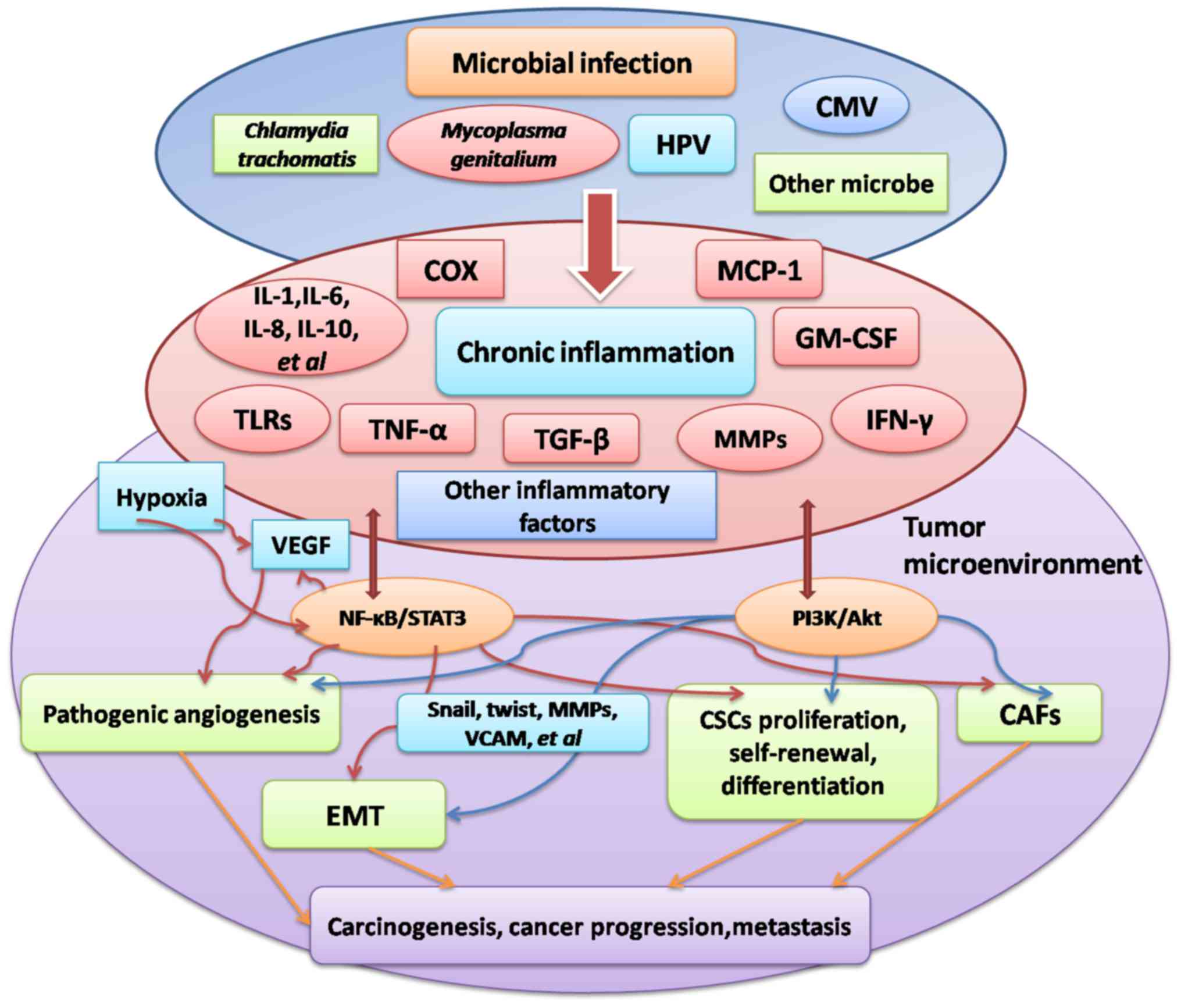
matrix metalloproteinase-1 upregulation (Fig. 1) (128,129).
Chronic inflammation serves an important function in
stimulating tumorigenesis. A number of large case-control studies
have identified an association between PID and ovarian cancer
(4–6).
