Introduction
Osteosarcoma is one of the most common pediatric
malignancies and accounts for up to 15% of childhood cancers
(1). Osteosarcoma is the major form
of bone and soft tissue primary malignant tumor, and is
characterized by specific tumor cell proliferation, early and rapid
metastasis that also occurs at the local primary site, and a high
mortality rate (2). Despite the
development of novel treatments for osteosarcoma, including
neo-adjuvant chemotherapy combined with wide excision of tumors or
the amputation of the affected limbs, which has resulted in
improved survival rates in patients who present with non-metastatic
osteosarcoma in their extremities, the survival rate in patients
with osteosarcoma in general has only demonstrated slight
improvements (3). In particular,
early metastasis is the key risk factor responsible for the low
survival rate. Previous studies have also demonstrated that ~30% of
patients with no evidence of metastasis at diagnosis who were
treated with wide tumor resection and intensive adjuvant
chemotherapy may develop lung metastases later (4,5), leading
to poor survival. Therefore, more effective and earlier diagnosis
of osteosarcoma is critical for the early initiation of treatment
and resultant improved survival of patients. In conjunction with
traditional factors that influence patient survival, including age,
sex, tumor location, size, differentiation and lymph node
metastasis, molecular genetics technology has been employed to
predict prognosis in osteosarcoma diagnosed at an earlier stage
(6–9).
Enhanced glucose metabolism is one of the principal
alterations observed in malignant tissue, and malignant cells often
exhibit augmented expression levels of glucose transport genes.
Glucose transporters (Gluts) are a group of proteins expressed on
the cytoplasmic side of the plasma membrane, which are involved in
energy-independent glucose transport. As a member of the Glut
family, Glut-1 is the most common form of human glucose transporter
and is crucial for glucose metabolism (10,11).
Glut-1 expression has been demonstrated to be associated with
enhanced glucose uptake, resulting improved glucose metabolism
which provides additional energy to meet the requirements tumor
cells as they proliferate and adapt to severe microenvironments
(12–14). In addition, previous studies have
demonstrated that Glut-1 is the predominant glucose transporter
that is significantly overexpressed in various types of tumor cell,
and its expression is correlated with poor prognosis (15–17).
Overexpression of Glut-1 may be associated with
clinical outcome in bone and soft tissue sarcomas (18), and expression levels of Glut-1 may be
negatively associated with survival time and tumor microvessel
density in patients with osteosarcoma (19). Furthermore, Glut-1 protein is
positively overexpressed in osteosarcoma, and downregulation of
Glut-1 has the capacity to inhibit the formation, growth and
invasion of osteosarcoma cells in vitro and in vivo
(20,21), further indicating the potential of
using Glut-1 to assess the malignancy of bone tumors and as a
predictor of survival in patients with osteosarcoma. However, the
potential function and clinical value of Glut-1 expression in
osteosarcoma still remains unclear, particularly in terms of the
prospective association between Glut-1 expression and
clinicopathological factors. To the best of our knowledge, no
previous studies have investigated the association between Glut-1
expression and other pathological variables including age, sex,
tumor location, size, differentiation, T stage, lymph node
metastasis, tumor-node-metastasis (TNM) stage, inner metastasis,
recurrence and reaction to chemotherapy. It is possible to use this
information to demonstrate the relationships between Glut-1
expression levels and the prognosis of patients with
osteosarcoma.
In the present study, to evaluate the potential
value of Glut-1 in predicting the prognosis of osteosarcoma
patients, 51 paired human osteosarcoma specimens and adjacent
non-cancerous tissues from the last ten years were retrospectively
collected and analyzed to investigate the associations between
Glut-1 expression levels and clinicopathological variables.
Materials and methods
Patients
A total of 51 patients with osteosarcoma with
complete clinical data who underwent surgical resection in the
Orthopedic Department of Tongji Hospital, Tongji University
(Shanghai, China) between April 1993 and March 2012 were
retrospectively reviewed. The surgical specimens included
paraffin-embedded primary osteosarcoma tissues and paired control
tissues adjacent to the carcinoma specimens. The 51 specimens of
osteosarcoma were from 28 male and 23 female patients between
13.3–71.2 years old (average age, 34.6 years). All patients
received adjuvant chemotherapy, including conventional doxorubicin
in combination with methotrexate treatment without radiotherapy
prior to surgery. The adjuvant chemotherapy consisted of 30 mg
doxorubicin combined with 40 mg methotrexate once a week and
continued for three weeks as one period of treatment. Each period
was had interval of 3 weeks and three periods were usually used for
each patient. The histological responses to adjuvant chemotherapy
were determined by the Huvos grading scale (22). Surgical procedures consisted of wide
or marginal resection as described by Enneking et al
(23). Age, sex, tumor location,
size, differentiation, T stage, lymph node metastasis, TNM stage,
inner metastasis, recurrence and reaction to chemotherapy were
recorded prior to surgery (Table I).
