Introduction
Hepatocellular carcinoma (HCC) ranks fifth among the
primary causes of cancer-associated mortality in males and ninth in
females (1). In China, HCC is the
most common type of cancer and the highest cause of cancer
mortality in males <60 years (2),
due to widespread hepatitis B virus (HBV) infection which almost
90% of patients with HCC experience. Hepatitis C virus (HCV)
infection, alcoholism and obesity are additional risk factors for
HCC in the western world (3). At
present, the only treatment for HCC is hepatic resection which is
restricted to the patients who have localized lesions arising in
non-cirrhotic livers, or in cirrhotic livers with well-preserved
hepatic function (4). However, the
majority of patients are initially diagnosed with advanced HCC and
therefore the majority of patients with HCC are unable to be
treated by resection. Despite substantial progress achieved in the
treatment for advanced HCC in previous years, including targeted
therapy (sorafenib, a kinase inhibitor drug) and interventional
therapy, the overall 5-year survival rate remains unsatisfactory.
The prognosis and treatment of HCC depends on the tumor stage, the
patient's physical status and liver function (4,5).
Clinicopathological factors including microvascular invasion, novel
extrahepatic lesions and portal vein tumor thrombus (PVTT) have
been used to predict survival time in patients with advanced HCC
(6,7).
However, these aforementioned clinical tumor parameters may only
partially explain the prognostic heterogeneity of HCC.
The inflammatory microenvironment serves an
important function in the development and progression of HCC. In
China, chronic liver inflammation primarily contributes to the
development of HCC (8). In addition,
the host response in systemic inflammation has been considered to
be an independent prognostic factor for patients with HCC (9–12).
C-reactive protein (13,14), white blood cell counts, platelet
counts (15), neutrophil counts
(16) and monocyte counts (17) have been primarily investigated as
clinical indicators of inflammation for cancer. Previous studies
have indicated that the development of HCC is associated with
alterations of peripheral blood white blood cells. The principal
alterations include neutrophilia with relative lymphocytopenia or
thrombocytosis. White blood cells, along with the cytokines and
chemokines secreted, serve a role in the viability, maturation and
differentiation of cells within the tumor microenvironment.
Peripheral blood lymphocyte-to-monocyte ratio (LMR), an indication
of lymphocytopenia and an increased monocyte count, has been
identified to be a novel inflammatory biomarker. LMR combines an
estimate of host immune homeostasis and tumor microenvironment and
has been identified as a predictor for clinical outcomes in a
variety of malignancies, including breast (18), renal (19,20), lung
(21) and colorectal (22) cancer. Recently, a study elucidated
that LMR was an independent prognostic factor in patients with
HBV-associated HCC following curative resection (23). However, to the best of our knowledge,
there have been no studies regarding the prognostic value of LMR in
advanced HCC until the present study, which aimed at clarifying the
prognostic value of LMR in patients with HBV-associated advanced
HCC.
Materials and methods
Patients
The Clinical Ethics Review Board at The Third
Affiliated Hospital of Sun Yat-sen University (Guangdong, China)
approved the present study, and written informed consent was
obtained from the patients or their family members. Medical records
of 174 consecutive Chinese patients with HBV-associated advanced
HCC presented to The Third Affiliated Hospital of Sun Yat-sen
University (Guangdong, China) between September 2008 and June 2010,
were reviewed and retrospectively analyzed in the present study.
The inclusion criteria of the patients were as follows: i) Patients
were diagnosed with advanced HCC. The diagnosis of HCC was
confirmed using pathological analysis or the patients met the
radiological criteria from The American Association for the Study
of Liver Diseases (4), including
computed tomography (CT) and magnetic resonance imaging (MRI).
Patients with advanced HCC exhibited stage C or D, according to The
Barcelona Clinic Liver Cancer Staging for Hepatocellular Carcinoma
(BCLC) (24). ii) All the patients
had chronic HBV infection and were negative for anti-HCV antibody.
iii) Antiviral therapy with oral nucleos(t)ide analogues was
recommended for all patients, although the patients did not receive
antitumor treatment recommended by the National Comprehensive
Cancer Network guidelines (25)
including chemotherapy, targeted therapy or locoregional therapies
(percutaneous ethanol injection or transarterial
chemoembolization). The exclusion criteria of the patients were as
follows: i) Patients exhibited a fever or additional manifestations
of acute infections; ii) patients exhibited previous or secondary
cancers and coexistent hematological disorders; and iii) patients
did not possess information required for the present study or lost
follow-up within 6 months.
Collection of patient information
Acquisition of patient results from electronic
charts was approved by the institutional ethics committee of the
Third Affiliated Hospital of Sun Yat-sen University (Guangdong,
China). Clinical examination, laboratory studies and imaging
studies (CT or MRI) were required for baseline evaluation.
Information was collected at the time of the initial diagnosis of
advanced HCC. The overall survival (OS) time was calculated between
the date of diagnosis and the date of mortality or last contact,
for surviving patients.
Demographic information, laboratory results
[including routine blood tests, liver function parameters, serum
α-fetoprotein (AFP) level], BCLC stage and Karnofsky performance
status (26) were retrospectively
analyzed from the patients' clinical records. Tumor-associated
variables, including maximal tumor diameter, number of tumor
nodules and PVTT were obtained from medical image diagnostic
results. The peripheral blood absolute lymphocyte count (ALC) and
absolute monocyte count (AMC) were derived from the complete blood
cell count prior to initiation of treatment, with the LMR
calculated by dividing the lymphocyte count by the monocyte
count.