Microorganisms including C. trachomatis, M.
genitalium, HPV and CMV have been identified to induce ovarian
cancer and other types of malignancy. In addition, angiogenesis,
EMT, CSCs and CAFs are important factors that lead to ovarian
cancer progression, viability and metastasis, and may be stimulated
by microbial infection. Furthermore, the four processes are
essential for the interaction between inflammation and the tumor
microenvironment. The pathogenesis of inflammation-associated
cancer remains unclear and a limited number of therapeutic methods
are currently used to treat cancer. Therefore, the present review
aimed at describing the function of microbial infection in inducing
ovarian cancer and in the tumor microenvironment, and to assist the
development of novel strategies to prevent and treat ovarian
cancer.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herzog TJ and Pothuri B: Ovarian cancer: A
focus on management of recurrent disease. Nat Clin Pract Oncol.
3:604–611. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hunn J and Rodriguez GC: Ovarian cancer:
Etiology, risk factors, and epidemiology. Clin Obstet Gynecol.
55:3–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin HW, Tu YY, Lin SY, Su WJ, Lin WL, Lin
WZ, Wu SC and Lai YL: Risk of ovarian cancer in women with pelvic
inflammatory disease: A population-based study. Lancet Oncol.
12:900–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Risch HA and Howe GR: Pelvic inflammatory
disease and the risk of epithelial ovarian cancer. Cancer Epidemiol
Biomarkers Prev. 4:447–451. 1995.PubMed/NCBI
|
|
6
|
Shu XO, Brinton LA, Gao YT and Yuan JM:
Population-based case-control study of ovarian cancer in Shanghai.
Cancer Res. 49:3670–3674. 1989.PubMed/NCBI
|
|
7
|
Kuper H, Adami HO and Trichopoulos D:
Infections as a major preventable cause of human cancer. J Intern
Med. 248:171–183. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garrett WS: Cancer and the microbiota.
Science. 348:80–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Centers for Disease Control and
Prevention1, ; Workowski KA and Berman SM: Sexually transmitted
diseases treatment guidelines, 2006. MMWR Recomm Rep. 55:1–94.
2006.
|
|
10
|
Ladany S and Sarov I: Recent advances in
chlamydia trachomatis. Eur J Epidemiol. 1:235–256. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moulder JW: Interaction of chlamydiae and
host cells in vitro. Microbiol Rev. 55:143–190. 1991.PubMed/NCBI
|
|
12
|
Molano M, Meijer CJ, Weiderpass E, Arslan
A, Posso H, Franceschi S, Ronderos M, Muñoz N and van den Brule AJ:
The natural course of Chlamydia trachomatis infection in
asymptomatic Colombian women: A 5-year follow-up study. J Infect
Dis. 191:907–916. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tosi MF: Innate immune responses to
infection. J Allergy Clin Immunol. 116:241–249; quiz 250. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
den Hartog JE, Morré SA and Land JA:
Chlamydia trachomatis-associated tubal factor subfertility:
Immunogenetic aspects and serological screening. Hum Reprod Update.
12:719–730. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sellami H, Said-Sadier N, Znazen A, Gdoura
R, Ojcius DM and Hammami A: Chlamydia trachomatis infection
increases the expression of inflammatory tumorigenic cytokines and
chemokines as well as components of the Toll-like receptor and
NF-κB pathways in human prostate epithelial cells. Mol Cell Probes.
28:147–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
King AE, Wheelhouse N, Cameron S, McDonald
SE, Lee KF, Entrican G, Critchley HO and Horne AW: Expression of
secretory leukocyte protease inhibitor and elafin in human
fallopian tube and in an in-vitro model of Chlamydia trachomatis
infection. Hum Reprod. 24:679–686. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Derbigny WA, Shobe LR, Kamran JC, Toomey
KS and Ofner S: Identifying a role for Toll-like receptor 3 in the
innate immune response to Chlamydia muridarum infection in murine
oviduct epithelial cells. Infect Immun. 80:254–265. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frazer LC, Darville T, Chandra-Kuntal K,
Andrews CW Jr, Zurenski M, Mintus M, AbdelRahman YM, Belland RJ,
Ingalls RR and O'Connell CM: Plasmid-cured Chlamydia caviae
activates TLR2-dependent signaling and retains virulence in the
guinea pig model of genital tract infection. PLoS One.
