Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of malignancy worldwide, and its incidence rate is
increasing (1–3). Although intensive studies have improved
early diagnosis and therapeutic options, providing minimally
invasive approaches for hepatic resection and liver
transplantation, the overall prognosis of HCC remains poor due to
high rates of metastasis and recurrence (4,5). The
accompanying high rate of mortality for metastatic HCC presents an
urgent need to identify key molecules that can be used as
prognostic and diagnostic markers to further improve early
detection, as well as approaches towards prevention, antagonizing
metastatic progression and therapy.
The neural precursor cell-expressed developmentally
downregulated gene 4 (NEDD4, also termed NEDD4-1) is a novel E3
ubiquitin-protein ligase that targets proteins for ubiquitination
and degradation. In addition, NEDD4 regulates a large number of
membrane proteins, including ion channels and membrane receptors
via its ubiquitination activities and ability to mediate
endocytosis (6). Ubiquitination is
crucial for controlling the turnover rate, localization and
activity of cellular proteins (7).
While NEDD4 was initially identified as a critical regulator of
neuronal function and plasticity in the brain (8), other studies have shown that NEDD4 also
performs a role in tumorigenesis and cancer development.
Specifically, aberrant expression of NEDD4 was shown to lead to
malignant transformation (9,10). Additionally, a further study into the
liver revealed an important regulatory role for NEDD4 in tissue
regeneration (11). The
phosphoinositide 3-kinase (PI3K)-protein kinase B (AKT) signaling
pathway is hyper-activated in the majority of cancers. Phosphatase
and tensin homolog deleted on chromosome 10 (PTEN) is a central
negative regulator of PI3K-Akt signal transduction by
dephosphorylating phosphoinositide (PI) (3–5), P3 and
inhibiting downstream signals (12).
The function of NEDD4 in HCC and its interactions
with the tumor suppressor PTEN remain to be elucidated. Therefore,
the present study aimed to investigate the function of NEDD4 in HCC
in relation to PTEN and PI3K/AKT signaling. The results provide
additional information regarding the role of NEDD4 as an oncogene
in HCC and its potential as a diagnostic and therapeutic
target.
Materials and methods
Patients and tissue specimens
Hepatic surgical specimens of HCC and matched
adjacent non-tumorous tissues were collected from 78 patients who
underwent curative surgery between April 2007 and July 2012 at the
First People's Hospital of Yancheng (Yancheng, China). All
specimens were fixed in formalin, embedded in paraffin and
sectioned for analysis. All patients had histologically-confirmed
HCC. None of the patients had undergone preoperative interventions,
including neo-adjuvant radio- or chemotherapy, percutaneous
ablation or chemo-embolization. The Ethics Committee of the First
People's Hospital of Yancheng approved the study protocol. Written
informed consent was obtained from every patient preoperatively,
and study participation was voluntary. The clinical baseline
characteristics of the HCC patients are presented in Table I.
 | Table I.Clinicopathological correlation of
NEDD4 expression in patients with hepatocellular carcinoma. |
Table I.
Clinicopathological correlation of
NEDD4 expression in patients with hepatocellular carcinoma.
| Variables | NEDD4+, n
(%) | NEDD4−, n
(%) | P-value |
|---|
| Age, years |
|
| 0.826 |
|
>50 | 32 (58.2) | 14 (60.9) |
|
| ≤50 | 23 (41.8) | 9
(39.1) |
|
| Sex |
|
| 0.670 |
| Male | 38 (69.1) | 17 (73.9) |
|
|
Female | 17 (30.9) | 6
(26.1) |
|
| Hepatitis |
|
| 0.922 |
| HBV | 36 (65.5) | 16 (69.6) |
|
| HCV | 10 (18.2) | 4
(17.4) |
|
| None | 9
(16.3) | 3
(13.0) |
|
| Alpha fetal protein
(ng/ml) |
|
| 0.264 |
|
>400 | 36 (65.5) | 18 (78.3) |
|
| ≤400 | 19 (34.5) | 5
(21.7) |
|
| Tumor size (cm) |
|
| 0.047 |
|
>3 | 35 (63.6) | 9
(39.1) |
|
| ≤3 | 20 (36.4) | 14 (60.9) |
|
| Liver cirrhosis |
|
| 0.734 |
| Yes | 31 (56.4) | 12 (52.2) |
|
| No | 24 (43.6) | 11 (47.8) |
|
| Tumor number |
|
| 0.367 |
|
Single | 18 (32.7) | 10 (43.5) |
|
|
Multiple | 37 (67.3) | 13 (56.5) |
|
| Degree of
differentiation |
|
| 0.032 |
|
Well | 11 (20.0) | 11 (47.8) |
|
|
Moderate | 19 (34.5) | 7
(30.5) |
|
|
Poor | 25 (45.5) | 5
(21.7) |
|
| Tumor node
metastasis stage |
|
| 0.608 |
|
I+II | 30 (54.5) | 14 (60.9) |
|
|
III+IV | 25 (45.5) | 9
(39.1) |
|
| Vascular
invasion |
|
| <0.001 |
|
Yes | 49 (89.1) | 11 (47.8) |
|
| No | 6
(10.9) | 12 (52.2) |
|
| Lymph node
metastasis |
|
| 0.005 |
|
Yes | 42 (76.4) | 10 (43.5) |
|
| No | 13 (23.6) | 13 (56.5) |
|
Cell culture
The human HCC Huh7, Hep3B, PLC/PRF/5 and SMMC7721
cell lines, and the human normal liver cell line LO2 were purchased
from the Cell Bank of the Chinese Academy of Science (Shanghai,
China). All cells were cultured in DMEM medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 U/ml streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.), and maintained in a 5% CO2-humidified
atmosphere at 37°C.