Statistical analysis
Fisher's exact test (two-tailed) or χ2
test was used to analyze categorical variables, whereas Student's
t-test or non-parametric tests were used to analyze continuous
variables. Using OS time as the end-point, the optimal threshold
value of LMR using receiver operating curve (ROC) analysis was
obtained when the Youden index (sensitivity + specificity-1)
(27) was maximal. Subsequently,
patients with an LMR greater than the threshold value were
classified as high LMR (HLMR) and the remaining patients were
classified as low LMR (LLMR). Survival curves for the two groups
were plotted using the Kaplan-Meier estimator method and the
significance was analyzed using the log-rank test. Significant
factors for OS time in Cox's univariate analysis were assessed
using Cox's proportional hazards model (forward method) to
determine the independent prognostic predictors. Associations
between LMR levels and baseline characteristics, including
clinicopathological features, were analyzed using χ2
test. All statistical analysis was conducted using SPSS (version
20.0; IBM Corp., Armonk, NY, USA) and Stata software (version 12.0;
StataCorp LP, College Station, TX, USA). For all tests, P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
In the present study, of the 174 patients with
HBV-associated advanced HCC, 157 (90.2%) were male and the mean age
was 55 years (range, 19–85 years). The mean counts of white blood
cells, neutrophils, ALC, AMC and platelets were 6.84×109
cells/l [range, (1.84–25.09)x109 cells/l],
4.89×109 cells/l [range, (1.11–22.44)x109
cells/l], 1.23×109 cells/l [range,
(0.32–4.18)x109 cells/l], 0.60×109 cells/l
[range, (0.08–3.85)x109 cells/l] and 156.3 cells/l
[range, (4–503)x109 cells/l], respectively. The median
LMR was 2.57 (range, 0.39–7.0). A total of 53 (30.5%) patients with
lymph node metastasis and 36 (20.7%) patients with initial distant
metastasis were recorded from newly diagnosed patients. According
to BCLC staging criteria, the number of patients with stage C and D
advanced HCC was 95 (54.6%) and 79 (45.4%), respectively. Of the
patients included in the present study, 152 (87.4%) patients
succumbed to cancer-associated disease. The 3-month and 6-month
mortality rates were 59.2 and 74.1%, respectively. Additional
information for the patients' baseline demographics are presented
in Table I.
 | Table I.Baseline demographics of all patients
included in the present study. |
Table I.
Baseline demographics of all patients
included in the present study.
| Characteristic | Patients
(n=174) |
|---|
| Sex |
|
Male | 90.2%
(157/174) |
|
Female | 9.8% (17/174) |
| Age, years | 55 (19–85) |
| Ascites | 66.1%
(115/174) |
| Encephalopathy | 2.9% (5/174) |
| Karnofsky
performance status | 60 (20–90) |
| Laboratory
parameters |
|
Leukocytes, ×109
cells/l | 6.84
(1.84–25.09) |
|
Neutrophils, ×109
cells/l | 4.89
(1.11–22.44) |
| ALC,
×109 cells/l | 1.23
(0.32–4.18) |
| AMC,
×109 cells/l | 0.60
(0.08–3.85) |
|
Hemoglobin, g/l | 115.3
(56.0–177.0) |
|
Platelets, ×109
cells/l | 156.3
(4.0–503.0) |
|
LMR | 2.57
(0.39–7.0) |
|
≤2.22 | 49.4% (86/174) |
|
>2.22 | 50.6% (88/174) |
| 3-Month mortality
rate, % | 59.2%
(103/174) |
| 6-Month mortality
rate, % | 74.1%
(129/174) |
| Child-Pugh
grade |
| 0 | 20.7% (36/174) |
| 1 | 44.8% (78/174) |
| 2 | 34.5% (60/174) |
| TNM stage |
| I | 9.8% (17/174) |
| II | 11.5% (20/174) |
|
III | 56.3% (98/174) |
| IV | 22.4% (39/174) |
| Lymph node
metastasis | 30.5% (53/174) |
| Distant
metastases | 20.7% (36/174) |
| Portal vein
thrombosis | 72.4%
(126/174) |
| NCCN-TNM |
| T1 | 10.9% (19/174) |
| T2 | 13.8% (24/174) |
|
T3-T4 | 75.3%
(131/174) |
Independent prognostic factors for
overall survival
To identify the optimal peripheral blood
inflammatory and immune biomarker for patient prognosis, the impact
of leukocyte, neutrophil, ALC, AMC and LMR on survival outcomes was
investigated. In the univariate analysis, leukocytes, neutrophils,
AMC and LMR were identified to be significant prognostic factors,
with P<0.001 for leukocytes, neutrophils and LMR, P=0.04 for AMC
and no significant difference indicated for ALC. Additional
independent prognostic factors that may be associated with overall
survival time included age, platelets, aspartate transaminase
(AST), AFP, ascites, tumor number, tumor size and PVTT (P<0.05).
