Introduction
Morbidity and mortality arising from lung carcinomas
account for 17% of novel cancer cases in humans each year (1), and lung cancer metastasis is the
principal reason for organ failure and patient mortality (2). Mast cells are common immune cells that
are widely distributed in the respiratory mucosa. Mast cells derive
from specific bone marrow cluster of differentiation 34+
precursor cells and migrate to other tissues where the cells
mature, depending on the internal environmental conditions
(3). Previous studies have revealed
that the number of mast cells is increased in various types of
cancer, including lung (4), breast
(5), prostate (6) and colon (7) cancer. Performing bronchoalveolar lavage
on patients with bronchial carcinoma revealed that these patients
possess an increased number of mast cells (8–10). In
addition, mast cell density has been identified to be associated
with cancer progression, angiogenesis and poor prognosis in human
adenocarcinomas (11,12).
Mast cell chymase (MCC) (EC 3.4.21.39) is a
chymotrypsin-like protease enzyme which is expressed in the
secretory granules of mast cells. MCC is able to degrade the
extracellular matrix (ECM) of animal tissue (13). ECM turnover involves the alteration of
the cellular microenvironment within tissue, and is able to
influence carcinoma cell migration, adhesion and relocalization
(14). Matrix metalloproteinase-9
(MMP-9) belongs to the class of tissue matrix metalloproteinases
which primarily degrade and remodel the ECM (15). MMP-9 has been identified to be an
integral part of numerous diseases, including cancer, where
modulation of the ECM is a key step (16–18).
Epithelial (E-) cadherin is present in various
epithelial cells and tumor cells (19); it is a fundamental component of the
adherens junctions (the cytoplasmic connection between neighboring
cells) and is known to mediate aggregation-dependent cell survival
(20). Loss of E-cadherin gene
expression in carcinoma cells may lead to increased cell apoptosis,
cell death, cell invasion and metastasis (21,22). The
protein p53 is a known carcinoma suppressor which is commonly
associated with the pathogenesis of human carcinoma (23). The p53 protein is involved in the
response to DNA damage, cell cycle regulation and cell apoptosis
(23). This protein also controls
cellular progression from G1 to S phase in the cell
cycle. When cellular DNA is damaged, p53 may initiate the synthesis
of p21, which is a cyclin-dependent kinase (CDK) inhibitor protein.
In turn, p21 may combine with cyclin-CDK to form a trimer which
prevents the damaged cells progressing from G1 to S
phase (24).
The aim of the present study was to investigate
whether MCC is involved in carcinoma cytology, the progression to
metastasis through degradation of the ECM, cleavage of
intercellular connections by proteolysis of E-cadherin and how
expression of MMP-9, p53 and p21 proteins were triggered which
determine cell apoptosis or cell survival. In the present study, a
schematic model of the role of MCC in the fate of lung carcinoma
cells was proposed, and it was hypothesized that MCC may affect the
biological features of lung cancer cells.
Materials and methods
Cell lines
A549 human adenocarcinoma alveolar basal epithelial
cells and H520 human lung squamous carcinoma cells were purchased
from the Cell Bank of the Chinese Academy of Science (Shanghai,
China).
Cell viability via the MTT assay
Suspensions of each cell line were conventionally
prepared in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), and the density was adjusted to
105 cells/ml. Aliquots of 100 µl were added to each well
of 96-well plates and treated with various concentrations of
serum-free human MCC (a gift from Dr A.F. Walls, Southampton
University, Southampton, UK) [0(control), 5, 25, 50 and 100 mU/ml
or heat-inactivated (65°C for 30 min) MCC (100 mU/ml, incubated at
37°C in 5% CO2 for 6, 24, 48 or 72 h)]. Following
incubation for the prescribed time, 10 µl MTT (5 mg/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to each
well prior to incubation for another 4 h. In order to solubilize
the formazan dye, 150 µl dimethyl sulfoxide (Sigma-Aldrich; Merck
KGaA) was added to each well and the absorbance was determined at
492 nm using a microplate reader (Thermo Fisher Scientific, Inc.).
This protocol was followed three times for each cell line.
Cell adhesion
A suspension of the A549 cell line was adjusted to
106 cells/ml and co-cultured with MCC [0, 5, 25, 50 and
100 mU/ml or heat-inactivated MCC (100 mU/ml)] in centrifuge tubes,
with gentle agitation for 30 min at 50 × g to avoid cell clumping.