The TNM stage was determined according to the American Joint
Committee on Cancer (24). A total of
15 patients were diagnosed with inner metastasis and metastases,
including lung (n=8), liver (n=5) and bone (n=2). All patients were
followed with chest X-ray or computed tomography scans every 3
months during the first year following the completion of treatment,
then every 6 months for at least 5 years to investigate the
recurrence and survival of these cases. Survival time was defined
as the period from diagnosis to mortality from any cause except
emergency traffic accidents or physical diseases of numerous
patients following identification of the tumor and developed during
the study period. The follow-up duration was dated from the day of
diagnosis, and the median follow up time was 6 years 5 months
(range, 62–242 months). The postoperative pathology specimens were
all confirmed for osteosarcoma. The present retrospective study was
approved by the Institutional Human Research Ethics Review Board of
Tongji Hospital.
 | Table I.Polymerase chain reaction primer
sequences used in the present study. |
Table I.
Polymerase chain reaction primer
sequences used in the present study.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| Glut-1 |
CCATCCACCACACTCACCAC |
GCCCAGGATCAGCATCTCAA |
| GAPDH |
TGCACCACCAACTGCTTAGC |
GGCATGGACTGTGGTCATGAG |
Immunohistochemical analysis of Glut-1
protein expression
Paired specimens of osteosarcoma and tissues
adjacent to the carcinoma were routinely embedded in paraffin and
sectioned (5 µm). The fresh sections were subsequently dewaxed with
xylene and dehydrated with a graded ethanol series (100 and 70%)
two times for 10 min each. Endogenous peroxidase was blocked by
incubating the sections with 3% hydrogen peroxide in 50% methanol
for 30 min at room temperature. Pre-warmed Dako target retrieval
solution (pH 6; Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA) was used for the antigen retrieval and non-specific protein
binding was blocked by incubation with 10% normal rabbit serum
(Dako; Agilent Technologies, Inc.) in 1% bovine serum albumin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)/PBS for 1.5 h in a
humidified chamber at room temperature. Subsequent to washing with
PBS, the slides were incubated at 4°C overnight with polyclonal
Glut-1 antibodies (dilution, 1:200; catalog no. MA5-11315; Thermo
Scientific Lab Vision; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), after which they were incubated with horseradish
peroxidase-conjugated secondary antibody (dilution, 1:500; catalog
no., PA1-28587, Thermo Scientific Lab Vision) for 1 h at room
temperature. These slides were then processed for
3,3′-Diaminobenzidine (DAB) substrate solution (Sigma-Aldrick;
Merck KGaA) reaction following the manufacturer's protocol. Ten
random fields of view from each section were examined and analyzed
using an imaging system (catalog no. HMIAS-2000; Champion Medical
Imaging Co., Wuhan, China). Cells with characteristic membranous
and/or cytoplasmic staining were identified as Glut-1 positive
(Glut-1+) cells. The Glut-1+ staining
intensity was also expressed as the number of Glut-1+
cells/the total number of cells ×100, and was divided into three
categories: <10%, negative; 10–50%, weak positive; >50%,
strong positive, as previously described (18).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to further verify the
expression levels of Glut-1 in 6 paired specimens of osteosarcoma
and tissue adjacent to the carcinoma, in which the Glut-1+ staining
intensities were identified as positive (>10%) by
immunohistochemistry. Extraction and purification of total RNA was
conducted using the TRIzol RNA isolation kit (Invitrogen; Thermo
Fisher Scientific, Inc.). All RNA samples were diluted to 1 µg/l
and were reverse-transcribed using the PrimeScript RT-PCR kit
(Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
instructions. The PCR primers for Glut-1 were obtained from
Fermentas; Thermo Fisher Scientific, Inc. (Table I). PCR assays were run in a Real-Time
PCR System (ABI 7500; Applied Biosystems; Thermo Fisher Scientific,
Inc.) using iTaq Universal SYBR-Green Supermix (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). PCR was conducted as
follows: 95°C for 10 sec; 40 cycles of 95°C for 5 sec; and 60°C for
34 sec. Analysis of RT-PCR data was performed using the comparative
Cq (2-ΔCq) method to calculate levels of gene expression relative
to the internal control gene, glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) as previously described (25).
Statistical analysis
Statistical analysis was performed using the
statistical software R (version 3.01, Nokia Bell Labs, Murray Hill,
NJ, USA). A paired Student's t-test was used to identify
significant differences in Glut-1 mRNA expression levels between
osteosarcoma and tissues adjacent to carcinoma. Fisher's test was
used to test the association between Glut-1 expression levels and
clinicopathological variables. Cumulative survival rate was
estimated using the Kaplan-Meier method, log-rank tests were
performed to test the survival time difference, and univariate and
multivariate proportional hazards (Cox) regressions were used to
test the associations between survival time, and
clinicopathological variables and Glut-1 expression. In the
multivariate Cox regression, those associated variables were
step-wisely selected according to Akaike's information criterion
(26). P<0.05 was considered to
indicate a statistically significant difference. In the figures,
the symbols * and ** represent P<0.05 and P<0.01,
respectively.