Small interfering (si)RNA
The Hep3B cells that overexpress NEDD4 were selected
for siRNA experiments. Briefly, the cells were seeded (5×105
cells/well) in 6-well plates and grown at 37°C for 24 h to 60–80%
confluence. Transient-transfection was carried out with
NEDD4-targeting siRNA sequence (5-UUC AAU UGC CAU CUG AAG UUU AUC
C-3; Thermo Fisher Scientific, Inc.) or non-targeting siRNA
(5′-CCGGAUUUAAAGCCGAAACCCGGUU-3′) as a control (13) using the previously determined optimal
concentration (2 µg/well respectively) and
Lipofectamine® 2000 for 48 h at 37°C (Thermo Fisher
Scientific, Inc.).
Cell viability assay
Cellular viability was assessed by the MTT assay.
Briefly, 5×103 cells/well were plated in 96-well plates, incubated
at 37°C overnight, and transfected with NEDD4-siRNA or
non-targeting siRNA. Following 24 and 48 h of incubation, 20 µl of
MTT (5 mg/ml) was added to each well. Subsequent to an additional 4
h of incubation, 150 µl of dimethyl sulfoxide was added to each
well and optical density values were measured at 570 nm (14).
Wound healing and invasion assays
Cells were seeded at 4×105 cells/well in 6-well
plates and incubated for 24 h at 37°C. A wound was made by
scratching a straight line in the monolayer of cells and
NEDD4-siRNA transfection was carried out. At 0, 24 and 48 h, dead
cells were removed by washing with PBS and the migration ability
was observed using an Olympus BX61 upright microscope equipped with
imaging technology (Olympus Corporation, Tokyo, Japan). Migration
distance was measured and migration area was calculated
automatically by using the Image J version 1.42 software (National
Institutes of Health, Bethesda, MA, USA).
Cell invasion assays were carried out using the
Transwell chamber assay with Matrigel (EMD Millipore, Billerica,
MA, USA) (14). Matrigel
(Sigma-Aldrich; Merck KGaA) was added to the filter to form a thin
gel layer. Following incubation at 37°C for 48 h, the cells
adherent to the upper surface of the filter were removed using a
cotton applicator, then stained with crystal violet. Ten random
fields were selected and the values obtained were calculated by
averaging the total numbers of cells from triplicate
determinations.
Western blot analysis
Collected cells were lysed with RIPA lysis buffer
(1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 150 mmol/l NaCl and
10 mmol/l Tris-HCl) containing 1/100 phenylmethanesulfonyl fluoride
solution. The total protein concentration was measured by using the
BCA method (Beyotime Institute of Biotechnology, Haimen, China).
Aliquots (20 µg) of each sample were separated by 10% SDS-PAGE
(14) and transferred to
polyvinylidene fluoride membranes for probing with the following
primary antibodies: rabbit anti-human NEDD4 (dilution, 1:1,000;
cat. no. ab14592; Abcam, Cambridge, UK), mouse anti-human AKT,
rabbit anti-human p-AKT (dilutions, 1:1,000/1:500 and cat nos.
sc-5298/135650, respectively from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), rabbit anti-human PTEN (dilution, 1:1,000; cat.
no. ab32199; Abcam), mouse anti-human E-cadherin (dilution,
1:1,000; cat. no. ab1416; Abcam), mouse anti-human vimentin
(dilution, 1:2,000; cat. no. ab8979; Abcam) and mouse anti-human
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; dilution, 1:5,000;
cat. no. ab8245, Abcam). Immunoreactive bands were assessed by
optical densitometry analysis using the Image J version 1.42
software. Signal values were adjusted using GAPDH as the loading
control.