The aforementioned factors identifying statistical significance
were assessed using Cox's multivariate regression analysis to
determine the significance of independent factors. In the
multivariate analysis, LMR [hazard ratio (HR), 0.858; 95%
confidence interval (CI), 0.754–0.976; P=0.020], neutrophil (HR,
1.104; 95% CI, 1.046–1.165; P<0.001), PVTT (HR, 1.984; 95% CI,
1.296–3.037; P<0.001) and AFP >400 g/ml (HR, 1.739; 95% CI,
1.221–2.478; P=0.002) remained independent prognostic predictors
(Table II).
 | Table II.Independent prognostic factors for
overall survival in patients with hepatitis B virus-associated
advanced hepatocellular carcinoma according to univariate and
multivariate analysis. |
Table II.
Independent prognostic factors for
overall survival in patients with hepatitis B virus-associated
advanced hepatocellular carcinoma according to univariate and
multivariate analysis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 0.983 | 0.971–0.996 | 0.009 |
|
|
|
| Leukocytes,
×109 cells/l | 1.085 | 1.042–1.130 | 0.000 |
|
|
|
| Neutrophils,
×109 cells/l | 1.130 | 1.077–1.186 | 0.000 | 1.104 | 1.046–1.165 | 0.000 |
| AMC,
×109 cells/l | 1.348 | 1.013–1.794 | 0.040 |
|
|
|
| ALC,
×109 cells/l | 0.832 | 0.613–1.129 | 0.237 |
|
|
|
| LMR | 0.765 | 0.675–0.867 | 0.000 | 0.858 | 0.754–0.976 | 0.020 |
| Platelets,
×109 cells/l | 1.002 | 1.001–1.004 | 0.006 |
|
|
|
| AST, U/l | 1.001 | 1.000–1.001 | 0.000 |
|
|
|
| AFP, ng/dl, >400
vs. ≤400 | 2.30 | 1.649–2.309 | 0.000 | 1.739 | 1.221–2.478 | 0.002 |
| Ascites, yes vs.
no | 2.05 | 1.444–2.909 | 0.000 |
|
|
|
| Tumor number, <3
vs. ≥3 | 1.306 | 1.106–1.542 | 0.002 |
|
|
|
| Tumor size, cm,
<1 vs. ≥1 | 3.450 | 1.682–7.077 | 0.001 |
|
|
|
| PVTT, yes vs.
no | 2.661 | 1.792–3.951 | 0.000 | 1.984 | 1.296–3.037 | 0.002 |
Optimal threshold value for LMR
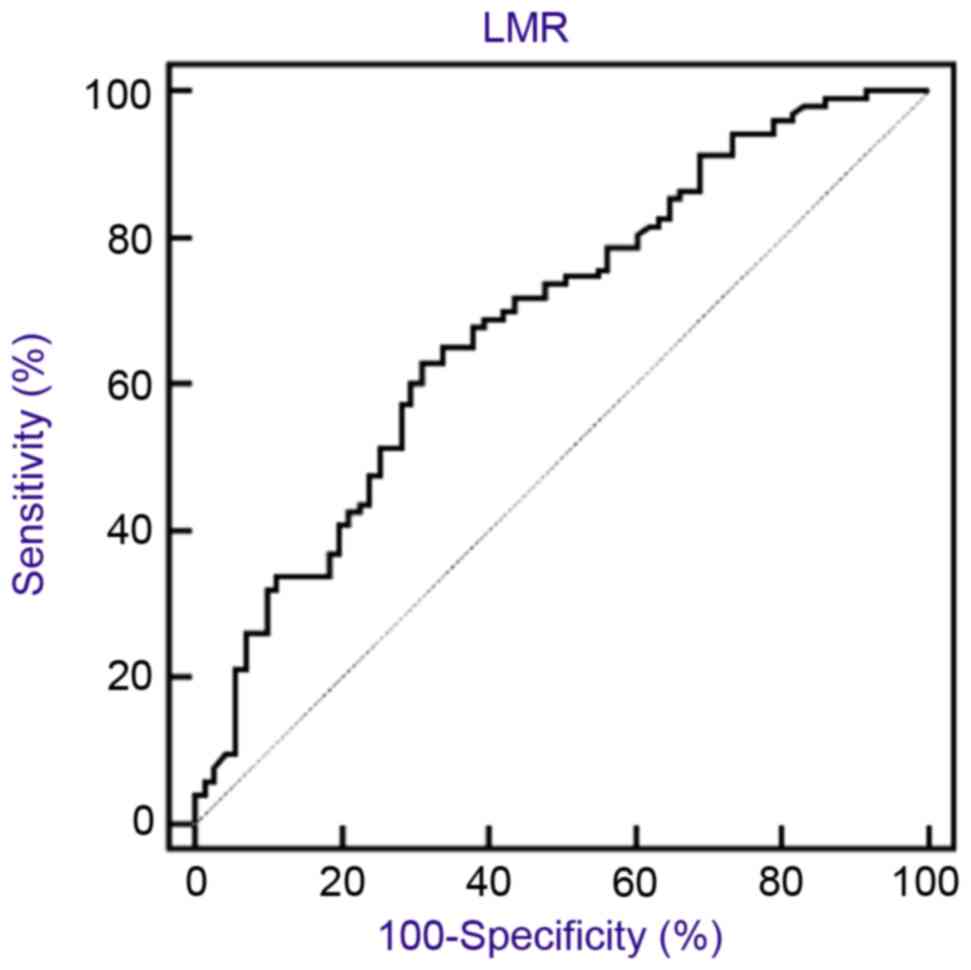
LMR was used as the test variable and the OS time
was employed as the state variable for LMR. According to the ROC
curve, the optimal threshold value for LMR was 2.22 (sensitivity,
63.1%; specificity, 69.0%) (Fig. 1).