7:e307472012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mascellino MT, Boccia P and Oliva A:
Immunopathogenesis in Chlamydia trachomatis infected women. ISRN
Obstet Gynecol. 2011:4369362011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans: Human papillomaviruses. IARC Monogr
Eval Carcinog Risks Hum. 90:1–636. 2007.PubMed/NCBI
|
|
21
|
Shanmughapriya S, Senthilkumar G,
Vinodhini K, Das BC, Vasanthi N and Natarajaseenivasan K: Viral and
bacterial aetiologies of epithelial ovarian cancer. Eur J Clin
Microbiol Infect Dis. 31:2311–2317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Idahl A, Lundin E, Jurstrand M, Kumlin U,
Elgh F, Ohlson N and Ottander U: Chlamydia trachomatis and
Mycoplasma genitalium plasma antibodies in relation to epithelial
ovarian tumors. Infect Dis Obstet Gynecol. 2011:8246272011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carvalho JP and Carvalho FM: Is
Chlamydia-infected tubal fimbria the origin of ovarian cancer? Med
Hypotheses. 71:690–693. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kessler M, Zielecki J, Thieck O,
Mollenkopf HJ, Fotopoulou C and Meyer TF: Chlamydia trachomatis
disturbs epithelial tissue homeostasis in fallopian tubes via
paracrine Wnt signaling. Am J Pathol. 180:186–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zügel U and Kaufmann SH: Role of heat
shock proteins in protection from and pathogenesis of infectious
diseases. Clin Microbiol Rev. 12:19–39. 1999.PubMed/NCBI
|
|
26
|
Pockley AG: Heat shock proteins as
regulators of the immune response. Lancet. 362:469–276. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Di Felice V, David S, Cappello F, Farina F
and Zummo G: Is chlamydial heat shock protein 60 a risk factor for
oncogenesis? Cell Mol Life Sci. 62:4–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsai YP, Yang MH, Huang CH, Chang SY, Chen
PM, Liu CJ, Teng SC and Wu KJ: Interaction between HSP60 and
beta-catenin promotes metastasis. Carcinogenesis. 30:1049–1057.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giaginis C, Daskalopoulou SS, Vgenopoulou
S, Sfiniadakis I, Kouraklis G and Theocharis SE: Heat Shock
Protein-27, −60 and −90 expression in gastric cancer: Association
with clinicopathological variables and patient survival. BMC
Gastroenterol. 9:142009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bodzek P, Partyka R and Damasiewicz-Bodzek
A: Antibodies against Hsp60 and Hsp65 in the sera of women with
ovarian cancer. J Ovarian Res. 7:302014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lim R, Lappas M, Ahmed N, Permezel M,
Quinn MA and Rice GE: 2D-PAGE of ovarian cancer: Analysis of
soluble and insoluble fractions using medium-range immobilized pH
gradients. Biochem Biophys Res Commun. 406:408–413. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tully JG, Taylor-Robinson D, Cole RM and
Rose DL: A newly discovered mycoplasma in the human urogenital
tract. Lancet. 1:1288–1291. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gibson DG, Benders GA, Andrews-Pfannkoch
C, Denisova EA, Baden-Tillson H, Zaveri J, Stockwell TB, Brownley
A, Thomas DW, Algire MA, et al: Complete chemical synthesis,
assembly, and cloning of a Mycoplasma genitalium genome. Science.
319:1215–1220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fraser CM, Gocayne JD, White O, Adams MD,
Clayton RA, Fleischmann RD, Bult CJ, Kerlavage AR, Sutton G, Kelley
JM, et al: The minimal gene complement of Mycoplasma genitalium.
Science. 270:397–403. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Manhart LE, Holmes KK, Hughes JP, Houston
LS and Totten PA: Mycoplasma genitalium among young adults in the
United States: An emerging sexually transmitted infection. Am J
Public Health. 97:1118–1125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Taylor-Robinson D and Jensen JS:
Mycoplasma genitalium: From Chrysalis to multicolored butterfly.