Immunocytochemistry (ICC)
Cells were grown on coverslips in 6-well dishes and
transfected with NEDD4-siRNA. Subsequent to 48 h, the cells were
washed with PBS, fixed with 2% (w/v) paraformaldehyde and
permeabilized with 1% (v/v) Triton X-100. Cells were then blocked
by incubating with 10% (w/v) normal goat serum in PBS at room
temperature for 1 h and were then reacted with one of the primary
antibodies by incubation at 4°C overnight. The following day, the
cells were washed and incubated at 37°C for 1 h with Cy3-labeled
secondary antibody (Beyotime Institute of Biotechnology, Haimen,
China) at room temperature, and then co-stained with DAPI
(Sigma-Aldrich; Merck Millipore). Double staining with NEDD4 (cat.
no. ab14592; Abcam) and PTEN (cat. no. sc7974; Santa Cruz
Biotechnology, Inc.) was carried out, and the Cy3-(cat. no. A0516)
and Alexa Fluor 488-labeled secondary antibodies (cat. no. A0423)
(dilution, 1:400; Beyotime Institute of Biotechnology) were used.
Images of the immunostained cells were obtained using a
fluorescence microscope (magnification, ×200) (14).
Statistical analysis
Statistical analyses were conducted with SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). All
quantitative data were calculated as the mean ± standard deviation
using data from at least 3 independent experiments. Statistical
evaluation of the data was performed with one-way analysis of
variance (Bonferroni method). Pair-wise comparisons were conducted
using a Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
NEDD4 and PTEN protein expression
levels in normal hepatocyte and HCC cell lines
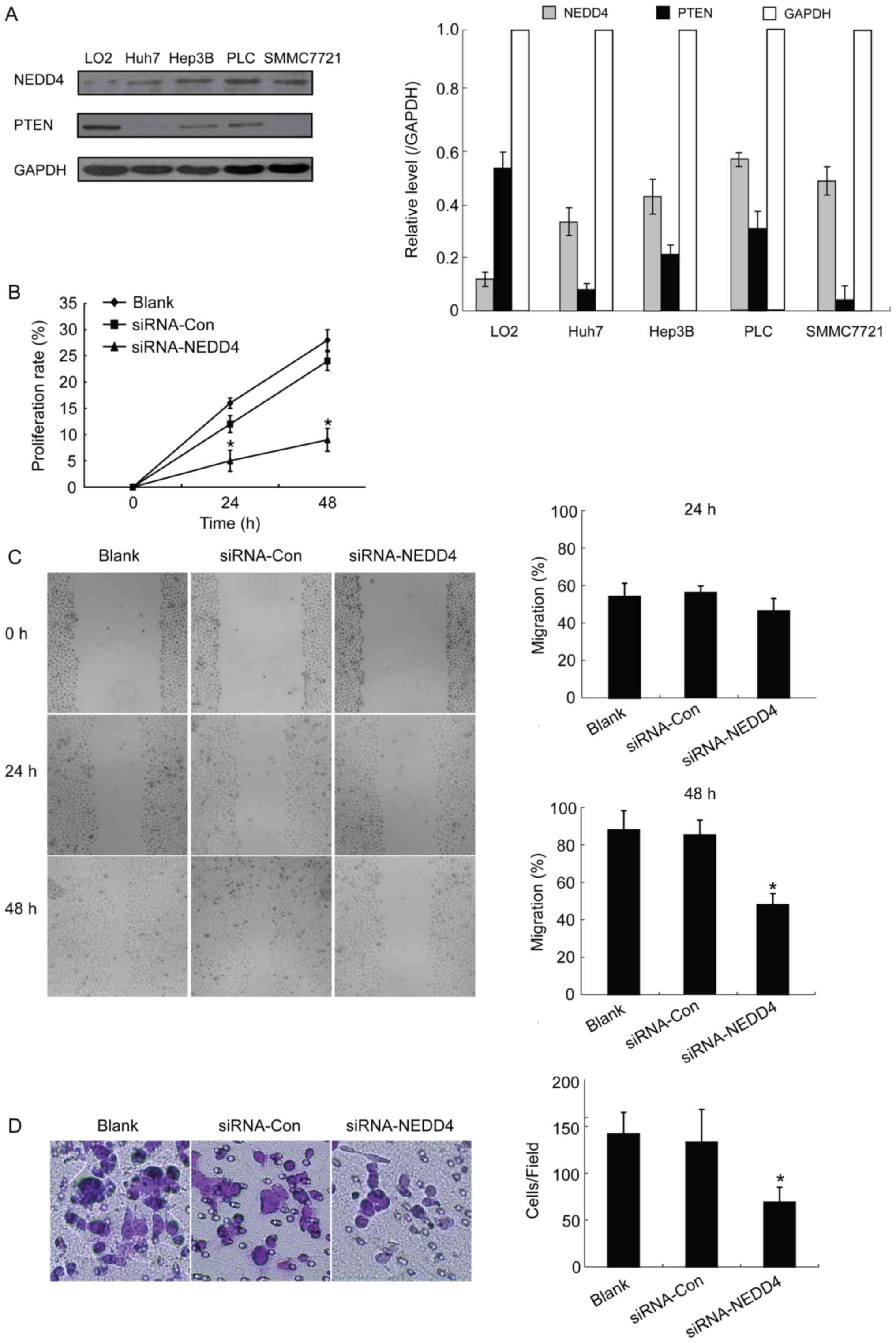
Western blot analysis detection of NEDD4 and PTEN
expression revealed different levels of the two proteins among the
normal liver cell LO2 and HCC cells including Huh7, Hep3B,
PLC/PRF/5 and SMMC7721 cell lines. In general, the protein
expression of NEDD4 and PTEN were different in normal liver cell
compared with all detected HCC cells. According to the results, all
detected HCC cells presented higher expression levels of NEDD4 and
lower expression levels of PTEN compared with LO2. Since the Hep3B
cell line exhibited high expression of NEDD4, it was selected for
the subsequent siRNA-mediated knockdown experiments.