Aliquots of 100 µl each treated cell suspension were transferred to
a 96-well plate and incubated for 2 h; this was to allow
aggregation of adherent cells, prior to removing the suspension
medium, and MTT staining of the unsuspended adherent cells. The MTT
assay was performed described as above, the cell adhesion rate (%)
relative to that of the untreated controls was calculated as
follows: [Optical density (OD) value of the experimental group/OD
value of the control group]x100%.
Cell re-adhesion and survival
Following MCC treatment as aforementioned, a number
of cells detached from adherent clusters and remained suspended in
the culture medium, and were therefore not included in the MTT
analysis. To investigate whether those suspended cells had
undergone apoptosis, or whether the cells may become re-adherent
under altered conditions, fresh cell suspensions (1×105
cells/ml) were prepared as aforementioned and co-cultured with MCC
[(0, 25 and 100 mU/ml or heat-inactivated MCC (100 mU/ml) for 6,
24, 48 or 72 h]. Following stimulation with MCC, the medium
containing the detached cells was carefully transferred from each
of the treated new wells, using separate sterile pipettes, into
separate wells containing fresh MCC-free RPMI-1640 medium. The
plate was incubated at 37°C for 2 h to allow the cells to aggregate
at the bottom of the wells. The MTT method as described above was
used to determine the re-adhesion rate of the cells.
Western blot analysis
Following treatment with MCC (0, 25 or 50 mU/ml) or
heat-inactivated MCC (100 mU/ml) for 24 h, the cells were lysed
using radioimmunoprecipitation assay buffer, and protein was
quantified using the bicinchoninic acid protein assay
(Sigma-Aldrich; Merck KGaA). A total of 20 µg extracted
protein/lane was treated with sample buffer containing 5 mM
dithiothreitol at 95°C for 10 min. The samples were then separated
by SDS-PAGE (10% gel) and transferred onto a polyvinylidene
fluoride membrane (EMD Millipore, Billerica, MA, USA). The
non-specific binding sites were blocked with a solution of 5%
skimmed milk in PBS containing 0.1% Tween-20 (Sigma-Aldrich; Merck
KGaA). The primary antibodies against p53 (cat. no. sc-099; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), p21 (cat. no. sc-817;
Santa Cruz Biotechnology, Inc.) and E-cadherin (cat. no. sc-1500;
Invitrogen; Thermo Fisher Scientific, Inc.) were diluted 1:500. The
membrane was incubated overnight at 4°C. The corresponding
horseradish peroxidase-conjugated anti-mouse secondary antibody was
diluted 1:500 and added to the membrane for 1 h at room
temperature. Enhanced chemiluminescence (PerkinElmer, Inc.,
Waltham, MA, USA) was used to develop the blot and the images were
captured using a ChemiDoc™ CRS+ Molecular Imager
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Zymography
Aliquots of 20 µl serum-free cell culture medium
containing MCC-treated (0, 5, 25 or 50 mU/ml) cells were taken from
the incubated plate wells, mixed with 10 µl sample buffer solution
(0.5 M Tris-HCl, 50% glycerol, 10% SDS and 0.1% bromophenol blue),
loaded onto 8% polyacrylamide gels containing 1 mg/ml gelatin
(Sigma-Aldrich; Merck KGaA) and run at 100 V until the blue dye had
reached the bottom of the gel. Following electrophoresis, SDS was
removed from the gel using 2.5% Triton X-100 (Sigma-Aldrich; Merck
KGaA) for 1 h at room temperature. The gels were subsequently
incubated overnight in an MMP developing buffer (50 mM Tris-HCl, 25
mM NaCl and 7 mM CaCl2) at 37°C with agitation.
Subsequently, the gels were stained with 0.2% Coomassie brilliant
blue for 30 min and counterstained using a mixture of 10% acetic
acid, 50% methanol and 40% distilled water. Semi-quantification was
carried out using Image Lab software (version 7.0; Bio-Rad
Laboratories, Inc.), and alterations in the density of the bands
were calculated as percentages relative to untreated controls.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA,
USA). Student's t-test was carried out, and the data were presented
as the mean ± standard deviation (n=3–6). P<0.05 was considered
to indicate a statistically significant difference, and P<0.01
was considered to indicate a highly statistically significant
difference.