Results
Glut-1 protein expression in
osteosarcoma and tissues adjacent to carcinoma
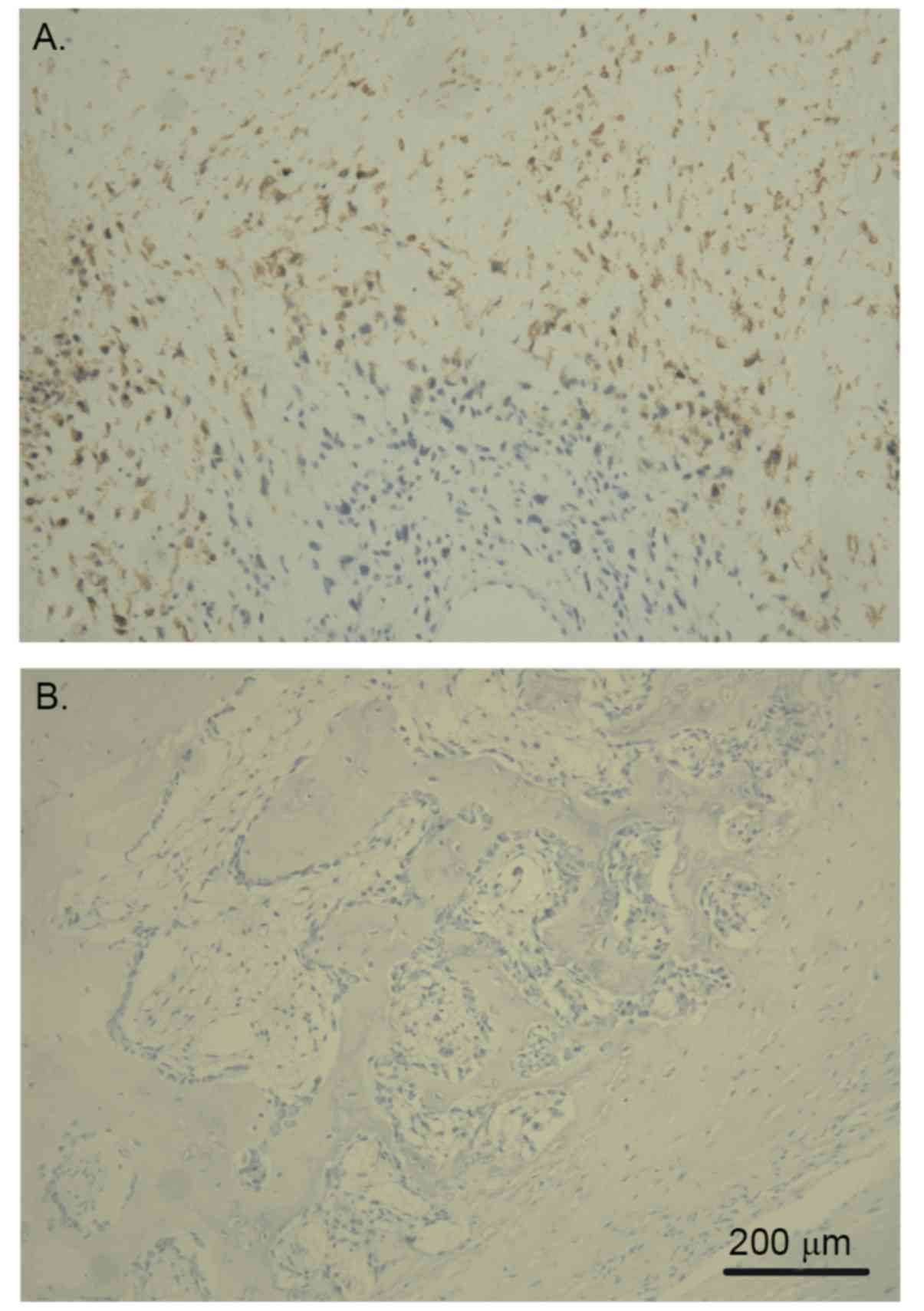
In general, Glut-1 protein was revealed to primarily
be expressed in osteosarcoma cell membranes and cytoplasm, with the
immunostaining having a focal or diffuse distribution pattern. The
intensity of Glut-1+ cellular staining in osteosarcoma
was significantly higher than that in paired tissue adjacent to
carcinoma. In 38 (74.5%) of 51 patients with osteosarcoma, the
expression of Glut-1 was positive. Indeed, half (19) of these patients demonstrated strong
expression (Table I). On the other
hand, only 6 (11.8%) of 51 patients had positive expression of
Glut-1 in tissue adjacent to carcinoma and none of them had a
strong expression intensity (Table
II). In addition, in osteosarcoma samples, more intensely
positive staining was observed in the center of the tumor tissue,
with the positive intensity becoming stronger with increased
distance from the stromal blood vessel (Fig. 1).
 | Table II.Qualitative analysis of glucose
transporter protein-1 immunostaining in osteosarcoma and tissue
adjacent to carcinoma. |
Table II.