Patients were dichotomized into either the HLMR group or LLMR
group, on the basis of the optimal threshold value. A total of 86
patients (49.4%) were included in the LLMR group (≤2.22) and 88
patients (50.5%) were included in the HLMR group (>2.22).
Comparison of patients between the
HLMR and LLMR groups
To determine the association between LMR and
clinicopathological features of patients with HBV-associated
advanced HCC, comparisons between the HLMR group and LLMR group
were made (Table III). The results
indicated that HLMR group exhibited a tendency to present distant
metastasis, ascites and a larger tumor size (P<0.01). The LLMR
level was significantly associated with increased leukocytes,
neutrophils, AMC, ALC, AST, total bilirubin, alkaline phosphatase
(P<0.001) and albumin (P=0.045). In the survival analysis, the
3-month and 6-month survival rates of the HLMR group were
significantly increased compared with those of the LLMR group
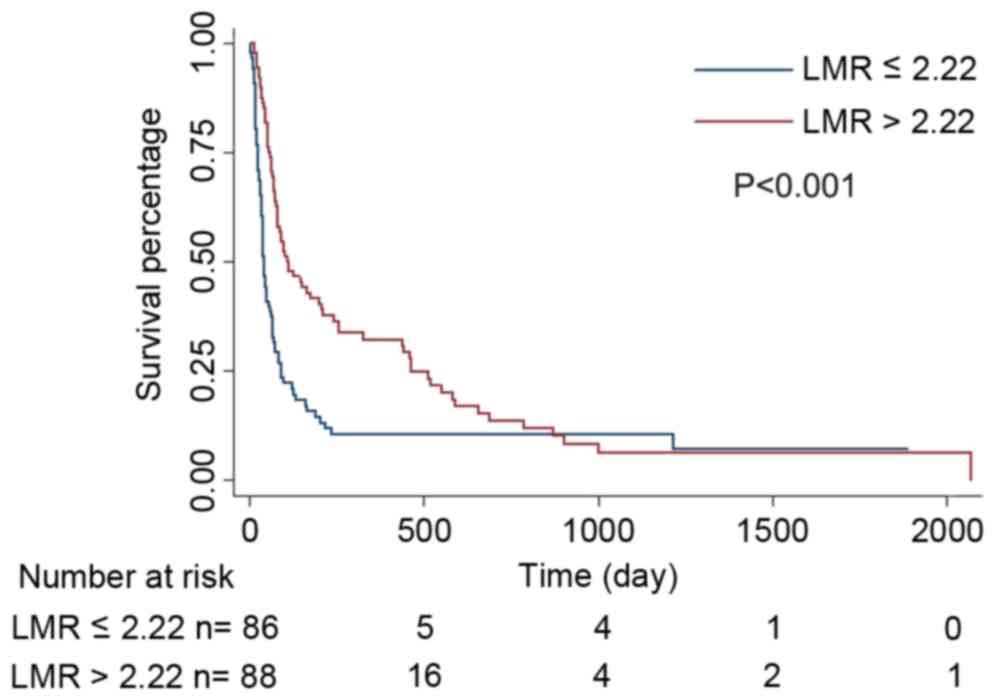
(P<0.001). A Kaplan-Meier estimator curve identified that the OS
rate was significantly decreased in the LLMR group compared with
that of the HLMR group (P<0.001, Fig.
2).
 | Table III.Clinical features between the high
and low LMR groups. |
Table III.
Clinical features between the high
and low LMR groups.
