Introduction
Lung cancer (LC) remains a major cause of
cancer-associated mortality worldwide (1). Despite the recent development of novel
treatments, the survival rates of patients with lung cancer remain
low, with a 5-year survival rate ≤15% (2). To improve the prognosis of patients,
further research on lung cancer, particularly focusing on the
underlying molecular mechanisms, is warranted.
There are two major types of lung cancer based on
clinical classification: Non-small cell lung cancer (NSCLC) and
small cell lung cancer, which account for ~80 and 20% of all lung
cancer cases, respectively. NSCLC is a heterogeneous group of
carcinomas derived from epithelial cells and they can be separated
into three major histological subtypes: Adenocarcinoma (ADC),
squamous cell carcinoma (SCC), and large cell carcinoma (3).
Considering the risk posed by NSCLC and relatively
small progress in NSCLC treatment, efforts have been made to
investigate specific molecular markers to understand the primary
molecular mechanisms of this malignancy. Thus, this may benefit the
diagnosis, therapy design and prognostic assessment of NSCLC.
The speckle-type POZ domain protein (SPOP) gene,
which encodes the substrate-recognition component of a
cullin3-based E3-ubiquitin ligase (Cul3) (4), is located at the 17q21 locus where a
high allelic imbalance has been observed in primary tumors
(5). SPOP is a 374-amino acid protein
comprising an N-terminal meprin and TRAF-C homology (MATH) domain
for recruiting substrate proteins, and a C-terminal poxvirus and
zinc finger (POZ) domain (also known as a BTB domain) for
interaction with Cul3 (4). It has
been demonstrated that the MATH-BTB SPOP protein in mammals serves
as an adapter of macroH2A in ubiquitination for regulating its
deposition on the inactive X-chromosome (6). In addition, it is associated with the
Daxx ubiquitination process in which Cul3-based ubiquitin ligase in
the Hedgehog/Gli signaling pathway is involved for regulating
transcriptional repression of proapoptotic proteins, including p53
(7). Ubiquitin modifications regulate
various cellular processes and are involved in cancer pathogenesis
to a certain extent (8). As an
adaptor for Cul3-based ubiquitination, the SPOP gene may be mutated
in certain malignancies (8–13). Based on previous analyses of
genome-wide somatic mutation, frequent mutations of SPOP gene were
observed in certain types of human cancer (9,10).
Although SPOP protein is expressed in normal gastric, colonic and
prostate epithelial cells, 30% of gastric cancer, 20% of colorectal
cancer and 37% of prostate cancer do not express SPOP protein
(14).
Not much is known regarding SPOP somatic mutations
in lung cancer, and the prevalence of decreased SPOP expression has
rarely been reported in lung cancer, in particular with respect to
the influence of decreased SPOP expression in lung cancer. The
present study aimed to examine the difference between in SPOP mRNA
and protein levels in lung cancer tissues, and corresponding normal
lung tissues derived from patients with NSCLC. In addition, the
association between SPOP expression and clinicopathological
features of patients with NSCLC was investigated. Potential
candidate prognostic markers of NSCLC were identified by evaluating
the role of SPOP in carcinogenesis, development and prognosis of
NSCLC.
Materials and methods
Patients and tissue specimens
A total of 157 surgical specimens and 23 normal lung
tissues were obtained from the Affiliated Hospital of Nantong
University (Nantong, China) between January 2004 and January 2010,
which included 115 adenocarcinoma cases and 42 squamous cell
carcinoma cases. The carcinoma tissues and the adjacent normal
tissues were snap frozen in liquid nitrogen and stored at −80°C.