Depletion of NEDD4 suppresses the
ability of growth and migration in HCC cells
It has been reported that NEDD4 may be an important
factor in the malignant transformation processes (9,10).
Therefore, the present study investigated whether depletion of
NEDD4 would have an impact on HCC cells' ability to grow and
migrate. An MTT assay revealed that NEDD4 siRNA-transfected Hep3B
cells exhibited a significantly decreased proliferation rate
compared with control blank (no treatment) cells at 24 and 48 h
following transfection (15 to 4% at 24 h and 28 to 9% at 48 h;
P<0.05; Fig. 1B). These results
suggest that depletion of NEDD4 negatively influences cell
proliferation in HCC cells.
In order to study the migration potential of
NEDD4-depleted HCC cells, and thus the NEDD4 role in HCC cell
metastasis, wound-healing experiments were performed. Results at 48
h demonstrated a statistically significant decrease in migration
ability for the NEDD4-siRNA transfected HCC cells compared with the
blank control cells (45 vs. 88%, respectively; P<0.05; Fig. 1C). However, no significant differences
were observed at 24 h. The present study also conducted Transwell
assays with matrigel in order to investigate whether NEDD4 is
capable of promoting the invasive capacity of HCC cells and whether
NEDD4 depletion leads to decreased invasion. Results from the
invasion assay demonstrated significantly decreased invasiveness
for the NEDD4-siRNA cells compared with the blank control and
siRNA-control groups (P<0.05; Fig.
1D). Thus, depletion of NEDD4 decreased the HCC cells'
capacities for proliferation, migration and invasion, all of which
are key features of tumorigenesis and tumor development.
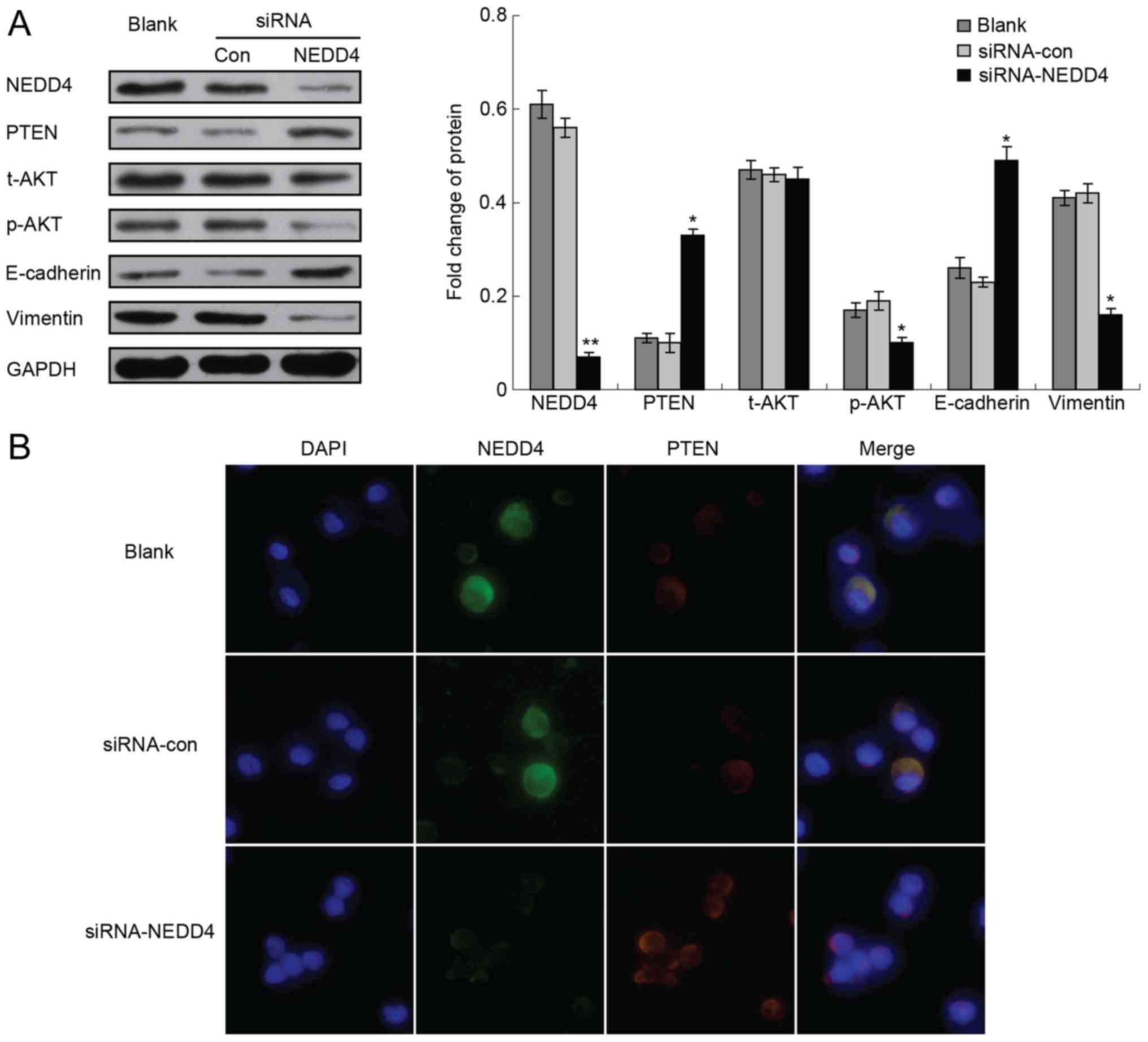
Furthermore, the present study studied the impact of
NEDD4 depletion on PTEN and the PI3K/AKT signaling pathway in HCC
cells. The NEDD4-siRNA transfected cells demonstrated a significant
reduction of NEDD4 protein expression, compared with the blank
control and siRNA-control groups (Fig.
2). Depletion of NEDD4 increased the expression of PTEN, and
had no effect on the expression level of total AKT but reduced the