|
| LMR |
|
|---|
|
|
|
|
|---|
|
Characteristics | ≤2.22 (n=86) | >2.22
(n=88) | P-value |
|---|
| Age, years | 54.4±4.51 | 56.8±13.2 | 0.217 |
| Sex |
|
Male | 76 | 81 | 0.415 |
|
Female | 10 | 7 |
|
| Leukocytes,
×109 cells/l | 8.0±4.3 | 5.7±2.1 | 0.000 |
| Neutrophils,
×109 cells/l | 6.1±3.5 | 3.7±1.8 | 0.000 |
| Monocytes,
×109 cells/l | 0.8±0.6 | 0.4±0.2 | 0.000 |
| Lymphocytes,
×109 cells/l | 1.1±0.6 | 1.4±0.5 | 0.000 |
| Platelets,
×109 cells/l | 164.1±109.4 | 148.7±95.0 | 0.584 |
| Hemoglobin,
g/l | 112.3±21.7 | 118.3±21.7 | 0.068 |
| AST, U/l | 266.4±323.9 | 164.6±200.8 | 0.002 |
| Total bilirubin,
µmol/l | 105.2±136.7 | 72.6±108.0 | 0.002 |
| Albumin, g/l | 32.0±6.3 | 33.9±6.1 | 0.045 |
| ALP, U/l | 212.7±144.2 | 148.3±76.1 | 0.006 |
| BUN, mmol/l | 6.8±4.2 | 6.1±3.2 | 0.546 |
| Creatinine,
µmol/l | 75.2±30.2 | 76.4±27.6 | 0.137 |
| AFU, U/l | 30.3±17.4 | 29.7±18.5 | 0.758 |
| GGT, U/l | 292.9±268.4 | 215.3±179.7 | 0.091 |
| Prothrombin time,
sec | 70.6±18.1 | 75.4±19.5 | 0.088 |
| INR | 1.3±0.3 | 1.3±0.3 | 0.125 |
| Lymph node
metastasis |
|
Absent | 54 | 67 | 0.056 |
|
Present | 32 | 21 |
|
| Distant
metastasis |
|
Absent | 60 | 78 | 0.002 |
|
Present | 36 | 0 |
|
| PVTT |
|
Absent | 18 | 30 | 0.052 |
|
Present | 68 | 58 |
|
| Tumor number |
| ≤3 | 34 | 43 | 0.215 |
|
>3 | 52 | 45 |
|
| Tumor size, cm |
|
<2 | 12 | 28 | 0.005 |
| ≥2 | 74 | 60 |
|
| Ascites |
|
Absent | 15 | 44 | 0.000 |
|
Present | 71 | 44 |
|
| 3-Month survival
rate, % | 25.6 (22/86) | 55.7 (49/88) | 0.000 |
| 6-Month survival
rate, % | 14.0 (12/86) | 37.5 (33/88) | 0.000 |
Discussion
HCC has been established as life-threatening disease
and it has been reported that majority of patients are initially
diagnosed with advanced HCC. Despite the efforts of clinicians to
prolong the survival time of patients with advanced HCC, effective
therapies are limited. A number of clinicopathological
characteristics, including tumor size and thrombosis, have become
the primary determinants of existing therapies and predictors of
prognosis of patients with HCC. However, these aforementioned
factors are far from meeting medical needs.
In 1863, Virchow (28)
first proposed the association between inflammation and cancer.
Subsequently, a number of studies have indicated the important
function of inflammation in early carcinogenesis, development,
metastasis and therapeutic response of cancers (29–31). HCC
was a typical example of inflammation-associated cancer as the
majority of HCC developed in the setting of chronic liver damage
and inflammation, particularly in China. Therefore, in the present
study, to illustrate the function of LMR in HBV-associated advanced
HCC, only patients with HBV infection were included. Pretreatment
numbers of peripheral blood cells, including neutrophils, ALC, AMC
and platelets, have been suggested to be marked prognostic factors
in a number of types of malignancies. Neutrophils, as the largest
proportion of leukocytes in human peripheral blood, secrete
mediators including cytokines and matrix metalloproteinase-9 to
react to cancer (32,33) and alter their phenotype, similar to
granulocytic myeloid-derived suppressor cells, allowing them to
exhibit distinct characteristics of maturity, tumor cytotoxicity
and immune suppression (34). During
tumor development, the inflammatory response may cause
lymphocytopenia, leading to the inhibition of suppression of tumor
progression (35). Macrophages, which
are tissue-resident cells that develop from circulating monocytes
in local tissues, are reported to influence tumor migration and
invasion, and suppress antitumor immunity (36,37). In
addition, a number of studies have demonstrated platelet
involvement in tumor metastasis. The activation of platelets
protect tumor cells from immune elimination by promoting their
arrest at the endothelium, thereby supporting the establishment of
secondary lesions (38,39).
On the basis of the association between systemic
inflammation and carcinoma, increasing numbers of inflammatory
markers have been associated with poor prognosis of a variety of
types of cancers. Chen et al (40) identified that patients with resectable
gastric cancer who exhibited increased preoperative white blood
cells and p-leukopenia had a poorer prognosis, compared with those
with exhibited lower baseline white blood cells and no
p-leukopenia. Kitayama et al (41) revealed that in patients with
colorectal cancer, the lymphocyte count was markedly associated
with tumor response to neoadjuvant chemoradiotherapy. Peripheral
blood LMR, as a novel inflammatory biomarker, has been identified
as a predictor of clinical outcome in a number of types of cancer
(18–22). In regard to HCC, to the best of our
knowledge, only one study has demonstrated an association between
LMR and HCC (42). Lin et al
(23) identified that preoperative
LMR may serve as an independent prognostic factor for patients with
HBV-associated HCC undergoing curative resection. However, to the
best of our knowledge, the function of LMR in HBV-associated
advanced HCC remains unclear.
The present study, to the best of our knowledge, was
the first to demonstrate that LMR level was independently
associated with OS for patients with advanced HCC. Only patients
with HBV-associated HCC who do not exhibit a fever, or any other
manifestations of acute infections, were included to avoid
potential confounding factors from distinct etiologies, since
infection alters blood cell number. The results of the present
study identified LMR, but not ALC or AMC, to be an independent
predictor of the OS rate in patients with HBV-associated advanced
HCC. The aforementioned patients with an LMR ≤2.22 exhibited a
decreased 3-month and 6-month survival rate compared with those
with an LMR >2.22, which was consistent with previous studies
that identified low LMR to be an independent poor prognostic factor
(18–23). However, the optimal thresholds for LMR
in distinct studies differ, which may be due to the different
baseline characteristics, type of malignancy, clinical stages and
treatments in each study.
The underlying molecular mechanism which enables LMR
to be used as a prognostic factor in cancers remains unknown.