Results
Effects of MCC on lung cancer cell
viability
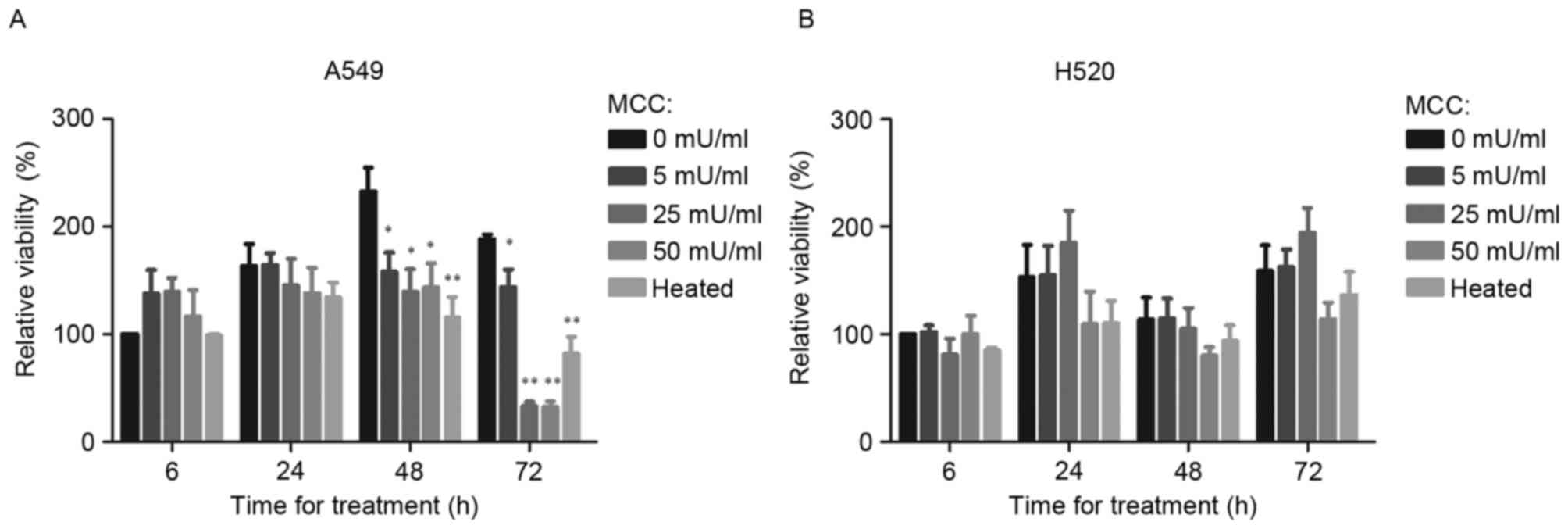
When A549 cells were treated with MCC for 6 h, their
relative viability was slightly increased. Compared with the
untreated control, treatment of A549 cells for 48 h with 5, 25 and
50 mU/ml MCC significantly decreased the relative viability
(P<0.05; Fig. 1A) as did
heat-inactivated MCC (P<0.01; Fig.
1A). In H520 cells, a small inhibitory effect from 50 mU/ml MCC
on cell viability was observed, although no significant difference
between cells treated with MCC and the untreated control group were
identified (Fig. 1B).
Effects of MCC on A549 cell
adhesion
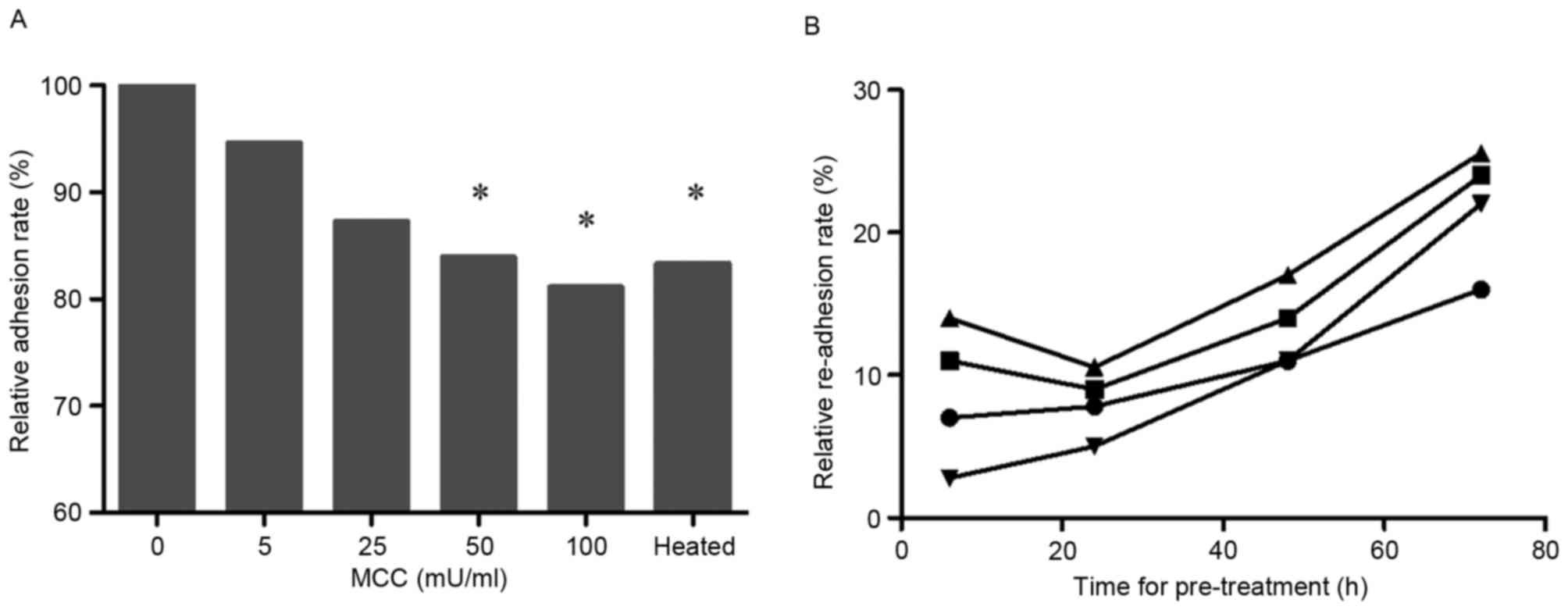
The MTT assay, carried out on suspended cells mixed
with various concentrations of MCC, revealed that, with increasing
MCC concentration, the relative adhesion rate of lung cancer cell
decreased (50 and 100 mU/ml; P<0.05; Fig. 2A).
A549 cell re-adhesion and
survival
The detached cells induced by MCC were washed and
relocated to new wells without MCC in order to promote re-adhesion
of these cells (Fig. 2B). The
re-adhesion rate was time- and dose-dependent.
Effects of MCC on E-cadherin
expression and regulation of p53 and p21 levels in A549 cells
As presented in Fig.
3, following 24 h of treatment with 25 and 50 mU/ml MCC, the
expression level of E-cadherin was decreased and E-cadherin
fragment expression was identified using normal medium and
heat-inactivated MCC. The p53 tumor suppressor protein was
expressed in limited quantities in the A549 cells; conversely,