The patients involved in the present study did not receive any
chemotherapy and/or radiation therapy prior to surgery. Written
informed consent was obtained from patients or their legal
guardians for the surgical procedures and permission to utilize
resected tissue specimens for the current study. The present study
was approved by the Ethics Committee of the Affiliated Hospital of
Nantong University. All specimens had been confirmed through
pathological diagnosis. The histological subtype was assessed using
the current World Health Organization classification guidelines and
the stage was classified in conformity with the 7th edition of the
Tumor Node Metastasis classification of malignant tumors by two
independent, experienced pathologists (15,16). The
follow-up data of patients with lung cancer in the present study
were available and complete. Overall survival, which was defined as
the length of time between the surgery date and date of mortality
or last follow-up, was used as a measure for prognosis.
Postoperative follow-up was performed at the outpatient department
of the Affiliated Hospital of Nantong University, and comprised
clinical and laboratory examinations every quarter for the first 2
years, every 2 quarters during the 3rd to 5th years, and annually
for an additional 5 years or until mortality.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue lysate using a
TRIzol reagent extraction kit (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) followed by RT using the High
Capacity cDNA Reverse Transcription kit (Qiagen, Inc., Valencia,
CA, USA) in accordance with the manufacturer's protocol. The Roche
Light Cycler 480 system (Roche Diagnostics, Burgess Hill, UK) was
used for qPCR analysis. Primer pairs designed by Primer Premier 5.0
(Premier Biosoft International, Palo Alto, CA, USA) were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China), the
sequences of which were as follows: SPOP forward,
5′-TGACCACCAGGTAGACAGCG-3′ and reverse, 5′-CCCGTTTCCCCCAAGTTA-3′.
The GAPDH gene served as an internal control. The sequences of the
primers for GAPDH were as follows: GAPDH forward,
5′-AACTTCCGTTGCTGCCAT-3′ and reverse, 5′-TTTCTTCCACAGGGCTTTG-3′.
The PCR system (25 µl) comprised 1 µl of cDNA, 12.5 µl of 2X Fast
EvaGreen™ qPCR Master mix (Biotium Inc., Freemont, CA, USA), 1 µl
of primers (10 µM) and 10.5 µl of RNase/DNase-free water. The
following thermocycling conditions were maintained: 96°C for 2 min;
40 cycles at 96°C for 15 sec; and 60°C for 1 min. Each sample was
performed in triplicate, and the average value was computed. For
comparative expression of SPOP, the 2−∆∆Cq method
(17) was used as an indication of
relative changes.
Western blot analysis
Lung cancer tissues from patients were homogenized
in lysis buffer (50 mM Tris, pH 7.5, 5 mM EDTA, 1% SDS, 1% NP-40,
1% Triton X-100, 1% sodium deoxycholate, 1 mM phenylmethylsulfonyl
fluoride, 10 µg/ml leupeptin and 10 µg/ml aprotinin) and then
centrifuged in a microcentrifuge at 12,800 × g at 4°C for 20 min.
The supernatants were accumulated and protein concentrations were
measured using a Bradford assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Approximately 20 µg protein was loaded into
each well and samples were separated using 10% SDS-PAGE then
electrophoretically transferred to a nitrocellulose membrane, which
was blocked with 5% non-fat milk for 2 h at room temperature.
Subsequently, the membrane was washed with TBS-Tween 20 three
times, incubated with primary goat polyclonal antibodies directed
against SPOP (1:500; cat. no. sc-66649; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and GAPDH (anti-rabbit, cat. no. sc-25778;
1:1,000; Santa Cruz Biotechnology, Inc.) at 4°C overnight, and
incubated with horseradish peroxidase-conjugated goat-anti-rabbit
secondary antibody (1:10,000; cat. no. sc-2007; Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature in the antibody
buffer. The protein levels were normalized by GAPDH. The proteins
were then detected using an Enhanced Chemiluminescence Detection
system (Pierce; Thermo Fisher Scientific, Inc.). The band intensity
was quantified using ImageJ (version 1.44p; National Institutes of
Health, Bethesda, MD, USA).