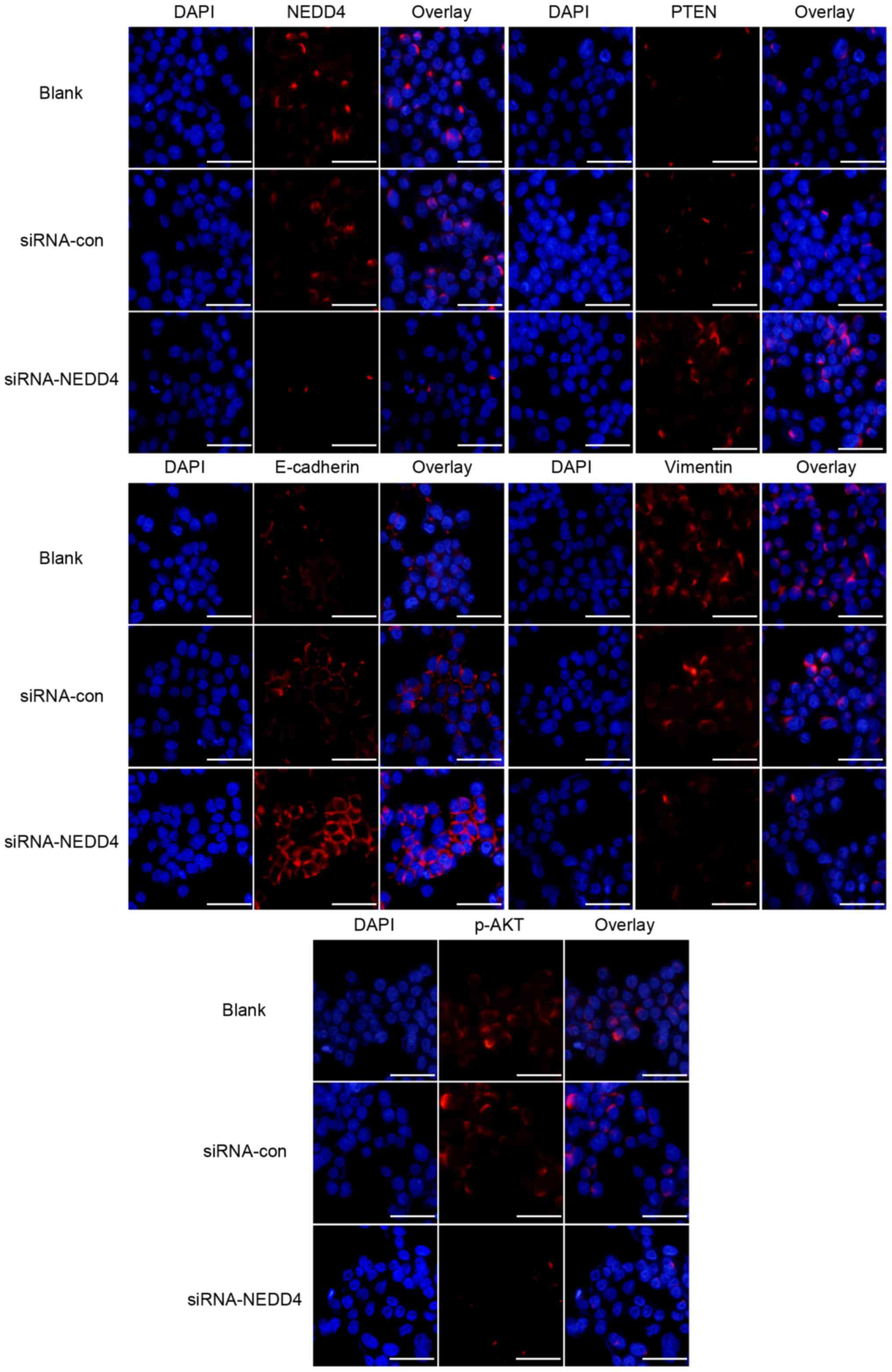
expression of p-AKT (Fig. 3).
Immunofluorescence experiments confirmed these results (Fig. 3).
Depletion of NEDD4 affects
epithelial-mesenchymal transition (EMT)
To investigate whether the expression level of NEDD4
has an impact on EMT, the present study measured EMT markers,
including the epithelial marker E-cadherin and the mesenchymal
marker vimentin, in HCC and NEDD4-depleted HCC cells. As expected,
the EMT marker vimentin was clearly expressed in tumor cells.
Notably, NEDD4-depleted HCC cells exhibited increased E-cadherin
and decreased vimentin protein levels (Fig. 3) Immunofluorescence experiments
confirmed these results (Fig. 3).
Overexpression of NEDD4 in HCC
correlates with tumor size, differentiation degree, vascular
invasion and lymph node metastasis
NEDD4 expression was evaluated in paraffin-embedded
serial sections of cancerous and non-cancerous tissues from 78
patients with HCC. On average, the HCC tissues exhibited higher
expression of NEDD4, lower expression of PTEN and higher expression
of p-AKT compared with the adjacent non-tumor liver tissues
(Fig. 4). NEDD4 overexpression was
identified in 70.5% (55/78) of the patients with HCC.
Immunohistochemically, NEDD4 was principally detected in the
cytoplasm and nucleus of tumor cells. Table I summarizes the association of
immunohistochemical NEDD4 expression with the various
clinicopathological parameters of the patients. The expression of
NEDD4 was significantly associated with tumor size (P=0.032),
vascular invasion (P<0.001), differentiation grade (P=0.032) and
lymph node status (P=0.005). These observations suggest that NEDD4
expression level may be a predictive factor for the recurrence and
survival rate of patients with HCC.
Discussion
HCC was ranked as the third most lethal type of
cancer in 2008 (15,16). The mortality rate of HCC is almost
identical to its rate of incidence, a fact that underlies the high
fatality rate of HCC (17–19). In Asia, incidence rates of HCC are
continuing to rise and its level is considered to be nearing an
epidemic proportion (20). Early