Lymphocytes are a key mediator in the antitumor immunity of the
host and infiltration into the tumor microenvironment is a
prerequisite to an immunological antitumor reaction. ALC may alter
along with the antitumor immune reaction (35,43,44). ALC
decreased when there was an insufficient immunological reaction to
the tumor, thus promoting tumor progression and metastasis
(45). Although, in the present
study, ALC was not identified as a significant factor for the OS
rates in patients with advanced HCC, ALC exhibited an antitumor
effect. In addition to the quantity of lyphocytes, the phenotype
and subset composition may change; however, additional studies are
required to determine the underlying molecular mechanism (46–48). As a
component of the tumor microenvironment, monocytes serve a markedly
active function in tumor development, progression and metastasis
(30,49). Inflammation activates the mobilization
of monocytes from the bone marrow to the peripheral blood (50) and the differentiation of monocytes
into tumor-associated macrophages (TAMs) following recruitment into
tumor tissue (51,52), suppressing adaptive immunity and
exerting protumoral functions, promoting tumor cell invasion,
metastasis and angiogenesis (36,51). The
circulating level of monocytes in the peripheral blood is reported
to reflect the formation and/or presence of TAMs (45). Therefore, an increased level of
monocytes reflects a high tumor burden in patients with cancer
(53–55). However, in the present study, AMC was
not considered a prognostic factor for the OS rate using
multivariate analysis. It is hypothesized that ALC or AMC alone may
not explain the effect of inflammation on patients with
HBV-associated advanced HCC.
As aforementioned, LMR reflects the interaction
between the immune status of the host and the tumor
microenvironment. In the present study, the combination of ALC and
AMC, compared with ALC and AMC alone, enabled LMR to be an improved
predictor of OS rate in patients with HBV-associated advanced HCC,
which means that decreased ALC and increased AMC simultaneously
reflect the essence of the disease. Decreased LMR is associated
with a poorer prognosis. A previous study demonstrated that low LMR
may be considered as an independent biomarker for predicting
mortality in patients with HBV-related liver cirrhosis (56), which supports the predictive value of
LMR identified in the present study, as the majority of
HBV-associated advanced HCC developed from liver cirrhosis.
Additionally, decreased LMR was markedly associated with a number
of clinicopathological characteristics, including distant
metastasis, tumor size and ascites, which may reflect either higher
tumor burden or a more prolonged chronic inflammatory process,
indicating poor prognosis.
LMR is a readily available and low-cost objective
marker of systemic inflammation and may be obtained from routine
blood testing. Therefore, LMR may be used for prognostic
stratification of patients with HBV-associated advanced HCC and may
be a potential criterion for patient selection in clinical
trials.
However, the present study had limitations. First,
it is a retrospective study, the number of patients involved was
small and patients were restricted to be from a single center.
Secondly, LMR is a non-specific parameter of inflammation and the
results may be influenced by the presence of other systemic
diseases. Thirdly, the results of the present study were limited to
patients with HBV-associated advanced HCC. Additional etiologies of
HCC, including HCV and alcohol, require study. Finally, the
heterogeneity of patients with advanced HCC includes the
distinctions between metastatic sites, which may bias the results.
Additional large-scale prospective studies and standard
investigations are required to validate the results of the present
study.
The present study, to the best of our knowledge, was
the first study to analyze the prognosis of patients with
HBV-associated advanced HCC on the basis of a novel biomarker, LMR.
LMR was identified to be an independent prognostic factor of
patients with HBV-associated advanced HCC with increased baseline
LMR levels indicating an improved prognosis. A prospective and
well-designed study of LMR with larger cohorts is warranted for
further validation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 31600710 and
81372374), the Natural Science Foundation of Guangdong (grant nos.
2014A030313146 and 2016A030313302) and Project on the Integration
of Industry, Education and Research of Guangdong Province (grant
no. 2012B091100460)
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan JM, Govindarajan S, Arakawa K and Yu
MC: Synergism of alcohol, diabetes and viral hepatitis on the risk
of hepatocellular carcinoma in blacks and whites in the U.S.
Cancer. 101:1009–1017. 2014. View Article : Google Scholar
|
|
4
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases.
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Wang L, Cong N, Shi C, Bu W, Song J
and Chen H: Efficacy of sorafenib for advanced hepatocellular
carcinoma and prognostic factors. Hepatogastroenterology.
61:954–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogasawara S, Chiba T, Ooka Y, Suzuki E,
Kanogawa N, Saito T, Motoyama T, Tawada A, Kanai F and Yokosuka O:
Post-progression survival in patients with advanced hepatocellular
carcinoma resistant to sorafenib. Invest New Drugs. 34:255–260.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hong YF, Chen ZH, Ma XK, Li X, Wu DH, Chen
J, Dong M, Wei L, Wang TT, Ruan DY, et al: Comparison of five
models for end-stage liver disease in predicting the survival rate
of patients with advanced hepatocellular carcinoma. Tumour Biol.
37:5265–5273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sherman M: Hepatocellular carcinoma:
Epidemiology, surveillance, and diagnosis. Semin Liver Dis.
30:3–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bugada D, Allegri M, Lavand'homme P, De
Kock M and Fanelli G: Inflammation-based scores: A new method for
patient-targeted strategies and improved perioperative outcome in
cancer patients. Biomed Res Int. 2014:1424252014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deng Q, He B, Liu X, Yue J, Ying H, Pan Y,
Sun H, Chen J, Wang F, Gao T, et al: Prognostic value of
pre-operative inflammatory response biomarkers in gastric cancer
patients and the construction of a predictive model. J Transl Med.