Qualitative analysis of glucose
transporter protein-1 immunostaining in osteosarcoma and tissue
adjacent to carcinoma.
| Cases | Osteosarcoma
tissue | Tissue adjacent to
carcinoma |
|---|
| Total number of
cases, n | 51 | 51 |
| Cases with negative
staining, n (%) | 13 (25.5) | 45 (88.2) |
| Cases with weak
positive staining, n (%) | 19 (37.3) | 6 (11.8) |
| Cases with strong
positive staining, n (%) | 19 (37.3) | 0 (0) |
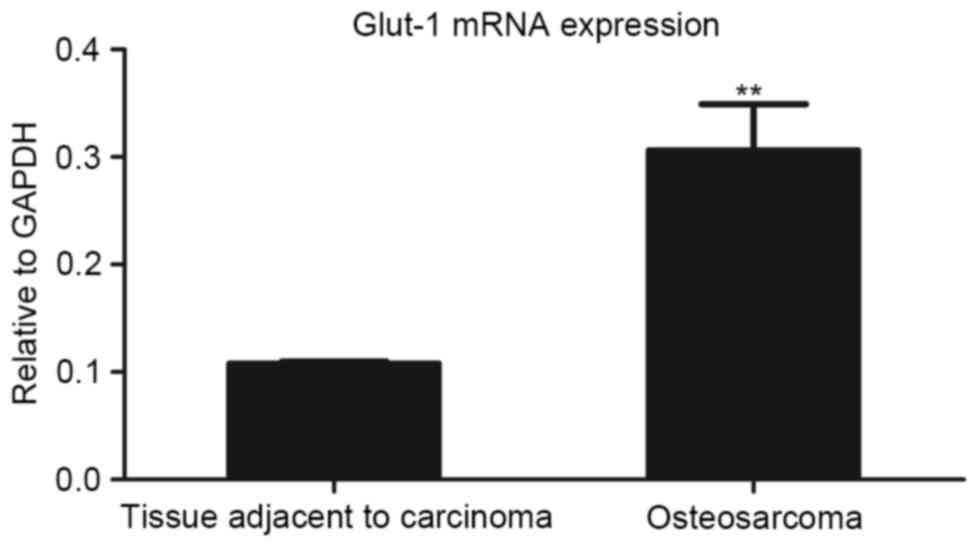
Glut-1 mRNA expression in fresh
specimens of osteosarcoma and tissues adjacent to carcinoma
To determine the differences in Glut-1 mRNA
expression levels within or adjacent to carcinoma tissues, RT-qPCR
analysis was conducted with freshly frozen specimens. The mRNA
expression levels of Glut-1 in osteosarcoma tissues were
significantly higher than those in tissues adjacent to carcinoma
(P<0.01; Fig. 2).
Associations between Glut-1 expression
and osteosarcoma clinicopathological parameters
Fisher's test was conducted to identify associations
between Glut-1 expression and clinicopathological parameters. Sex,
age, tumor site, T stage, inner metastasis and reaction to
chemotherapy were not associated with Glut-1 expression (Table III). On the other hand, tumor
volume, differentiation, lymph node metastasis, TNM stage and
recurrence were observed to have a significant association with
Glut-1 expression (Table II). Due to
missing data in a few patients, the sample sizes for recurrence and
reaction to chemotherapy were 49 and 50, respectively.
 | Table III.Relationship between glucose
transporter protein-1 expression and clinicopathological
characteristics. |
Table III.
Relationship between glucose
transporter protein-1 expression and clinicopathological
characteristics.
| Clinicopathological
characteristic | n | Postive (n, %) | Negative (n, %) | P-value |
|---|
| Sex |
| Male | 28 | 22 (43.1) | 6
(11.8) |
0.529 |
|
Female | 23 | 16 (31.4) | 7
(13.7) |
|
| Age |
| <30
year | 28 | 23 (45.1) | 5 (9.8) |
0.207 |
| ≥30
year | 23 | 15 (29.4) | 8
(15.7) |
|
| Tumor site |
| Distal
femur | 26 | 20 (39.2) | 6
(11.8) |
0.755 |
| Proximal
tibia | 25 | 18 (35.3) | 7
(13.7) |
|
| Tumor volume |
| <3
cm | 5 | 4 (7.8) | 1 (2.0) |
0.012 |
| ≥3
cm | 46 | 37 (72.5) | 9
(17.6) |
|
|
Differentiation |
|
Well-differentiated | 13 | 5 (9.8) | 8
(15.7) |
0.001 |
|
Moderately differentiated | 38 | 33 (64.7) | 5 (9.8) |
|
| T stage |
|
T1+T2 | 20 | 13 (25.5) | 7
(13.7) |
0.513 |
| T3 | 23 | 18 (35.3) | 5 (9.8) |
|
| T4 | 8 | 7