Immunohistochemistry analysis
Tissues were fixed in 10% formalin for 12 h at room
temperature, embedded in paraffin for 4 h at 4°C, then cut into
5-µm thick serial sections. The tissue sections were deparaffinized
in dimethylbenzene 15 min twice and rehydrated in graded ethanol
solutions at room temperature. The slides were washed with running
water and then washed in PBS to deactivate endogenous peroxidase 3
min three times at room temperature. With respect to the retrieval
of antigen, the sections were immersed in buffer containing 0.01 M
sodium citrate-hydrochloric acid (pH 6.0) and boiled by microwaving
for 15 min. When the slides were naturally cooled to room
temperature, they were subjected to washing twice in distilled
water and three times in PBS. To block endogenous peroxidase,
petroleum jelly was smeared on the tissue edge, and one drop of 3%
H2O2 was added. After incubation at room temperature for 15 min,
the slides were washed three times in PBS. The tissue sections went
through the following steps: Incubation with goat polyclonal
anti-human antibody directed against SPOP (1:100; no. sc-66649;
Santa Cruz Biotechnology, Inc.) for 1 h at room temperature;
rinsing in 3% peroxidase quenching solution (Invitrogen; Thermo
Fisher Scientific, Inc.) to block endogenous peroxidase 20 min at
room temperature; incubation with a donkey anti-goat horseradish
peroxidase-conjugated secondary antibody (1:5,000; cat. no.
ab-6885; Abcam, Cambridge, UK) for 30 min at room temperature; and
washing three times in PBS. The signal was developed with
3,3′-diaminobenzidine solution and all of the slides were
counterstained with hematoxylin 5 min at room temperature. Normal
lung tissues were processed simultaneously as negative controls
with omission of the primary antibody. The specimens were analyzed
by two consultant pathologists who were blinded to the patient
data. The total SPOP immunoreactivity was determined by combining
the percentage of positively stained tumor cells with the staining
intensity. A total of five randomly selected high power fields were
observed and scored based on the percentage of positive cells.
Briefly, the percentage of positive cells were scored as 0 (0–5%),