decreased expression was exhibited by A549 cells treated with 25
and 50 mU/ml MCC, compared with untreated cells.
Effects of MCC on MMP-9 expression in
A549 and H520 carcinoma cells
The results of the zymography assay demonstrated
that increased concentrations of MCC treatment resulted in
increased MMP-9 expression levels in A549 (Fig. 4A) and H520 (Fig. 4B) cell lines.
Discussion
Previous studies have demonstrated that the
accumulation of bone marrow-derived cells, including mast cells,
serves an important role in cancer tumor growth and angiogenesis
(8,12). Despite this, and the historic
speculation surrounding this topic, a limited number of studies
have addressed the interactions between mast cells and cancer cells
(9,25). Furthermore, although MCC is
well-characterized and its proteolytic effect on the extracellular
matrix is well known, a broader role for this enzyme in carcinoma
cytology and metastasis has, to the best of our knowledge, not been
investigated previously. Accordingly, the effect of MCC on the
proliferation and adhesion of lung cancer cells, as well as cell
growth-associated factors p53 and p21 were investigated in the
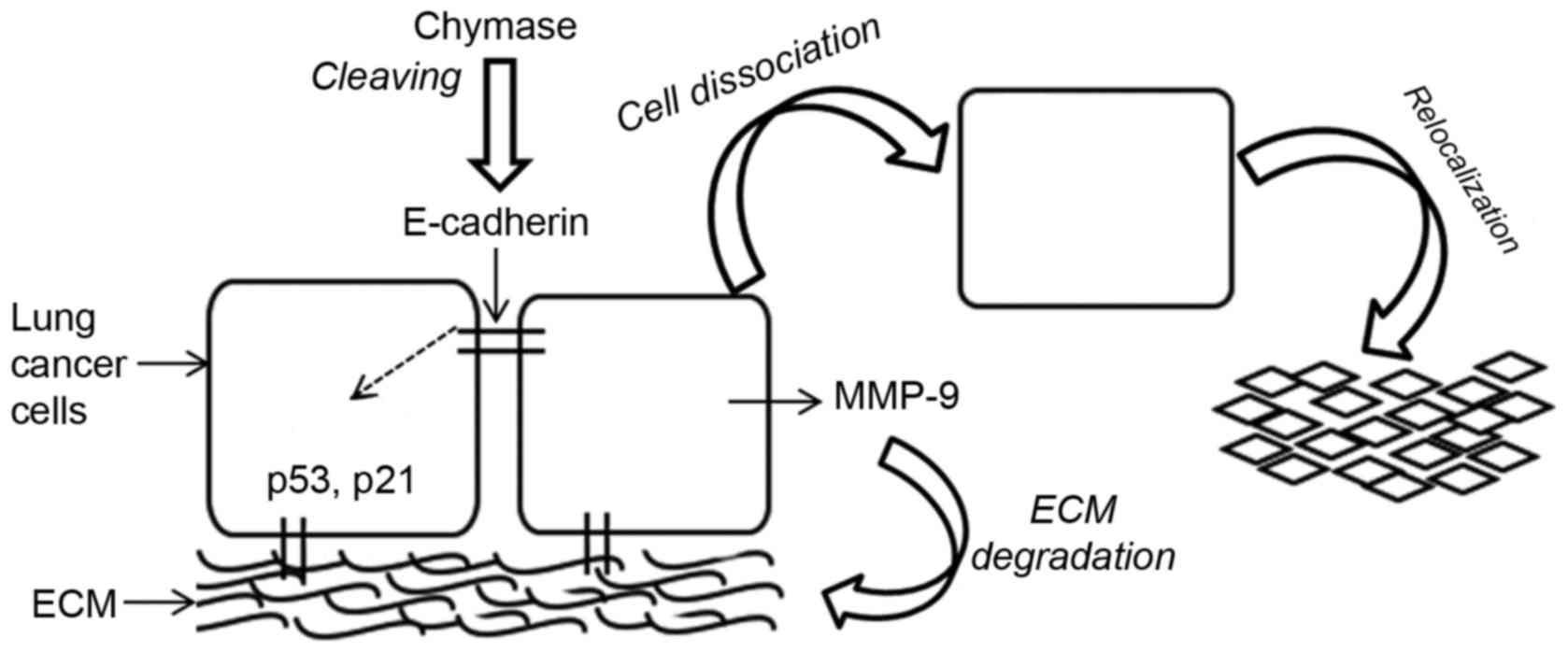
present study. Fig. 5 presents a
schematic diagram of the events triggered by MCC.
The results of the present study indicated that MCC
exerts various effects on lung cancer cells, depending on its
activity and exposure time. Compared with the untreated control,
MCC treatment for 6 h resulted in a slight increase in A549 cell
numbers; however, MCC treatment for 24 h caused the numbers of A549