(13.7) | 1 (2.0) |
|
| Lymph node
metastasis |
| N0 | 16 | 8
(15.7) | 8
(15.7) |
0.013 |
| N1 | 35 | 30 (58.8) | 5 (9.8) |
|
| TNM stage |
| I | 13 | 5 (9.8) | 8
(15.7) |
0.001 |
| II | 29 | 24 (47.1) | 5 (9.8) |
|
|
III | 9 | 9
(17.6) | 0 (0.0) |
|
| Inner
metastasis |
| No | 36 | 25 (49.0) | 11 (21.6) |
0.297 |
|
Yes | 15 | 13 (25.5) | 2 (3.9) |
|
| Recurrence |
| No | 14 | 13 (26.5) | 1 (2.0) | <0.001 |
|
Yes | 35 | 34 (69.4) | 1 (2.0) |
|
| Reaction to
chemotherapy |
| No | 23 | 17 (34.0) | 6
(12.0) |
0.510 |
|
Yes | 27 | 20 (40.0) | 7
(14.1) |
|
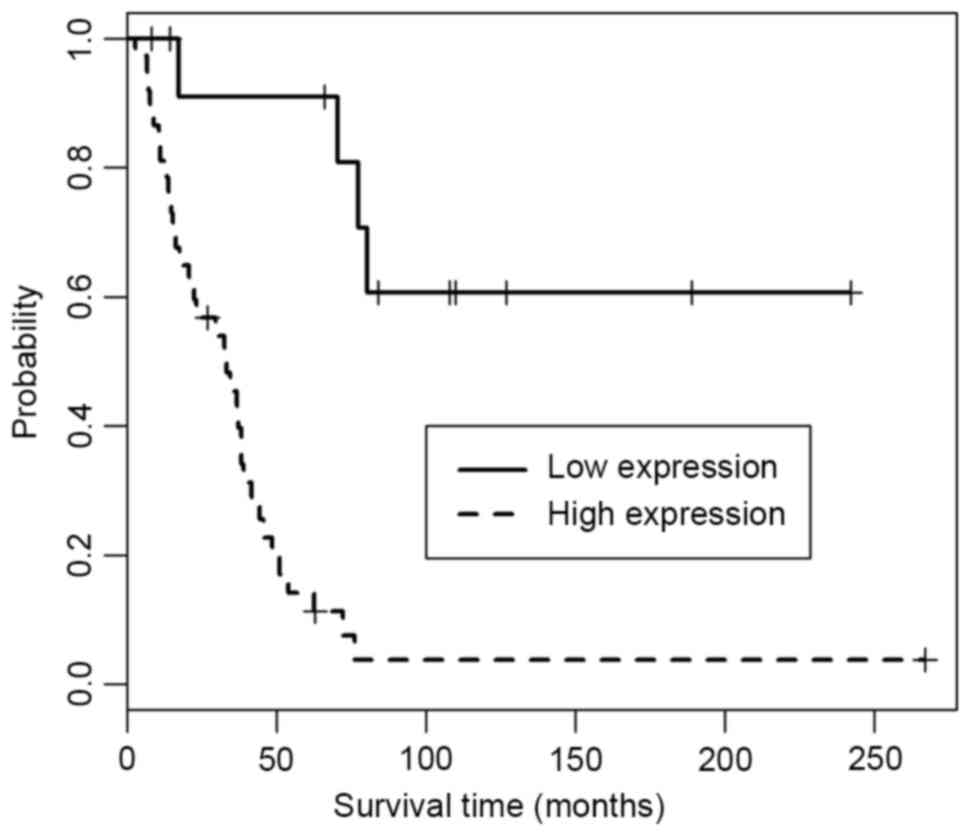
Association between Glut-1 expression
and postoperative survival of osteosarcoma patients
While the survival time was the period between
diagnosis and death for patients with tumor recurrence, in a few
cases, patients died of other diseases during the follow-up phase,
and this was considered as a truncated event. In the present study,
the median survival time was defined as the time of 50% cumulative
survival rates, following which half of the patients were still
living. Kaplan-Meier survival curves were presented for the
osteosarcoma patients with high or low Glut-1 expression (Fig. 3). From the survival curves, the median
survival time for patients with low Glut-1 expression was observed
to be 540 days, while for patients with high Glut-1 expression it
was 317 days. In addition, Glut-1 overexpression was observed to be
associated with a poor survival time. The survival curve of the
patients with high expression significantly differed from that of
the patients with low expression (P=1.39×10-05, as determined by
the log-rank test).
Single and multivariate Cox regression
analyses of prognosis and survival
Single proportional hazards (Cox) regression
analysis revealed that sex, tumor site, tumor size and reaction to
chemotherapy were not significantly associated with survival time
(P>0.05; Table III). On the
other hand, age, differentiation, inner metastasis, recurrence, T
stage, lymph, TNM stage and Glut-1 expression were revealed to have
a significant association with survival time (P<0.05; Table III). However, in the following
multivariate Cox regression analysis, the effects of
differentiation, inner metastasis and recurrence were masked by the
other risk factors due to collinearity. T stage, lymph, TNM stage
and Glut-1 expression were still observed to be associated with
survival time (P<0.05; Tables IV
and V).
 | Table IV.Single Cox regression analysis. |
Table IV.