1 (6–25%), 2 (26–50%, focal), 3 (51–75%) or 4 (>75%), and the
intensity as 0, no staining; 1, weak staining; 2, moderate staining
or 3, strong staining. The two scores were then added, and the
expression level of SPOP was defined as follows: ‘−’ (negative,
score of 0); ‘+’ (weakly positive, score of 1–3); ‘++’ (positive,
score of 4–7); or ‘+++’ (strongly positive, score of 8–12). A total
score of >3 was defined as strong SPOP expression, and a total
score of ≤3 was defined as weak SPOP expression.
Statistical analysis
SPSS 21.0 (IBM Corp., Armonk, NY, USA) was utilized
for statistical analysis, with the results presented as the mean ±
standard deviation. One-way analysis of variance among groups or
two-tailed Student's t-tests between groups was used to compare
expression of SPOP protein and mRNA in tissues. Chi-squared or
Fisher's exact tests were used to distinguish categorical
variables. Survival curves were constructed using Kaplan-Meier
method. Multivariate analysis was conducted using Cox proportional
hazards method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Downregulated expression of SPOP in
human NSCLC tissue samples
To investigate the role of SPOP in NSCLC, qPCR and
western blot analysis was performed to detect the expression of
SPOP in 12 pairs of newly collected NSCLC tissues, and adjacent
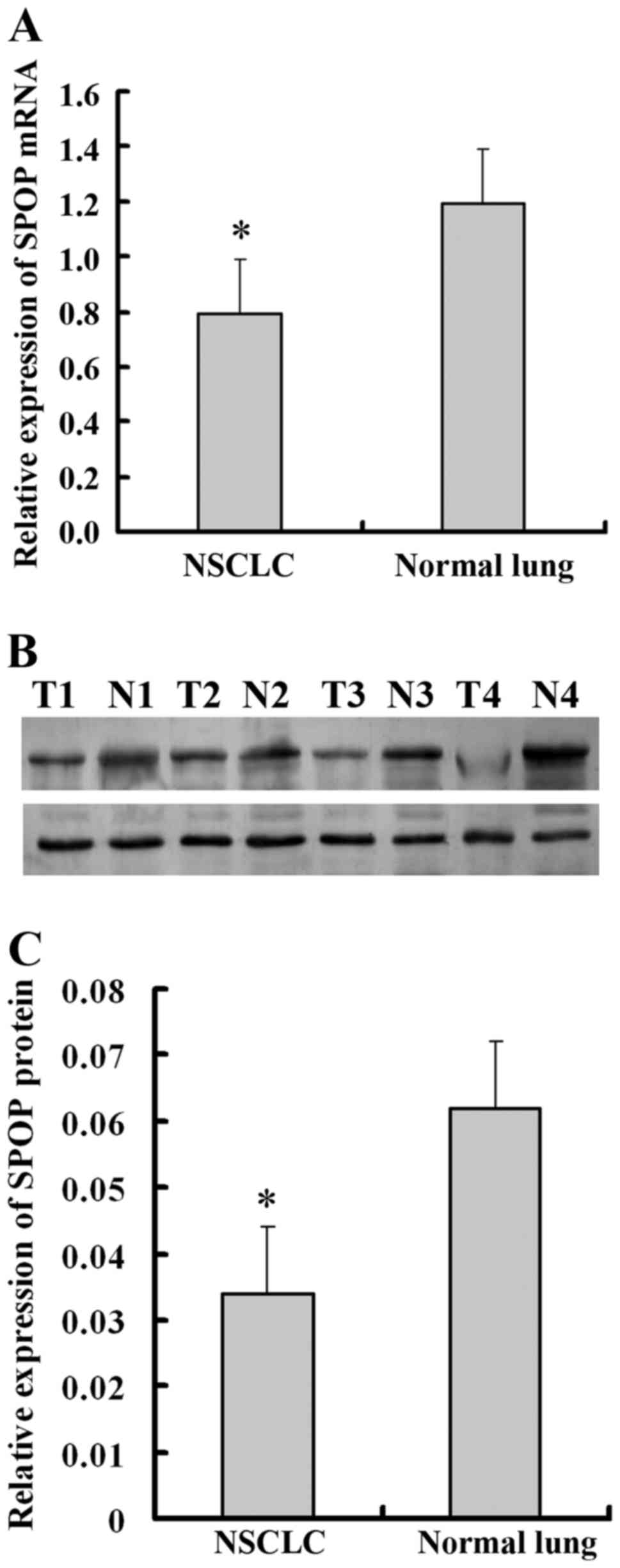
normal lung tissues. It was demonstrated that SPOP mRNA was
significantly downregulated in NSCLC tissues compared with normal
lung tissues (P<0.05; Fig. 1A).
Similar to mRNA concentration, the cellular concentration of SPOP
protein in NSCLC tissues was significantly lower compared with that
of normal lung tissues (P<0.05; Fig.
1B and C). For further verification, immunohistochemistry
experiments were performed to detect the level of SPOP expression
in 157 NSCLC tissues and 23 normal lung tissues. SPOP
immunoreactivity was identified in the cytoplasm of representative
NSCLC cells. Specifically, low expression level of SPOP protein was
present in 84.1% (132/157) of lung cancer samples, while high
expression level of SPOP was present in 52.2% (12/23) of normal
lung samples (P<0.001; Table I and
Fig. 2).