and H520 cells to decrease. Notably, the inhibitory effect of MCC
on cell proliferation was time-dependent, as observed in the cells
following 24 h of treatment. Treating cells with the lowest
concentration of MCC (5 mU/ml) resulted in increased cell
viability, whereas the higher doses of MCC (25 and 50 mU/ml) caused
decreased viability of A549 and H520 cell lines. Similar results
were observed with MCC treated human epithelial cells (26). The duration and the activity of MCC
also influenced the cell cycle where p53 and p21 are involved.
The weakening of cell-cell adhesion is particularly
important for the metastasis of cancer cells (27). Cadherins are considered to be the most
important group of molecules involved in cell-cell and cell-matrix
adhesion (28). Decreased expression
of E-cadherin has been associated with more advanced tumor stages
and grades for lung (29), gastric
tract (30), breast (31), bladder (32), colorectal (33) and prostate (34) cancer. Lower expression of E-cadherin
in cancer cells rendered them prone to invasion and promoted
metastasis (21,35). Following 24 h of treatment with MCC
(25 and 50 mU/ml), the expression of E-cadherin was decreased; a
result that was consistent with cell adhesion data for A549. The
loss of E-cadherin expression may induce cell dissociation and
trigger a downstream intracellular signaling pathway, resulting in
deregulation of the cell cycle. However, as the results of the
present study demonstrated, cell dissociation may eventually result
in apoptosis, metastasis and re-adherence, under altered
conditions.