Single Cox regression analysis.
| Factors | Coding | Hazard ratio | 2.5% limit | 97.5% limit | P-value |
|---|
| Sex | Male vs.
female | 0.924 | 0.488 |
1.749 |
0.808 |
| Age |
| 0.360 | 0.182 | 0.715 |
0.004 |
| Site | Distal femur vs.
proximal tibia | 0.887 | 0.468 |
1.684 |
0.715 |
| Size | ≥3 cm vs. <3
cm | 3.329 | 0.793 | 13.983 |
0.101 |
|
Differentiation | Poor vs. well | 4.458 | 1.824 | 10.897 |
0.001 |
| T stage |
T4>T3>T1&T2 | 4.982 | 2.713 |
9.150 | <0.001 |
| Lymph | N1 vs. N0 | 9.590 | 3.608 | 25.490 | <0.001 |
| TNM stage | III>II>I | 6.780 | 3.196 | 14.383 | <0.001 |
| Inner
metastasis | Yes vs. no | 2.598 | 1.283 |
5.261 |
0.008 |
| Recurrence | Yes vs. no | 57.158 | 7.434 | 439.195 | <0.001 |
| Reaction to
chemotherapy | Poor vs. good | 1.137 | 0.597 |
2.168 |
0.696 |
| Glut-1
expression | High vs. low |
8.75007 | 2.902 | 26.386 | <0.001 |
 | Table V.Multivariate Cox regression
analysis. |
Table V.
Multivariate Cox regression
analysis.
| Factors | Hazard ratio | 2.5% limit | 97.5% limit | z statistics | P-value |
|---|
| Age |
0.301 | 0.136 |
0.611 |
0.0294 |
0.003 |
| T stage |
4.916 | 1.968 |
12.282 | 3.409 | <0.001 |
| Lymph | 14.473 | 2.875 |
72.858 | 3.241 |
0.001 |
| TNM stage |
8.519 | 3.194 |
22.722 | 4.280 | <0.001 |
| Glut-1
expression | 22.351 | 4.479 | 111.521 | 3.788 | <0.001 |
Discussion
Glut-1 is an essential carrier responsible for
glucose transportation across the plasma membrane of cells.
Cellular regulation of glucose intake is dependent on Glut-1
expression and function, either through active transport or
facilitated diffusion and even under the circumstance of a low
glucose concentration. Previous studies have demonstrated that
Glut-1 is usually expressed at low levels in mammalian embryos and
mature tissues, providing basic energy for normal cell growth and
function. On the other hand, Glut-1 is typically expressed at a
high level in multiple types of malignant carcinoma tissue and in
atypical hyperplasia tissues with a high cancer risk, and this is
believed to meet the requirements for increased absorption and
utilization of glucose of the tumor cells (27). Although Glut-1 expression levels have
been investigated in various types of tumor (15–17), no
further literature has reported the association between Glut-1
expression and osteosarcoma beyond those of Endo et al
(18), Kubo et al (19) and the present study. In the present
study, in 51 paired human osteosarcoma specimens and adjacent
non-cancerous specimens collected between April 1993 and March
2012, Glut-1 expression levels were examined using
immunohistochemistry and RT-qPCR. In total, 74.5% of osteosarcoma
tissues stained positive for Glut-1, but only 11.8% adjacent
tissues stained positively for Glut-1. The mRNA expression level of
Glut-1 was also higher in osteosarcoma compared with non-cancerous
tissues. These results were consistent with the results obtained by
Endo et al (18). Furthermore,
the associations between the Glut-1 expression and
clinicopathological parameters of osteosarcoma were
investigated.
The associations between Glut-1 expression and
clinicopathological parameters have previously been investigated in
certain other types of malignant tumor. Glut-1 expression in lung
cancer was demonstrated to be associated with its malignant stage,
with more advanced stages typically being accompanied with higher
expression levels of Glut-1 (28).
Expression of Glut-1 in endometrial lesions has also been
demonstrated to be associated with cancer differentiation, and it
is possible to use Glut-1 expression to effectively distinguish the
malignant tendency from a benign tissue to atypical hyperplasia of
the endometrium (29). Similarly, in
pancreatic ductal adenocarcinoma and laryngeal cancer, Glut-1
expression levels have been demonstrated to be positively
correlated with the clinical malignant stage (30). However, the associations between
Glut-1 expression and clinicopathological parameters of
osteosarcoma have not previously been reported. In the present
study, based on the recorded clinical pathological characteristics
for the collected specimens, the associations between Glut-1
expression levels, pathological variables and survival of the
patients were examined with statistical methods, to evaluate the
value of Glut-1 expression levels as a predictor of prognosis in
osteosarcoma. The results revealed that the expression levels of
Glut-1 were positively associated with osteosarcoma tumor volume,
differentiation, lymph node metastasis, TNM stage and recurrence,
indicating that Glut-1 is involved in the incidence of
osteosarcoma. Since Glut-1 provides an energy supply for the rapid
progression of malignant osteosarcoma, higher expression levels of
Glut-1 are consistent with the malignant status. The data from the
present study also demonstrated that Glut-1 expression levels are
associated with cancer recurrence and metastasis.
In the present study, the median survival time of
patients with positive expression of Glut-1 was decreased compared
with those with negative expression of Glut-1. From the univariate
analysis, patients with high Glut-1 expression and patients with
low Glut-1 expression were revealed to have significant differences
in survival rate. Multivariate analysis also revealed that the
hazard ratio of the patients with high expression of Glut-1 was
22.4 times (95% confidence interval=4.5–111.5;
P=1.51×10−4) higher compared with patients with low
expression. These results suggested that decreased survival time
caused by the proliferative and invasive behaviors of malignant
cells was significantly associated with Glut-1 overexpression.