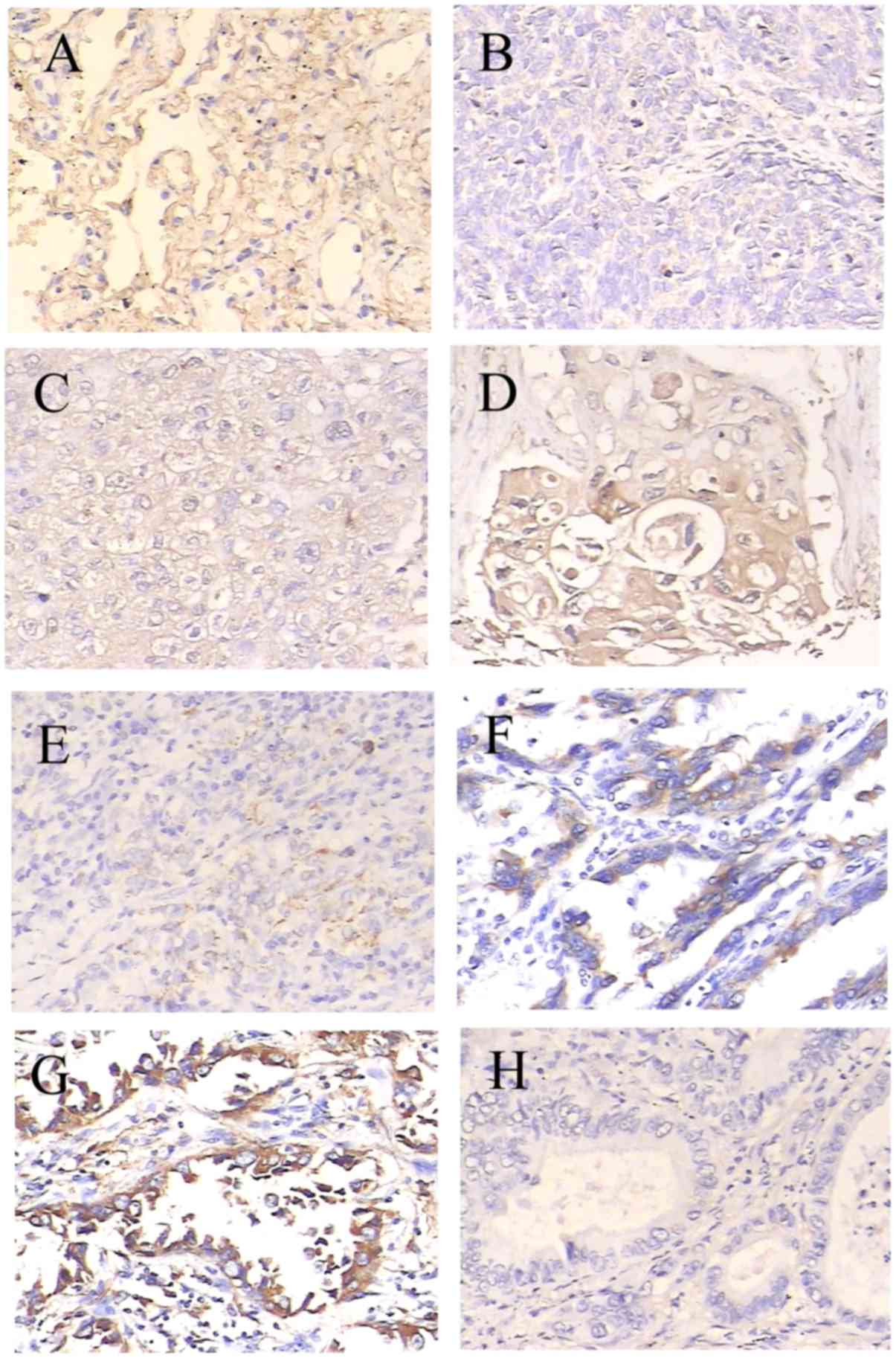 | Figure 2.Expression of SPOP in lung cancer
specimens of different tumor differentiation grades was determined
by immunohistochemical experiments. SPOP immunoreactivity shows
homogeneous brown-yellow staining in the cytoplasm of tumor cells
along with hematoxylin (blue) counterstain. Samples include (A)
normal lung tissue, (B) poorly differentiated squamous cell
carcinoma, (C) moderately differentiated squamous cell carcinoma,
(D) well differentiated squamous cell carcinoma, (E) poorly
differentiated adenocarcinoma, (F) moderately differentiated
adenocarcinoma, (G) well differentiated adenocarcinoma and (H)
negative control. Original magnification, ×200. With poorer tumor
differentiation, the SPOP expression in NSCLC was significantly
decreased (P<0.05 compared with normal lung). SPOP, speckle-type
POZ domain protein; NSCLC, non-small cell lung cancer. |
 | Table I.Summary of immunohistochemical results
in NSCLC and normal lung tissues. |
Table I.