resectable presentation is rare (only 10–20% of cases) since the
disease is clinically silent at this stage. The outcomes for
advanced, inoperable stages are unsatisfactory and treatments are
typically limited to palliation (21).
The molecular events of pathogenesis, tumorigenesis
and metastasis in HCC are still poorly understood. The HCC tumor
markers that have been developed for clinical use include:
a-fetoprotein, squamous cell carcinoma antigen, golgiprotein73 or
des-γ-carboxy prothrombin, however these have limited sensitivity
and specificity (22,23). Identification of other diagnostic and
prognostic biomarkers (and consequent therapeutic targets) is
imperative to improve detection of HCC as well as treatment and
follow-up of patients. To the best of our knowledge, there are
limited studies on the relevance of NEDD4 to the progression of
liver cancer and the molecular mechanisms underlying the
association of NEDD4 with liver cancer remain unclear. The present
data show that NEDD4 overexpression in HCC is associated with the
processes of tumorigenesis and tumor progression, which would
support a poor prognosis and reduced overall survival rate. Results
from HCC cell lines (vs. normal hepatocytes) and tumor specimens
from patients with HCC (vs. adjacent non-tumor liver tissue)
confirm that overexpression of NEDD4 is associated with the tumor
microenvironment. Furthermore, the present data suggest that NEDD4
depletion affects the phosphorylation of AKT, which supports the
hypothesis that NEDD4 is a potential oncoprotein and a promoting
factor of the PTEN/PI3K/AKT signaling pathway. In addition, NEDD4
depletion in HCC cells may allow PTEN to negatively regulate the
PI3K signaling and exert its functions as a tumor suppressor
protein, thereby inhibiting an overactive PTEN/PI3K/AKT pathway
cascade. NEDD4 transcription is known to be positively regulated by
the PI3K pathway, representing a positive feedback for PTEN
degradation and PI3K activation (12). Therefore, the effects of NEDD4
depletion on PTEN/PI3K/AKT in HCC cells may have resulted from this
upstream and feedback regulatory process.