Clin Microbiol Rev. 24:498–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Idahl A, Lundin E, Elgh F, Jurstrand M,
Møller JK, Marklund I, Lindgren P and Ottander U: Chlamydia
trachomatis, Mycoplasma genitalium, Neisseria gonorrhoeae, human
papillomavirus, and polyomavirus are not detectable in human tissue
with epithelial ovarian cancer, borderline tumor, or benign
conditions. Am J Obstet Gynecol. 202:71.e1–e6. 2010. View Article : Google Scholar
|
|
38
|
Chan PJ, Seraj IM, Kalugdan TH and King A:
Prevalence of mycoplasma conserved DNA in malignant ovarian cancer
detected using sensitive PCR-ELISA. Gynecol Oncol. 63:258–260.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Quirk JT, Kupinski JM and DiCioccio RA:
Detection of mycoplasma ribosomal DNA sequences in ovarian tumors
by nested PCR. Gynecol Oncol. 83:560–562. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Namiki K, Goodison S, Porvasnik S, Allan
RW, Iczkowski KA, Urbanek C, Reyes L, Sakamoto N and Rosser CJ:
Persistent exposure to mycoplasma induces malignant transformation
of human prostate cells. PLoS One. 4:e68722009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang S, Wear DJ and Lo S: Mycoplasmal
infections alter gene expression in cultured human prostatic and
cervical epithelial cells. FEMS Immunol Med Microbiol. 27:43–50.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
McGowin CL, Popov VL and Pyles RB:
Intracellular Mycoplasma genitalium infection of human vaginal and
cervical epithelial cells elicits distinct patterns of inflammatory
cytokine secretion and provides a possible survival niche against
macrophage-mediated killing. BMC Microbiol. 9:1392009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsai S, Wear DJ, Shih JW and Lo SC:
Mycoplasmas and oncogenesis: Persistent infection and multistage
malignant transformation. Proc Natl Acad Sci USA. 92:pp.
10197–10201. 1995; View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Haggerty CL and Taylor BD: Mycoplasma
genitalium: An emerging cause of pelvic inflammatory disease.
Infect Dis Obstet Gynecol. 2011:9598162011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baczynska A, Funch P, Fedder J, Knudsen
HJ, Birkelund S and Christiansen G: Morphology of human Fallopian
tubes after infection with Mycoplasma genitalium and Mycoplasma
hominis-in vitro organ culture study. Hum Reprod. 22:968–979. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Crum CP, McKeon FD and Xian W: The oviduct
and ovarian cancer: Causality, clinical implications, and ‘targeted
prevention’. Clin Obstet Gynecol. 55:24–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
McGowin CL, Annan RS, Quayle AJ, Greene
SJ, Ma L, Mancuso MM, Adegboye D and Martin DH: Persistent
Mycoplasma genitalium infection of human endocervical epithelial
cells elicits chronic inflammatory cytokine secretion. Infect
Immun. 80:3842–3849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
McGowin CL, Radtke AL, Abraham K, Martin
DH and Herbst-Kralovetz M: Mycoplasma genitalium infection
activates cellular host defense and inflammation pathways in a
3-dimensional human endocervical epithelial cell model. J Infect
Dis. 207:1857–1868. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dunne EF, Unger ER, Sternberg M, McQuillan
G, Swan DC, Patel SS and Markowitz LE: Prevalence of HPV infection
among females in the United States. JAMA. 297:813–819. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhao FH, Lewkowitz AK, Hu SY, Chen F, Li
LY, Zhang QM, Wu RF, Li CQ, Wei LH, Xu AD, et al: Prevalence of
human papillomavirus and cervical intraepithelial neoplasia in
China: A pooled analysis of 17 population-based studies. Int J
Cancer. 131:2929–2938. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hong H, He TF, Ni HX, Zhang S and Xu GZ:
Prevalence and genotype distribution of HPV infection among women
in Ningbo, China. Int J Gynaecol Obstet. 131:96–99. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen Q, Xie LX, Qing ZR, Li LJ, Luo ZY,
Lin M, Zhang SM, Chen WZ, Lin BZ, Lin QL, et al: Epidemiologic
characterization of human papillomavirus infection in rural
Chaozhou, eastern Guangdong Province of China. PLoS One.