13:662015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shiels MS, Katki HA, Hildesheim A,
Pfeiffer RM, Engels EA, Williams M, Kemp TJ, Caporaso NE, Pinto LA
and Chaturvedi AK: Circulating inflammation markers, risk of lung
cancer, and utility for risk stratification. J Natl Cancer Inst.
107:djv1992015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morrison L, Laukkanen JA, Ronkainen K,
Kurl S, Kauhanen J and Toriola AT: Inflammatory biomarker score and
cancer: A population-based prospective cohort study. BMC Cancer.
16:802016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang S, Wang Y, Sui D, Liu H, Ross MI,
Gershenwald JE, Cormier JN, Royal RE, Lucci A, Schacherer CW, et
al: C-reactive protein as a marker of melanoma progression. J Clin
Oncol. 33:1389–1396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aleksandrova K, Boeing H, Nöthlings U,
Jenab M, Fedirko V, Kaaks R, Lukanova A, Trichopoulou A,
Trichopoulos D, Boffetta P, et al: Inflammatory and metabolic
biomarkers and risk of liver and biliary tract cancer. Hepatology.
60:858–871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schumacher D, Strilic B, Sivaraj KK,
Wettschureck N and Offermanns S: Platelet-derived nucleotides
promote tumor-cell transendothelialmigration and metastasis via
p2y2 receptor. Cancer Cell. 24:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grosse-Steffen T, Giese T, Giese N,
Longerich T, Schirmacher P, Hansch GM and Gaida MM:
Epithelial-to-mesenchymal transition in pancreatic ductal
adenocarcinoma and pancreatic tumor cell lines: The role of
neutrophils and neutrophil-derived elastase. Clin Dev Immunol.
2012:7207682012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren QQ, Fu SJ, Zhao Q, Guo ZY, Ji F, Chen
MG, Wu LW and He XS: Prognostic value of preoperative peripheral
monocyte count in patients with hepatocellular carcinoma after
liver transplantation. Tumor Biol. 37:8973–8988. 2016. View Article : Google Scholar
|
|
18
|
Jia W, Wu J, Jia H, Yang Y, Zhang X, Chen
K and Su F: The peripheral blood Neutrophil-to-Lymphocyte ratio is
superior to the Lymphocyte-to-Monocyte ratio for predicting the
long-term survival of triple-negative breast cancer patients. PLoS
One. 10:e01430612015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang Y, Fu Q, Xu L, Zhou L, Liu Z, Yang
Y, Lin Z and Xu J: Prognostic value of preoperative lymphocyte to
monocyte ratio in patients with nonmetastatic clear cell renal cell
carcinoma. Tumor Biol. 37:4613–4620. 2016. View Article : Google Scholar
|
|
20
|
Chang Y, An H, Xu L, Zhu Y, Yang Y, Lin Z
and Xu J: Systemic inflammation score predicts postoperative
prognosis of patients with clear-cell renal cell carcinoma. Br J
Cancer. 113:626–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen YM, Lai CH, Chang HC, Chao TY, Tseng
CC, Fang WF, Wang CC, Chung YH, Wang YH, Su MC, et al: Baseline and
trend of Lymphocyte-to-Monocyte ratio as prognostic factors in
epidermal growth factor receptor mutant non-small cell lung cancer
patients treated with first-line epidermal growth factor tyrosine
kinase inhibitors. PLoS One. 10:e01362522015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shibutani M, Maeda K, Nagahara H, Ohtani
H, Sakurai K, Yamazoe S, Kimura K, Toyokawa T, Amano R, Tanaka H,
et al: Prognostic significance of the lymphocyte-to-monocyte ratio
in patients with metastatic colorectal cancer. World J
Gastroenterol. 21:9966–9973. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin ZX, Ruan DY, Li Y, Wu DH, Ma XK, Chen
J, Chen ZH, Li X, Wang TT, Lin Q, et al: Lymphocyte-to-monocyte
ratio predicts survival of patients with hepatocellular carcinoma
after curative resection. World J Gastroenterol. 21:10898–10906.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Llovet JM, Brú C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
National Comprehensive Cancer Network.
NCCN Guidelines Version 2.2105 Hepatocellular Carcinoma.
|
|
26
|
Schag CC, Heinrich RL and Ganz PA:
Karnofsky performance status revisited: Reliability, validity, and
guidelines. J Clin Oncol. 2:187–193. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fluss R, Faraggi D and Reiser B:
Estimation of the Youden Index and its associated cutoff point.
Biom J. 47:458–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keibel A, Singh V and Sharma MC:
Inflammation, microenvironment, and the immune system in cancer
progression. Curr Pharm Des. 15:1949–1955. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Niu XL, Qu Y, Wu J, Zhu YQ, Sun WJ
and Li LZ: Autocrine production of interleukin-6 confers cisplatin
and paclitaxel resistance in ovarian cancer cells. Cancer Lett.