Statistical analysis of the immunohistochemical
staining data and the HCC tumor clinicopathological data indicated
a significant correlation of NEDD4 positivity with various HCC
characteristics, including tumor size, differentiation degree,
vascular invasion and lymph node status. These observations suggest
that NEDD4 expression level could be a predictive factor for
recurrence and survival rate of patients with HCC. In addition,
NEDD4 overexpression was evidently linked to HCC metastasis, and
thus to a poor prognosis. It may be speculated that patients with a
NEDD4-negative HCC status would have improved prognosis, therapy
response and survival rate. If this assumption could be proven
true, NEDD4 would be an effective predictor for post-surgery
survival rate of HCC.
Knockdown experiments in the present study revealed
that depletion of NEDD4 in HCC cells markedly reduced cellular
migration and invasion, the two key cellular processes in
metastasis, and also impacted cellular proliferation. These results
may translate into clinical practice, as they suggest that NEDD4 is
positively associated with HCC metastatic potential and higher TNM
staging, and thus hint at its uses as a predictive biomarker of the
poor prognosis of HCC.
NEDD4 is homologous to E6-AP carboxyl terminus E3
ubiquitin ligase, which is involved in ubiquitination of the tumor
suppressor gene PTEN to cause its proteasomal degradation and
nuclear translocation. It has thus been suggested that NEDD4 may
have a pro-oncogenic role (10,24–27). In
agreement with this pathological function, overexpression of NEDD4
has been observed in various types of cancer, including gastric
(24), lung (23) and colorectal (10). Studies of NEDD4 in colorectal cancer
have demonstrated its role in promoting tumor cell growth (10). However, Eide et al (13) previously demonstrated that the
overexpression of NEDD4-1 in colon cancer tissues did not correlate
with the downregulation of PTEN. This seemingly contradictory
finding may actually suggest that in certain tumor entities NEDD4
may support oncogenesis and tumor progression independently of the
downregulation of PTEN. In non-small cell lung cancer (NSCLC),
inhibition of NEDD4 expression significantly suppresses
proliferation of NSCLC cells and tumor growth, as shown by in
vivo studies (24,28). Furthermore, NEDD4 overexpression was
shown to augment the tumorigenicity of lung cancer cells, while
producing no impact on PTEN gene expression (24). Recently, Liao et al (27) reported that NEDD4 is associated with
cancer metastasis through its regulation of the processes
underlying tumor invasion, apoptosis and colonization.
To the best of our knowledge, the present study
revealed that NEDD4 promotes HCC cell invasion and migration, which
is tightly associated with HCC metastasis. The present data
indicate that NEDD4 may be an exceptional biomarker for predicting
the poor prognosis of post-surgery HCC. Translating these finding
into clinical practice may embody the detection of NEDD4
overexpression in HCC tumors by immunohistochemistry techniques and
the results may be used to guide the treatment approach (i.e.,
tailoring the treatment to NEDD4-positive or -negative status).
Additionally, NEDD4 is suggested as a novel therapeutic target, due
to its positive correlation with the invasion and migration
capacities of the HCC cell lines. Depletion or inhibition of NEDD4
may be a strategic approach to reduce metastasis and delay tumor
recurrence, thereby improving survival rate. Further studies,
focusing in particular on the correlation of NEDD4 expression and
overall survival rate, should be carried out on larger populations
of patients with HCC.
Acknowledgements
The authors would like to thank Professor L. Dagna
for his intellectual and critical advice on the present study.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: From genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aravalli RN, Steer CJ and Cressman EN:
Molecular mechanisms of hepatocellular carcinoma. Hepatology.
48:2047–2063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye
QH, Wang L, Zhou J, Qiu SJ, Li Y, et al: A decade's studies on
metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol.