The p53 tumor suppressor protein is a key regulator
of programmed cell death (apoptosis) including anoikis (36), a form of apoptosis induced by the
detachment of cells from the ECM or cell clusters. Cell cycle
deregulation is common in human cancer. Alterations of the
tumor-suppressor protein p53 and its downstream effector, p21, have
been indicated in the development of a number of human malignancies
(24). The protein p21 is a regulator
of cell cycle progression at G1 and S phase, and,
additionally, mediates cellular senescence (37). The expression of p53 was
down-regulated in the A549 cells treated with MCC (25 and 50
mU/ml), compared with untreated cells. Notably, the A549 cells
treated with heat-inactivated MCC (50 mU/ml) expressed increased
levels of p53 protein, indicating that the effects of MCC on lung
cancer cells are associated with the activity of the enzyme.
The process of metastasis involves cell-cell and
cell-ECM interactions by the actions of proteolytic enzymes which
facilitate breakdown and invasion of the basement membrane
(38). Conversely, the loss of cell
attachment to ECM or cells may induce cell apoptosis (anoikis).
Therefore, cell adhesion, for survival, and cell disassociation,
for migration and re-establishment (metastasis), are key aspects of
the present study. MCC-dissociated cells formed clusters at the
bottom of the culture wells. At the highest MCC concentration (100
mU/ml), 20% of the cells lost attachment and migrated into the cell
culture medium; however, when transferred to a novel culture
environment, only 50% of those suspended cells proliferated and
became re-adherent. Additionally, subsequent co-culture of the cell
suspension with various concentrations of MCC led to a
dose-dependent effect on the cell adhesion rate.
Degradation of the ECM is the primary function of
matrix metalloproteinase, which allows cell clusters to be
separated. Previous studies have demonstrated that MMP-2 and MMP-9
are involved in tumor invasion and metastasis of gastric system
(17), colon (16), breast (39), head and neck (18) and lung (40) cancers. In the present study, MMP-9
expression levels in lung cancer cells was associated with the
activity of MCC. With the increase in concentration of MCC, MMP-9
expression in A549 and H520 cells were increased. In addition,
MCC-induced expression of MMP-9 was time-dependent and the present
study provides the first indication, to the best of our knowledge,
that MCC affects the expression of MMP-9 in lung cancer cells and
the activation of cancer cells themselves. The present study
additionally revealed that MCC is involved in ECM turnover and is
associated with lung cancer cell migration and metastasis.
The adhesion and relocalization of lung cancer cells
was affected by MCC-associated cell E-cadherin expression and the
expression of the cell cycle regulators p21 and p53. MCC was
revealed to influence MMP-9 expression and activation of lung
cancer cells, and serves a role in ECM degradation enabling the
cell clusters to separate, proliferate and relocate.
The results of the present study identified that MCC
triggers a cascade of responses associated with the proliferation,
adhesion and migration of lung cancer cells. Low doses were able to
induce proliferation and high doses of MCC inhibited the
proliferation of lung cancer cells. In addition, MCC may affect
adhesion molecules, resulting in tumor cell detachment and in cell
migration or apoptosis. The results of the present study indicate
that MCC is a promising candidate for lung cancer therapy.
Acknowledgements
The present study was supported by Changzou
University (grant no. ZMF14020066) and Changzhou Science and
Technology Bureau (grant no. KYJ1520305) to X.Z.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krishnaswamy G, Ajitawi O and Chi DS: The
human mast cell: An overview. Methods Mol Biol. 315:13–34.
2005.
|
|
4
|
Takanami I, Takeuchi K and Naruke M: Mast
cell density is associated with angiogenesis and poor prognosis in
pulmonary adenocarcinoma. Cancer. 88:2686–2692. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rajput AB, Turbin DA, Cheang MC, Voduc DK,
Leung S, Gelmon KA, Gilks CB and Huntsman DG: Stromal mast cells in
invasive breast cancer are a marker of favorable prognosis: A study
of 4,444 cases. Breast Cancer Res Treat. 107:249–257. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johansson A, Rudolfsson S, Hammarsten P,
Halin S, Pietras K, Jones J, Stattin P, Egevad L, Granfors T,
Wikström P and Bergh A: Mast cells are novel independent prognostic
markers in prostate cancer and represent a target for therapy. Am J
Pathol. 177:1031–1041. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blatner NR, Bonertz A, Beckhove P, Cheon
EC, Krantz SB, Strouch M, Weitz J, Koch M, Halverson AL, Bentrem DJ
and Khazaie K: In colorectal cancer mast cells contribute to
systemic regulatory T-cell dysfunction. Proc Natl Acad Sci USA.