Summary of immunohistochemical results
in NSCLC and normal lung tissues.
|
|
| SPOP expression |
|
|---|
|
|
|
|
|
|---|
| Group | N | Low (n) | High (n) | P-value |
|---|
| Tumor | 157 | 132 | 25 | <0.001 |
| Normal | 23 | 11 | 12 |
|
Association between SPOP protein
expression and clinicopathological variables in human NSCLC
tissues
The association between the SPOP protein expression
and clinicopathological variables in patients with NSCLC was
analyzed. For statistical analysis of the SPOP expression, the
NSCLC tissue specimens were separated into a low expression group
and a high expression group according to the final scores of
staining. According to Table II, the
level of SPOP was positively associated with histologic type
(P=0.003), tumor differentiation (P=0.046), tumor size (P=0.0036),
lymph node metastasis (P=0.041) and clinical stages (P=0.046) in
patients with NSCLC. However, no significant association was
identified between SPOP expression and other clinical factors.
 | Table II.Association between the
clinicopathological characteristics and the expression level of
SPOP in lung cancer tissues. |
Table II.
Association between the
clinicopathological characteristics and the expression level of
SPOP in lung cancer tissues.
|
|
| SPOP expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | N | Low | High | P-value |
|---|
| Sex |
|
|
| 0.266 |
| Male | 94 | 82 | 12 |
|
|
Female | 63 | 50 | 13 |
|
| Age |
|
|
| 0.266 |
| ≤60 | 94 | 50 | 13 |
|
|
>60 | 63 | 82 | 12 |
|
| Histologic type |
|
|