7:e321492012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang YY, Li L, Wei S, Peng J, Yuan SX, Xie
JS and Liu ZH: Human papillomavirus (HPV) infection in women
participating in cervical cancer screening from to 2010 in Shenzhen
city, South China. Asian Pac J Cancer Prev. 14:7483–7487. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sui S, Jiao Z, Niyazi MSS, Lu P and Qiao
YL: Genotype distribution and behavioral risk factor analysis of
human papillomavirus infection in Uyghur women. Asian Pac J Cancer
Prev. 14:5861–5865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Muñoz N, Bosch FX, de Sanjosé S, Herrero
R, Castellsagué X, Shah KV, Snijders PJ and Meijer CJ;
International Agency for Research on Cancer Multicenter Cervical
Cancer Study Group, : Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tang KW, Alaei-Mahabadi B, Samuelsson T,
Lindh M and Larsson E: The landscape of viral expression and host
gene fusion and adaptation in human cancer. Nat Commun. 4:25132013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rosa MI, Silva GD, de Azedo Simões PW,
Souza MV, Panatto AP, Simon CS, Madeira K and Medeiros LR: The
prevalence of human papillomavirus in ovarian cancer: A systematic
review. Int J Gynecol Cancer. 23:437–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Al-Shabanah OA, Hafez MM, Hassan ZK,
Sayed-Ahmed MM, Abozeed WN, Al-Rejaie SS and Alsheikh AA: Human
papillomavirus genotyping and integration in ovarian cancer Saudi
patients. Virol J. 10:3432013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bilyk OO, Pande NT, Pejovic T and
Buchinska LG: The frequency of human papilloma virus types 16, 18
in upper genital tract of women at high risk of developing ovarian
cancer. Exp Oncol. 36:121–124. 2014.PubMed/NCBI
|
|
60
|
Bilyk OO, Pande NT and Buchynska LG:
Analysis of p53, p16(INK4a), pRb and Cyclin D1 expression and human
papillomavirus in primary ovarian serous carcinomas. Exp Oncol.
33:150–156. 2011.PubMed/NCBI
|
|
61
|
Mahmood FM, Kadhim HS and Al Khuzaee LR
Mousa: Detection of human papillomavirus-16 e6-oncoprotein in
epithelial ovarian tumors samples of iraqi patients. Jundishapur J
Microbiol. 7:e119452014.PubMed/NCBI
|
|
62
|
Ghittoni R, Accardi R, Chiocca S and
Tommasino M: Role of human papillomaviruses in carcinogenesis.
Ecancermedicalscience. 9:5262015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Münger K and Howley PM: Human
papillomavirus immortalization and transformation functions. Virus
Res. 89:213–228. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Park JW, Nickel KP, Torres AD, Lee D,
Lambert PF and Kimple RJ: Human papillomavirus type 16 E7
oncoprotein causes a delay in repair of DNA damage. Radiother
Oncol. 113:337–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Honegger A, Schilling D, Bastian S,
Sponagel J, Kuryshev V, Sültmann H, Scheffner M, Hoppe-Seyler K and
Hoppe-Seyler F: Dependence of intracellular and exosomal microRNAs
on viral E6/E7 oncogene expression in HPV-positive tumor cells.
PLoS Pathog. 11:e10047122015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gregoire L, Rabah R, Schmelz EM, Munkarah
A, Roberts PC and Lancaster WD: Spontaneous malignant
transformation of human ovarian surface epithelial cells in vitro.
Clin Cancer Res. 7:4280–4287. 2001.PubMed/NCBI
|
|
67
|
Yu J, Solano FX Jr and Seethala RR:
Bilateral cytomegalovirus (CMV) oophoritis mimicking widely
metastatic carcinoma: A case report and review of the literature.
Diagn Pathol. 2:502007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wick W and Platten M: CMV infection and
glioma, a highly controversial concept struggling in the clinical
arena. Neuro Oncol. 16:332–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Richardson AK, Currie MJ, Robinson BA,
Morrin H, Phung Y, Pearson JF, Anderson TP, Potter JD and Walker
LC: Cytomegalovirus and Epstein-Barr virus in breast cancer. PLoS
One. 10:e01189892015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Han CP, Tsao YP, Sun CA, Ng HT and Chen
SL: Human papillomavirus, cytomegalovirus and herpes simplex virus
infections for cervical cancer in Taiwan. Cancer Lett. 120:217–221.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Harkins L, Volk AL, Samanta M, Mikolaenko
I, Britt WJ, Bland KI and Cobbs CS: Specific localisation of human
cytomegalovirus nucleic acids and proteins in human colorectal
cancer. Lancet. 360:1557–1563. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Samanta M, Harkins L, Klemm K, Britt WJ
and Cobbs CS: High prevalence of human cytomegalovirus in prostatic
intraepithelial neoplasia and prostatic carcinoma. J Urol.
170:998–1002. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Michaelis M, Baumgarten P, Mittelbronn M,
Driever PH, Doerr HW and Cinatl J Jr: Oncomodulation by human
cytomegalovirus: Novel clinical findings open new roads. Med
Microbiol Immunol. 200:1–5. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Söderberg-Nauclér C and Johnsen JI:
Cytomegalovirus in human brain tumors: Role in pathogenesis and
potential treatment options. World J Exp Med. 5:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bentz GL and Yurochko AD: Human CMV
infection of endothelial cells induces an angiogenic response
through viral binding to EGF receptor and beta1 and beta3
integrins. Proc Natl Acad Sci USA. 105:pp. 5531–5536. 2008;
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bishop RK, Oseguera CA Valle and Spencer
JV: Human cytomegalovirus interleukin-10 promotes proliferation and
migration of MCF-7 breast cancer cells. Cancer Cell Microenviron.