107:pp. 6430–6435. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ozdemir O: The role of mast cell density
in tumor-associated angiogenesis and survival of squamous cell
carcinoma of the lung. J Cancer Res Ther. 11:10412015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khazaie K, Blatner NR, Khan MW, Gounari F,
Gounaris E, Dennis K, Bonertz A, Tsai FN, Strouch MJ, Cheon E, et
al: The significant role of mast cells in cancer. Cancer Metastasis
Rev. 30:45–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagata M, Shijubo N, Walls AF, Ichimiya S,
Abe S and Sato N: Chymase-positive mast cells in small sized
adenocarcinoma of the lung. Archiv Für Pathologische Anatomie Und
Physiologie Und Für Klinische Medicin. 443:565–573. 2003.
|
|
11
|
Maltby S, Khazaie K and McNagny KM: Mast
cells in tumor growth: Angiogenesis, tissue remodelling and
immune-modulation. Biochim Biophys Acta. 1796:19–26.
2009.PubMed/NCBI
|
|
12
|
Theoharides TC, Angelidou A and Zhang B:
Mast cells and tumor microenvironment. Cancer Drug Discovery
Development. 1–370. 2010.
|
|
13
|
Stewart JA Jr, Wei CC, Brower GL, Rynders
PE, Hankes GH, Dillon AR, Lucchesi PA, Janicki JS and Dell'Italia
LJ: Cardiac mast cell- and chymase-mediated matrix
metalloproteinase activity and left ventricular remodeling in
mitral regurgitation in the dog. J Mol Cell Cardiol. 35:311–319.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seth D, D'Souza El-Guindy NB, Apte M, Mari
M, Dooley S, Neuman M, Haber PS, Kundu GC, Darwanto A, de Villiers
WJ, et al: Alcohol, signaling, and ECM turnover. Alcohol Clin Exp
Res. 34:4–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Christensen J and Shastri VP:
Matrix-metalloproteinase-9 is cleaved and activated by Cathepsin K.
Bmc Res Notes. 8:3222015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murnane MJ, Cai J, Shuja S, McAneny D,
Klepeis V and Willett JB: Active MMP-2 effectively identifies the
presence of colorectal cancer. Int J Cancer. 125:2893–2902. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cupić DF, Tesar EC, Ilijas KM, Nemrava J
and Kovacević M: Expression of matrix metalloproteinase 9 in
primary and recurrent breast carcinomas. Coll Antropol. 35 Suppl
2:S7–S10. 2011.
|
|
18
|
Werner G, Daniele M, Gabriella N, Lorenzo
M, Giovanni T and Renato G: Association between metalloproteinases
2 and 9 activity and ERK1/2 phosphorylation status in head and neck
cancers: An ex vivo study. Oncol Rep. 24:1073–1078. 2010.PubMed/NCBI
|
|
19
|
Lecuit T and Yap AS: E-cadherin junctions
as active mechanical integrators in tissue dynamics. Nat Cell Biol.
17:533–539. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao P, Guo S, Tu Z, Di L, Zha X, Zhou H
and Zhang X: Grhl3 induces human epithelial tumor cell migration
and invasion via downregulation of E-cadherin. Acta Biochim Biophys
Sin (Shanghai). 48:266–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Techasen A, Loilome W, Namwat N, Khuntikeo
N, Puapairoj A, Jearanaikoon P, Saya H and Yongvanit P: Loss of
E-cadherin promotes migration and invasion of cholangiocarcinoma
cells and serves as a potential marker of metastasis. Tumour Biol.
35:8645–8652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen A, Beetham H, Black MA, Priya R,
Telford BJ, Guest J, Wiggins GA, Godwin TD, Yap AS and Guilford PJ:
E-cadherin loss alters cytoskeletal organization and adhesion in
non-malignant breast cells but is insufficient to induce an
epithelial-mesenchymal transition. Bmc Cancer. 14:5522014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muller PA and Vousden KH: Mutant p53 in
cancer: New functions and therapeutic opportunities. Cancer Cell.