| 0.003a |
|
Adenocarcinoma | 115 | 91 | 24 |
|
| Squamous
cell carcinoma | 42 | 41 | 1 |
|
| Tumor
differentiation |
|
|
| 0.046a |
| Well | 29 | 21 | 8 |
|
|
Moderate | 100 | 84 | 16 |
|
| Poor | 28 | 27 | 1 |
|
| Tumor size |
|
|
| 0.0036a |
| T1 | 83 | 69 | 14 |
|
| T2 | 62 | 56 | 6 |
|
| T3 | 7 | 4 | 3 |
|
| T4 | 5 | 2 | 3 |
|
| Lymph node
metastasis |
|
|
| 0.041a |
|
Absent | 104 | 83 | 21 |
|
|
Present | 53 | 49 | 4 |
|
| Lung cancer
stages |
|
|
| 0.046a |
| 1 | 29 | 21 | 8 |
|
| 2 | 100 | 84 | 16 |
|
| 3 | 28 | 27 | 1 |
|
Prognostic significance of SPOP
expression level in human NSCLC samples
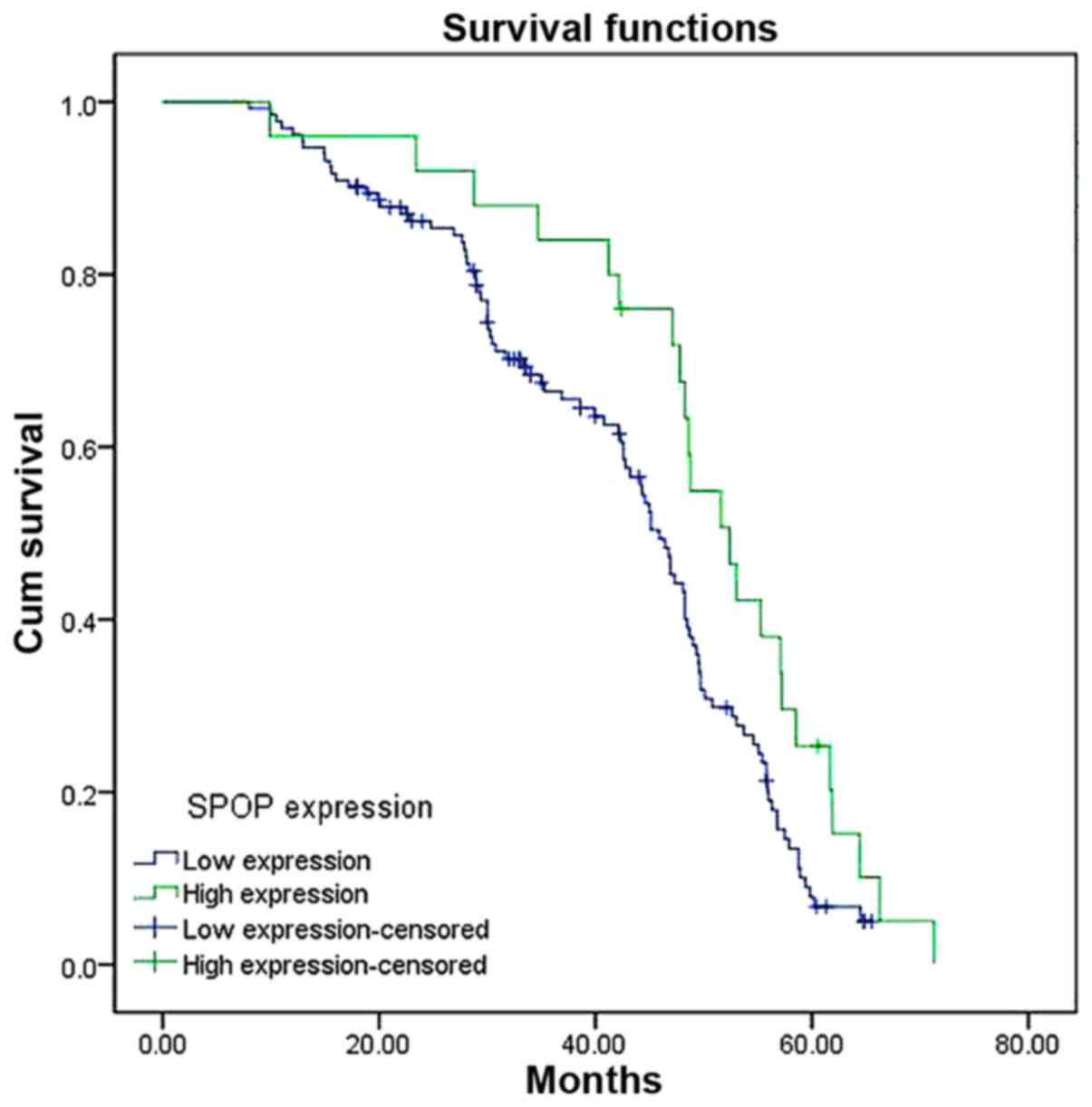
To determine the prognostic significance of SPOP in
NSCLC, the association between expression level of SPOP and
clinical outcome was analyzed. At the end of clinical follow-up,
survival information of all patients was available. Log-rank test
analysis indicated that the overall survival time of patients with
high SPOP expression level was significantly increased compared
with that of patients having low SPOP expression level (P=0.003;
Fig. 3). Based on univariate Cox
regression analyses, tumor size, clinical stages and SPOP
expression level were significantly associated with overall
survival. In addition, a multivariate Cox regression analysis
validated clinical stages and SPOP expression level for predicting
the overall survival of NSCLC patients (Table III).
 | Table III.Univariate and multivariate analyses
of overall survival of patients with NCSLC. |
Table III.