2(pii): e6782015.PubMed/NCBI
|
|
77
|
Price RL, Song J, Bingmer K, Kim TH, Yi
JY, Nowicki MO, Mo X, Hollon T, Murnan E, Alvarez-Breckenridge C,
et al: Cytomegalovirus contributes to glioblastoma in the context
of tumor suppressor mutations. Cancer Res. 73:3441–3450. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Scrivo R, Vasile M, Bartosiewicz I and
Valesini G: Inflammation as ‘common soil’ of the multifactorial
diseases. Autoimmun Rev. 10:369–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Karin M, Lawrence T and Nizet V: Innate
immunity gone awry: Linking microbial infections to chronic
inflammation and cancer. Cell. 124:823–835. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Whiteside TL: The role of immune cells in
the tumor microenvironment. Cancer Treat Res. 130:103–124. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Enzler T, Gillessen S, Manis JP, Ferguson
D, Fleming J, Alt FW, Mihm M and Dranoff G: Deficiencies of GM-CSF
and interferon gamma link inflammation and cancer. J Exp Med.
197:1213–1219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Macciò A and Madeddu C: Inflammation and
ovarian cancer. Cytokine. 58:133–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
86
|
Block MS, Charbonneau B, Vierkant RA,
Fogarty Z, Bamlet WR, Pharoah PD; Georgia Chenevix-Trench; for
AOCS; /ACS Group, ; Rossing MA, Cramer D, Pearce CL, et al:
Variation in NF-κB signaling pathways and survival in invasive
epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev.
23:1421–1427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Trabert B, Ness RB, Lo-Ciganic WH, Murphy
MA, Goode EL, Poole EM, Brinton LA, Webb PM, Nagle CM, Jordan SJ,
et al: Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and
acetaminophen use and risk of invasive epithelial ovarian cancer: A
pooled analysis in the Ovarian Cancer Association Consortium. J
Natl Cancer Inst. 106:djt4312014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hu T, Li LF, Shen J, Zhang L and Cho CH:
Chronic inflammation and colorectal cancer: The role of vascular
endothelial growth factor. Curr Pharm Des. 21:2960–2967. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ellis LM and Hicklin DJ: VEGF-targeted
therapy: mechanisms of anti-tumour activity. Nat Rev Cancer.
8:579–591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Noort AR, van Zoest KP, Weijers EM,
Koolwijk P, Maracle CX, Novack DV, Siemerink MJ, Schlingemann RO,
Tak PP and Tas SW: NF-κB-inducing kinase is a key regulator of
inflammation-induced and tumour-associated angiogenesis. J Pathol.
234:375–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
De Spiegelaere W, Cornillie P, Casteleyn
C, Burvenich C and Van den Broeck W: Detection of hypoxia inducible
factors and angiogenic growth factors during foetal endochondral
and intramembranous ossification. Anat Histol Embryol. 39:376–384.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yang Y, Sun M, Wang L and Jiao B: HIFs,
angiogenesis, and cancer. J Cell Biochem. 114:967–974. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sherer DM, Eliakim R and Abulafia O: The
role of angiogenesis in the accumulation of peritoneal fluid in
benign conditions and the development of malignant ascites in the
female. Gynecol Obstet Invest. 50:217–224. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li J, Li S, Chen R, Yu H and Lu X: The
prognostic significance of anti-angiogenesis therapy in ovarian
cancer: A meta-analysis. J Ovarian Res. 8:542015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li G, He L, Zhang E, Shi J, Zhang Q, Le
AD, Zhou K and Tang X: Overexpression of human papillomavirus (HPV)
type 16 oncoproteins promotes angiogenesis via enhancing HIF-1α and
VEGF expression in non-small cell lungcancer cells. Cancer Lett.