130:187–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Donovan P and Poronnik P: Nedd4 and
Nedd4-2: Ubiquitin ligases at work in the neuron. Int J Biochem
Cell Biol. 45:706–710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kerscher O, Felberbaum R and Hochstrasser
M: Modification of proteins by ubiquitin and ubiquitin-like
proteins. Annu Rev Cell Dev Biol. 22:159–180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar S, Tomooka Y and Noda M:
Identification of a set of genes with developmentally
down-regulated expression in the mouse brain. Biochem Biophys Res
Commun. 185:1155–1161. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakayama KI and Nakayama K: Ubiquitin
ligases: Cell-cycle control and cancer. Nat Rev Cancer. 6:369–381.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen C and Matesic LE: The Nedd4-like
family of E3 ubiquitin ligases and cancer. Cancer Metastasis Rev.
26:587–604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bellet MM, Piobbico D, Bartoli D, Castelli
M, Pieroni S, Brunacci C, Chiacchiaretta M, Del Sordo R, Fallarino
F, Sidoni A, et al: NEDD4 controls the expression of GUCD1, a
protein upregulated in proliferating liver cells. Cell Cycle.
13:1902–1911. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baker SJ: PTEN enters the nuclear age.
Cell. 128:25–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eide PW, Cekaite L, Danielsen SA,
Eilertsen IA, Kjenseth A, Fykerud TA, Ågesen TH, Bruun J, Rivedal
E, Lothe RA and Leithe E: NEDD4 is overexpressed in colorectal
cancer and promotes colonic cell growth independently of the
PI3K/PTEN/AKT pathway. Cell Signal. 25:12–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao L, Yan Y, Xu C, Ji W, Shen S, Xu G,
Zeng Y, Sun B, Qian H, Chen L, et al: MicroRNA-21 suppresses PTEN
and hSulf-1 expression and promotes hepatocellular carcinoma
progression through AKT/ERK pathways. Cancer Lett. 337:226–236.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: A global and regional perspective. Oncologist. 15:(Suppl
4). 5–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127:(5 Suppl 1). 5–16. 2004. View Article : Google Scholar
|
|
18
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuen MF, Hou JL and Chutaputti A: Asia
Pacific Working Party on Prevention of Hepatocellular Carcinoma:
Hepatocellular carcinoma in the Asia pacific region. J
Gastroenterol Hepatol. 24:346–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mann CD, Neal CP, Garcea G, Manson MM,
Dennison AR and Berry DP: Prognostic molecular markers in
hepatocellular carcinoma: A systematic review. Eur J Cancer.
43:979–992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okano H, Nakajima H, Tochio T, Suga D,
Kumazawa H, Isono Y, Tanaka H, Matsusaki S, Sase T, Saito T, et al:
A case of a resectable single hepatic epithelioid
hemangioendothelioma with characteristic imaging by ADC map. Clin J
Gastroentero. 8:406–413. 2015. View Article : Google Scholar
|
|
22
|
Zhao YJ, Ju Q and Li GC: Tumor markers for
hepatocellular carcinoma. Mol Clin Oncol. 1:593–598.
2013.PubMed/NCBI
|
|
23
|
Sakata T, Sakaguchi H, Tsuda L,
Higashitani A, Aigaki T, Matsuno K and Hayashi S: Drosophila Nedd4
regulates endocytosis of notch and suppresses its
ligand-independent activation. Curr Biol. 14:2228–2236. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amodio N, Scrima M, Palaia L, Salman AN,
Quintiero A, Franco R, Botti G, Pirozzi P, Rocco G, De Rosa N and
Viglietto G: Oncogenic role of the E3 ubiquitin ligase NEDD4-1, a
PTEN negative regulator, in non-small-cell lung carcinomas. Am J
Pathol. 177:2622–2634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao C, Pang L, Ren C and Ma T: Decreased
expression of Nedd4L correlates with poor prognosis in gastric
cancer patient. Med Oncol. 29:1733–1738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He S, Deng J, Li G, Wang B, Cao Y and Tu
Y: Down-regulation of Nedd4L is associated with the aggressive
progression and worse prognosis of malignant glioma. Jpn J Clin
Oncol. 42:196–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao CJ, Chi HC, Tsai CY, Chen CD, Wu SM,
Tseng YH, Lin YH, Chung IH, Chen CY, Lin SL, et al: A novel
small-form NEDD4 regulates cell invasiveness and apoptosis to
promote tumor metastasis. Oncotarget. 6:9341–9354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakashita H, Inoue H, Akamine S, Ishida T,
Inase N, Shirao K, Mori M and Mimori K: Identification of the
NEDD4L gene as a prognostic marker by integrated microarray
analysis of copy number and gene expression profiling in non-small
cell lung cancer. Ann Surg Oncol. 20:(Suppl 3). 590–598. 2013.
View Article : Google Scholar
|