Therefore, Glut-1 has the potential to be a prognostic marker for
osteosarcoma.
Aside from Glut-1 expression, there were other
potential prognostic factors observed by the present study to be
associated with survival time, including age, T stage, lymph and
TNM stage, which is similar to the results of Endo et al
(18). However, in clinical practice,
while the adoption by surgeons of these factors as prognostic
indicators may be more subjective, determining Glut-1 expression
levels by immunohistochemistry is comparatively more objective and
reliable. Therefore, Glut-1 expression levels may provide us with
an independent and valuable reference that distinguishes high risk
patients and develops an adapted therapeutic strategy (based on the
levels of risk) for osteosarcoma treatment.
Although the present study is based on clinical
data, other studies concerning the effects of inhibiting glucose
transport in osteosarcoma have been conducted. The results obtained
from these in vivo and in osteosarcoma cell in vitro
studies are consistent with those from the present study (4,16). They
demonstrated that Glut-1 expression is a key and independent
prognostic factor for survival in osteosarcoma patients, supporting
the idea that assessment of Glut-1 expression should be performed
prior to treatment to predict the potential clinical effects.
The present study contains a few notable
limitations. Due to the individual differences of the patient's
physique and treatment, it is challenging to obtain
clinicopathological data under the same circumstances. Meanwhile,
the judgment of certain clinical pathological parameters is
subjective and may lead to deviations. In addition, the follow-up
phase for evaluating patient survival rates and the total number of
patients remains limited, which may also affect the results.
Therefore, to provide more definitive conclusions, further
multi-institution studies are required with longer follow-up
durations and larger patient populations.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of Shanghai (grant no. 14ZR1437800), the
Foundation of Shanghai Municipal Bureau of Health (grant no.
20134328), and in part by the Australian National Health and
Medical Research Council Senior Research Fellowship. Dr Qian Tang
was supported by Australian Postgraduate Award Scholarship and Dr
Longhui Chen received support from the China Scholarship Council as
a joint PhD student at the University of Pennsylvania,
Philadelphia, PA, USA.
References
|
1
|
Arndt CA, Rose PS, Folpe AL and Laack NN:
Common musculoskeletal tumors of childhood and adolescence. Mayo
Clin Proc. 87:pp. 475–487. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mankin HJ, Hornicek FJ, Rosenberg AE,
Harmon DC and Gebhardt MC: Survival data for 648 patients with
osteosarcoma treated at one institution. Clin Orthop Relat Res.
1–291. 2004.PubMed/NCBI
|
|
3
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eselgrim M, Grunert H, Kühne T, Zoubek A,
Kevric M, Bürger H, Jürgens H, Mayer-Steinacker R, Gosheger G and
Bielack SS: Dose intensity of chemotherapy for osteosarcoma and
outcome in the Cooperative Osteosarcoma Study Group (COSS) trials.
Pediatr Blood Cancer. 47:42–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lewis IJ, Nooij MA, Whelan J, Sydes MR,
Grimer R, Hogendoorn PC, Memon MA, Weeden S, Uscinska BM, van
Glabbeke M, et al: Improvement in histologic response but not
survival in osteosarcoma patients treated with intensified
chemotherapy: A randomized phase III trial of the European
Osteosarcoma Intergroup. J Natl Cancer Inst. 99:112–128. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou SH, Fan J, Chen XM, Cheng KJ and Wang
SQ: Inhibition of cell proliferation and glucose uptake in human
laryngeal carcinoma cells by antisense oligonucleotides against
glucose transporter-1. Head Neck. 31:1624–1633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hasegawa T, Yamamoto S, Yokoyama R, Umeda
T, Matsuno Y and Hirohashi S: Prognostic significance of grading
and staging system using MIB-1 score in adult patients with soft
tissue sarcoma of the extremities and trunk. Cancer. 95:843–851.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Sun MX, Hua YQ and Cai ZD:
Prognostic significance of serum lactate dehydrogenase level in
osteosarcoma: A meta-analysis. J Cancer Res Clin Oncol.
140:1205–1210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim MS, Lee SY, Cho WH, Song WS, Koh JS,
Lee JA, Yoo JY, Jung ST and Jeon DG: Effect of increases in tumor
volume after neoadjuvant chemotherapy on the outcome of stage II
osteosarcoma regardless of histological response. J Orthop Sci.
14:292–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ito H, Duxbury M, Zinner MJ, Ashley SW and
Whang EE: Glucose transporter-1 gene expression is associated with
pancreatic cancer invasiveness and MMP-2 activity. Surgery.
136:548–556. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vleugel MM, Greijer AE, Shvarts A, van der
Groep P, van Berkel M, Aarbodem Y, van Tinteren H, Harris AL, van
Diest PJ and van der Wall E: Differential prognostic impact of
hypoxia induced and diffuse HIF-1alpha expression in invasive
breast cancer. J Clin Pathol. 58:172–177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mao ZP, Zhao LJ, Zhou SH, Liu MQ, Tan WF
and Yao HT: Expression and significance of glucose transporter-1,
P-glycoprotein, multidrug resistance-associated protein and
glutathione S-transferase-π in laryngeal carcinoma. Oncol Let.