311:160–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Matlaf LA, Harkins LE, Bezrookove V, Cobbs
CS and Soroceanu L: Cytomegalovirus pp71 protein is expressed in
human glioblastoma and promotes pro-angiogenic signaling by
activation of stem cell factor. PLoS One. 8:e681762013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Barriere G, Fici P, Gallerani G, Fabbri F
and Rigaud M: Epithelial mesenchymal transition: A double-edged
sword. Clin Transl Med. 4:142015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang YL, Zhao XM, Shuai ZF, Li CY, Bai QY,
Yu XW and Wen QT: Snail promotes epithelial-mesenchymal transition
and invasiveness in human ovarian cancer cells. Int J Clin Exp Med.
8:7388–7393. 2015.PubMed/NCBI
|
|
101
|
Wu J, Liu Z, Shao C, Gong Y, Hernando E,
Lee P, Narita M, Muller W, Liu J and Wei JJ: HMGA2
overexpression-induced ovarian surface epithelial transformation is
mediated through regulation of EMT genes. Cancer Res. 71:349–359.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Haslehurst AM, Koti M, Dharsee M, Nuin P,
Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, et al: EMT
transcription factors snail and slug directly contribute to
cisplatin resistance in ovarian cancer. BMC Cancer. 12:912012.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Cohen EN, Gao H, Anfossi S, Mego M, Reddy
NG, Debeb B, Giordano A, Tin S, Wu Q, Garza RJ, et al: Inflammation
mediated metastasis: Immune induced epithelial-to-mesenchymal
transition in inflammatory breast cancer cells. PLoS One.
10:e01327102015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Choi YJ, Kim N, Chang H, Lee HS, Park SM,
Park JH, Shin CM, Kim JM, Kim JS, Lee DH and Jung HC: Helicobacter
pylori-induced epithelial-mesenchymal transition, a potential role
of gastric cancer initiation and an emergence of stem cells.
Carcinogenesis. 36:553–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chandrakesan P, Roy B, Jakkula LU, Ahmed
I, Ramamoorthy P, Tawifik O, Papineni R, Houchen C, Anant S and
Umar S: Utility of a bacterial infection model to study
epithelial-mesenchymal transition, mesenchymal-epithelial
transition or tumorigenesis. Oncogene. 33:2639–2654. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Duan H, Qu L and Shou C: Mycoplasma
hyorhinis induces epithelial-mesenchymal transition in gastric
cancer cell MGC803 via TLR4-NF-κB signaling. Cancer Lett.
354:447–454. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang L, Tian WD, Xu X, Nie B, Lu J, Liu X,
Zhang B, Dong Q, Sunwoo JB, Li G and Li XP: Epstein-Barr virus
nuclear antigen 1 (EBNA1) protein induction of
epithelial-mesenchymal transition in nasopharyngeal carcinoma
cells. Cancer. 120:363–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Bose SK, Meyer K, Di Bisceglie AM, Ray RB
and Ray R: Hepatitis C virus induces epithelial-mesenchymal
transition in primary human hepatocytes. J Virol. 86:13621–13628.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li H, Li Y, Liu D and Liu J: LPS promotes
epithelial-mesenchymal transition and activation of TLR4/JNK
signaling. Tumour Biol. 35:10429–10435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhao L, Yang R, Cheng L, Wang M, Jiang Y
and Wang S: LPS-induced epithelial-mesenchymal transition of
intrahepatic biliary epithelial cells. J Surg Res. 171:819–825.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kondo Y, Higa-Nakamine S, Noguchi N, Maeda
N, Toku S, Isohama Y, Sugahara K, Kukita I and Yamamoto H:
Induction of epithelial-mesenchymal transition by flagellin in
cultured lung epithelial cells. Am J Physiol Lung Cell Mol Physiol.
303:L1057–L1069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Franchi L, Park JH, Shaw MH, Marina-Garcia
N, Chen G, Kim YG and Núñez G: Intracellular NOD-like receptors in
innate immunity, infection and disease. Cell Microbiol. 10:1–38.
2008.PubMed/NCBI
|
|
113
|
Park GB, Kim D, Kim YS, Kim S, Lee HK,
Yang JW and Hur DY: The Epstein-Barr virus causes
epithelial-mesenchymal transition in human corneal epithelial cells
via Syk/src and Akt/Erk signaling pathways. Invest Ophthalmol Vis
Sci. 55:1770–1779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Horikawa T, Yang J, Kondo S, Yoshizaki T,
Joab I, Furukawa M and Pagano JS: Twist and epithelial-mesenchymal
transition are induced by the EBV oncoprotein latent membrane
protein 1 and are associated with metastatic nasopharyngeal
carcinoma. Cancer Res. 67:1970–1978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Sides MD, Klingsberg RC, Shan B, Gordon
KA, Nguyen HT, Lin Z, Takahashi T, Flemington EK and Lasky JA: The
Epstein-Barr virus latent membrane protein 1 and transforming
growth factor-β1 synergistically induce epithelial-mesenchymal
transition in lung epithelial cells. Am J Respir Cell Mol Biol.