25:304–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sivoňová MK, Vilčková M, Kliment J,
Mahmood S, Jurečeková J, Dušenková S, Waczulíková I, Slezák P and
Dobrota D: Association of p53 and p21 polymorphisms with prostate
cancer. Biomed Rep. 3:707–714. 2015.PubMed/NCBI
|
|
25
|
Ribatti D and Crivellato E: Mast cells,
angiogenesis, and tumour growth. Biochim Biophys Acta. 1822:2–8.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou X, Cox C, Rajenthirar S, Mahrous AA,
Masters B, Roche WR and Walls AF: Mast cell Chymase Can Disrupt the
human bronchial epithelium and stimulate the loss of adhesion
molecules. J Allergy Clinical Immunol. 121:S1102008. View Article : Google Scholar
|
|
27
|
Lee HS and Daar IO: EphrinB reverse
signaling in cell-cell adhesion: Is it just par for the course?
Cell Adh Migr. 3:250–255. 2008. View Article : Google Scholar
|
|
28
|
Segal L, Katz LS, Shapira H, Sandbank J,
Geras-Raaka E, Gershengorn MC and Oron Y: PAR-3 knockdown enhances
adhesion rate of PANC-1 cells via increased expression of
integrinαv and E-cadherin. PLoS One. 9:e938792014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cui T, Srivastava AK, Han C, Yang L, Zhao
R, Zou N, Qu M, Duan W, Zhang X and Wang QE: XPC inhibits NSCLC
cell proliferation and migration by enhancing E-Cadherin
expression. Oncotarget. 6:10060–10072. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Waldum HL, Ringnes E, Nordbø H, Sørdal Ø,
Nordrum IS and Hauso Ø: The normal neuroendocrine cells of the
upper gastrointestinal tract lack E-cadherin. Scand J Gastroentero.
49:974–978. 2014. View Article : Google Scholar
|
|
31
|
Yamashita N, Tokunaga E, Inoue Y, Tanaka
K, Ueo H, Saeki H, Oki K and Maehara Y: Abstract P2-05-12:
Epithelial paradox; clinical significance of co-expression of
E-cadherin and vimentin in invasive breast cancer. Cancer Res.
76:2016. View Article : Google Scholar
|
|
32
|
Wu CL, Ho JY, Chou SC and Yu DS: MiR-429
reverses epithelial-mesenchymal transition by restoring E-cadherin
expression in bladder cancer. Oncotarget. 7:26593–26603. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dass SD, Cheah PL, Ong DB, Teoh KH and
Looi LM: E-cadherin downregulation at the infiltrating tumour front
is associated with histological grade and stage in colorectal
carcinoma of Malaysians. Malays J Pathol. 37:19–24. 2015.PubMed/NCBI
|
|
34
|
Nam RK, Benatar T, Wallis CJ, Amemiya Y,
Yang W, Garbens A, Naeim M, Sherman C, Sugar L and Seth A: MiR-301a
regulates E-cadherin expression and is predictive of prostate
cancer recurrence. Prostate. 76:869–884. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma B, Zhang HY, Bai X, Wang F, Ren XH,
Zhang L and Zhang MZ: ADAM10 mediates the cell invasion and
metastasis of human esophageal squamous cell carcinoma via
regulation of E-cadherin activity. Oncol Rep. 35:2785–2794.
2016.PubMed/NCBI
|
|
36
|
Wang X, Simpson ER and Brown KA: p53:
Protection against tumor growth beyond effects on cell cycle and
apoptosis. Cancer Res. 75:5001–5007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McNaughton M, Pitman M, Pitson SM, Pyne NJ
and Pyne S: Proteasomal degradation of sphingosine kinase 1 and
inhibition of dihydroceramidedesaturase by the sphingosine kinase
inhibitors, SKi or ABC294640, induces growth arrest in
androgen-independent LNCaP-AI prostate cancer cells. Oncotarget.
7:16663–16673. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Z, Liu Z, Zhang X, Xue P and Zhang H:
RY10-4 suppressed metastasis of MDA-MB-231 by stabilizing ECM and
E-cadherin. Biomed Pharmacother. 68:439–445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sullu Y, Demirag GG, Yildirim A, Karagoz F
and Kandemir B: Matrix metalloproteinase-2 (MMP-2) and MMP-9
expression in invasive ductal carcinoma of the breast. Pathol Res
Pract. 207:747–753. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peng WJ, Zhang JQ, Wang BX, Pan HF, Lu MM
and Wang J: Prognostic value of matrix metalloproteinase 9
expression in patients with non-small cell lung cancer. Clinica
Chim Acta. 413:1121–1126. 2012. View Article : Google Scholar
|