9:806–810. 2015.
|
|
13
|
Stewart GD, Gray K, Pennington CJ, Edwards
DR, Riddick AC, Ross JA and Habib FK: Analysis of
hypoxia-associated gene expression in prostate cancer: Lysyl
oxidase and glucose transporter-1 expression correlate with Gleason
score. Oncol Rep. 20:1561–1567. 2008.PubMed/NCBI
|
|
14
|
Kunkel M, Reichert TE, Benz P, Lehr HA,
Jeong JH, Wieand S, Bartenstein P, Wagner W and Whiteside TL:
Overexpression of Glut-1 and increased glucose metabolism in tumors
are associated with a poor prognosis in patients with oral squamous
cell carcinoma. Cancer. 97:1015–1024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manolescu AR, Witkowska K, Kinnaird A,
Cessford T and Cheeseman C: Facilitated hexose transporters: New
perspectives on form and function. Physiology (Bethesda).
22:234–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cooper R, Sarioğlu S, Sökmen S, Füzün M,
Küpelioğlu A, Valentine H, Görken IB, Airley R and West C: Glucose
transporter-1 (GLUT-1): A potential marker of prognosis in rectal
carcinoma? Br J Cancer. 89:870–876. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amann T, Maegdefrau U, Hartmann A, Agaimy
A, Marienhagen J, Agaimy A, Marienhagen J, Weiss TS, Stoeltzing O,
Warnecke C, et al: GLUT1 expression is increased in hepatocellular
carcinoma and promotes tumorigenesis. Am J Pathol. 174:1544–1552.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Endo M, Tateishi U, Seki K, Yamaguchi U,
Nakatani F, Kawai A, Chuman H and Beppu Y: Prognostic implications
of glucose transporter protein-1 (Glut-1) overexpression in bone
and soft-tissue sarcomas. Jpn J Clin Oncol. 37:955–960. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kubo T, Shimose S, Fujimori J, Furuta T,
Arihiro K and Ochi M: Does expression of glucose transporter
protein-1 relate to prognosis and angiogenesis in osteosarcoma?
Clin Orthop Relat Res. 473:305–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan J, Zhou JQ, Yu GR and Lu DD: Glucose
transporter protein 1-targeted RNA interference inhibits growth and
invasion of the osteosarcoma cell line MG63 in vitro. Cancer
Biother Radiopharm. 25:521–527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan J, Yuan F, Jiong M, Zhu XZ, Yu GR and
Lu DD: Silencing of glucose transporter protein-1 by RNA
interference inhibits human osteosarcoma Mg63 cells growth in vivo.
Technol Cancer Res Treat. 14:243–248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huvos AG: Osteogenic sarcoma: Pathologic
assessment of preoperative (neoadjuvant) chemotherapyBone Tumors:
Diagnosis, Treatment and Prognosis. 2nd. WB Saunders; Philadelphia,
PA: pp. 122–128. 1991
|
|
23
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. Clin
Orthop Relat Res. 153:106–120. 1980.
|
|
24
|
Yarbro JW, Page DL, Fielding LP, Partridge
EE and Murphy GP: American Joint Committee on Cancer prognostic
factors consensus conference. Cancer. 86:2436–2446. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pourhoseingholi MA, Hajizadeh E, Dehkordi
B Moghimi, Safaee A, Abadi A and Zali MR: Comparing Cox regression
and parametric models for survival of patients with gastric
carcinoma. Asian Pac J Cancer Prev. 8:412–416. 2007.PubMed/NCBI
|
|
27
|
Tateishi U, Yamaguchi U, Seki K, Terauchi
T, Arai Y and Hasegawa T: Glut-1 expression and enhanced glucose
metabolism are associated with tumour grade in bone and soft tissue
sarcomas: A prospective evaluation by [18F]fluorodeoxyglucose
positron emission tomography. Eur J Nucl Med Mol Imaging.
33:683–691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sasaki H, Shitara M, Yokota K, Hikosaka Y,
Moriyama S, Yano M and Fujii Y: Overexpression of GLUT1 correlates
with Kras mutations in lung carcinomas. Med Rep. 5:599–602.
2012.
|
|
29
|
Sadlecki P, Bodnar M, Grabiec M, Marszalek
A, Walentowicz P, Sokup A, Zegarska J and Walentowicz-Sadlecka M:
The role of Hypoxia-inducible factor-1 α, glucose transporter-1,
(GLUT-1) and carbon anhydrase IX in endometrial cancer patients.
Biomed Res Int. 2014:6168502014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Melstrom LG, Salabat MR, Ding XZ, Strouch
MJ, Grippo PJ, Mirzoeva S, Pelling JC and Bentrem DJ: Apigenin
down-regulates the hypoxia response genes: HIF-1α, GLUT-1, and VEGF
in human pancreatic cancer cells. J Surg Res. 167:173–181. 2011.
View Article : Google Scholar : PubMed/NCBI
|