44:852–862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Li T, Li D, Cheng L, Wu H, Gao Z, Liu Z,
Jiang W, Gao YH, Tian F, Zhao L and Wang S: Epithelial-mesenchymal
transition induced by hepatitis C virus core protein in
cholangiocarcinoma. Ann Surg Oncol. 17:1937–1944. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Bapat SA, Mali AM, Koppikar CB and Kurrey
NK: Stem and progenitor-like cells contribute to the aggressive
behavior of human epithelial ovarian cancer. Cancer Res.
65:3025–3029. 2005.PubMed/NCBI
|
|
118
|
Garson K and Vanderhyden BC: Epithelial
ovarian cancer stem cells: Underlying complexity of a simple
paradigm. Reproduction. 149:R59–R70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Korkaya H, Liu S and Wicha MS: Regulation
of cancer stem cells by cytokine networks: Attacking cancer's
inflammatory roots. Clin Cancer Res. 17:6125–6129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Leizer AL, Alvero AB, Fu HH, Holmberg JC,
Cheng YC, Silasi DA, Rutherford T and Mor G: Regulation of
inflammation by the NF-κB pathway in ovarian cancer stem cells. Am
J Reprod Immunol. 65:438–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chefetz I, Alvero AB, Holmberg JC,
Lebowitz N, Craveiro V, Yang-Hartwich Y, Yin G, Squillace L,
Soteras M Gurrea, Aldo P and Mor G: TLR2 enhances ovarian cancer
stem cell self-renewal and promotes tumor repair and recurrence.
Cell Cycle. 12:511–521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Xiang T, Long H, He L, Han X, Lin K, Liang
Z, Zhuo W, Xie R and Zhu B: Interleukin-17 produced by tumor
microenvironment promotes self-renewal of CD133+ cancer stem-like
cells in ovarian cancer. Oncogene. 34:165–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Castells M, Milhas D, Gandy C, Thibault B,
Rafii A, Delord JP and Couderc B: Microenvironment mesenchymal
cells protect ovarian cancer cell lines from apoptosis by
inhibiting XIAP inactivation. Cell Death Dis. 4:e8872013.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Farina A, Cirone M, York M, Lenna S,
Padilla C, McLaughlin S, Faggioni A, Lafyatis R, Trojanowska M and
Farina GA: Epstein-Barr virus infection induces aberrant TLR
activation pathway and fibroblast-myofibroblast conversion in
scleroderma. J Invest Dermatol. 134:954–964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
He Z, Gao Y, Deng Y, Li W, Chen Y, Xing S,
Zhao X, Ding J and Wang X: Lipopolysaccharide induces lung
fibroblast proliferation through Toll-like receptor 4 signaling and
the phosphoinositide3-kinase-Akt pathway. PLoS One. 7:e359262012.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Erez N, Glanz S, Raz Y, Avivi C and
Barshack I: Cancer associated fibroblasts express pro-inflammatory
factors in human breast and ovarian tumors. Biochem Biophys Res
Commun. 437:397–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yeung TL, Leung CS, Wong KK, Samimi G,
Thompson MS, Liu J, Zaid TM, Ghosh S, Birrer MJ and Mok SC: TGF-β
modulates ovarian cancer invasion by upregulating CAF-derived
versican in the tumor microenvironment. Cancer Res. 73:5016–5028.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Dimanche-Boitrel MT, Vakaet L Jr, Pujuguet
P, Chauffert B, Martin MS, Hammann A, Van Roy F, Mareel M and
Martin F: In vivo and in vitro invasiveness of a rat colon-cancer
cell line maintaining E-cadherin expression: An enhancing role of
tumor-associated myofibroblasts. Int J Cancer. 56:512–521. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Boire A, Covic L, Agarwal A, Jacques S,
Sherifi S and Kuliopulos A: PAR1 is a matrix metalloprotease-1
receptor that promotes invasion and tumorigenesis of breast cancer
cells. Cell. 120:303–313. 2005. View Article : Google Scholar : PubMed/NCBI
|