295:110–123. 2015. View Article : Google Scholar
|
|
32
|
Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu
Z, Yin XY and Zheng L: Peritumoral neutrophils link inflammatory
response to disease progression by fostering angiogenesis in
hepatocellular carcinoma. J Hepatol. 54:948–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee IK, Vansaun MN, Shim JH, Matrisian LM
and Gorden DL: Increased metastases are associated with
inflammation and matrix metalloproteinase-9 activity at incision
sites in a murine model of peritoneal dissemination of colorectal
cancer. J Surg Res. 180:252–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mishalian I, Granot Z and Fridlender ZG:
The diversity of circulating neutrophils in cancer. Immunobiology.
222:82–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dunn GP, Old LJ and Schreiber RD: The
immunobiology of cancer immunosurveillance and immunoediting.
Immunity. 21:137–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Condeelis J and Pollard JW: Macrophages:
Obligate partners for tumor cell migration, invasion, and
metastasis. Cell. 124:263–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leek RD and Harris AL: Tumor-associated
macrophages in breast cancer. J Mammary Gland Biol Neoplasia.
7:177–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gay LJ and Felding-Habermann B:
Contribution of platelets to tumour metastasis. Nat Rev Cancer.
11:123–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Buergy D, Wenz F, Groden C and Brockmann
MA: Tumor-platelet interaction in solid tumors. Int J Cancer.
130:2747–2760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen XF, Qian J, Pei D, Zhou C, Røe OD,
Zhu F, He SH, Qian YY, Zhou Y, Xu J, et al: Prognostic value of
perioperative leukocyte count in resectable gastric cancer. World J
Gastroenterol. 22:2818–2827. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kitayama J, Yasuda K, Kawai K, Sunami E
and Nagawa H: Circulating lymphocyte number has a positive
association with tumor response in neoadjuvant chemoradiotherapy
for advanced rectal cancer. Radiat Oncol. 5:472010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gu L, Li H, Chen L, Ma X, Li X, Gao Y,
Zhang Y, Xie Y and Zhang X: Prognostic role of lymphocyte to
monocyte ratio for patients with cancer: Evidence from a systematic
review and meta-analysis. Oncotarget. 7:31926–31942.
2016.PubMed/NCBI
|
|
43
|
Hoffmann TK, Dworacki G, Tsukihiro T,
Meidenbauer N, Gooding W, Johnson JT and Whiteside TL: Spontaneous
apoptosis of circulating T lymphocytes in patients with head and
neck cancer and its clinical importance. Clin Cancer Res.
8:2553–2562. 2002.PubMed/NCBI
|
|
44
|
Rabinowich H, Cohen R, Bruderman I,
Steiner Z and Klajman A: Functional analysis of mononuclear cells
infiltrating into tumors: Lysis of autologous human tumor cells by
cultured infiltrating lymphocytes. Cancer Res. 47:173–177.
1987.PubMed/NCBI
|
|
45
|
Stotz M, Pichler M, Absenger G, Szkandera
J, Arminger F, Schaberl-Moser R, Samonigg H, Stojakovic T and
Gerger A: The preoperative lymphocyte to monocyte ratio predicts
clinical outcome in patients with stage III colon cancer. Br J
Cancer. 110:435–440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Unitt E, Marshall A, Gelson W, Rushbrook
SM, Davies S, Vowler SL, Morris LS, Coleman N and Alexander GJ:
Tumour lymphocytic infiltrate and recurrence of hepatocellular
carcinoma following liver transplantation. J Hepatol. 45:246–253.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zikos TA, Donnenberg AD, Landreneau RJ,
Luketich JD and Donnenberg VS: Lung T-cell subset composition at
the time of surgical resection is a prognostic indicator in
nonsmall cell lung cancer. Cancer Immunol Immunother. 60:819–827.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY,
Xiao YS, Xu Y, Li YW and Tang ZY: Intratumoral balance of
regulatory and cytotoxic T cells is associated with prognosis of
hepatocellular carcinoma after resection. J Clin Oncol.
25:2586–2593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Evani SJ, Prabhu RG, Gnanaruban V, Finol
EA and Ramasubramanian AK: Monocytes mediate metastatic breast
tumor cell adhesion to endothelium under flow. FASEB J.
27:3017–3029. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shi C and Pamer EG: Monocyte recruitment
during infection and inflammation. Nat Rev Immunol. 11:762–774.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hagemann T and Lawrence T: Investigating
macrophage and malign interactions in vitro. Methods Mol Biol.
512:325–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tadmor T: Does monocyte count have
prognostic significance in cancer? Leuk Res. 37:1193–1194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sasaki A, Iwashita Y, Shibata K, Matsumoto
T, Ohta M and Kitano S: Prognostic value of preoperative peripheral
blood monocyte count inpatients with hepatocellular carcinoma.
Surgery. 139:755–764. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lin GN, Jiang XM, Peng JW, Xiao JJ, Liu DY
and Xia ZJ: Prognostic significance of the peripheral blood
absolute monocyte count inpatients with locally advanced or
metastatic hepatocellular carcinoma receiving systemic
chemotherapy. Asian Pac J Cancer Prev. 15:6387–6390. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang J, Feng G, Zhao Y, Zhang J, Feng L
and Yang J: Association between lymphocyte-to-monocyte ratio (LMR)
and the mortality of HBV-related liver cirrhosis: A retrospective
cohort study. BMJ Open. 5:e0080332015. View Article : Google Scholar : PubMed/NCBI
|