Univariate and multivariate analyses
of overall survival of patients with NCSLC.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 0.948 | 0.660–1.362 | 0.772 | 0.968 | 0.643–1.456 | 0.875 |
| Age (≤60 vs.
>60) | 1.159 | 0.810–1.658 | 0.419 | 1.216 | 0.996–2.681 | 0.303 |
| Histologic type
(adenocarcinoma vs. squamous cell carcinoma) | 0.855 | 0.477–1.529 | 0.597 | 1.099 | 0.574–2.105 | 0.776 |
| Tumor
differentiation (well vs. moderate vs. poor) | 0.862 | 0.462–1.548 | 0.829 | 1.289 | 0.656–1.857 | 0.721 |
| Tumor size (T1 vs.
T2 vs. T3 vs. T4) | 1.976 | 0.425–4.088 | 0.046a | 0.802 | 0.117–4.740 | 0.379 |
| Lymph node
metastasis (absent vs. present) | 1.261 | 0.441–1.192 | 0.365 | 1.543 | 0.619–3.848 | 0.352 |
| Lung cancer stages
(1 vs. 2 vs. 3) | 1.344 | 1.072–2.466 | 0.032a | 1.815 | 1.347–2.802 | 0.041a |
| SPOP expression
(low vs. high) | 0.652 | 0.306–0.861 | 0.039a | 0.634 | 0.422–0.712 | 0.045a |
Discussion
Although SPOP has been acknowledged as a tumor
suppressor gene in numerous types of cancer (9,18), little
has been reported regarding SPOP expression and the prognostic
implications thereof in lung cancer. In the current study, it was
demonstrated that, compared with normal lung tissues, the
expression of SPOP was significantly downregulated at the mRNA and
protein level in NSCLC tissues. Furthermore, low expression level
of SPOP was observed to be significantly associated with short
postsurgical survival. Notably, the data in the present study
demonstrated that SPOP is expressed in normal lung tissue,
providing a basis for further research on the potential role of
SPOP in lung development.
SPOP is an adaptor protein facilitating the
degradation of several substrates that are essential to cellular
development and physiology (18,19).
Specifically, it is an E3 ubiquitin ligase adaptor protein that has
been frequently mutated in prostate and endometrial cancer, which
cluster in the MATH domain (11,20). All
cancer-associated SPOP mutations presumably affect substrate
binding in the ubiquitination process. Ubiquitination regulates
essential cellular processes and is associated with tumor
progression (21). Ubiquitin is
activated by the E1 activating enzyme, provisionally carried by the
E2 conjugating enzyme, and then transferred to its specific
substrates by the E3 ubiquitin ligase (18), which confers substrate specificity to
ubiquitin ligation. Of the E3 ligase family, the Cullin-RING E3
ubiquitin ligase is the most notable (22). In addition, SPOP is a unique
substrate-binding adaptor protein that renders the E3 ubiquitin
ligase complex specific, and gains increasing attention due to its
far-reaching effects in cellular physiology and in pathological
conditions (18). It has been
demonstrated that the deregulation of the ubiquitin system results
in the development of numerous types of tumors, and alterations in
the ubiquitin system occur during the initiation and progression of
cancer (23). Thus, various
approaches have been made to target the ubiquitin system in cancer
therapy, in which E3 ubiquitin ligases are considered as the most
important components as they bind directly to target proteins
(23,24). As an E3 ubiquitin ligase adaptor
protein, SPOP serves a tumor suppressor role in numerous types of
cancer (18).
In the present study, the downregulated expression
of SPOP in NSCLC tissues suggests that SPOP is mutated in NSCLC and
may contribute to cancer development. The level of SPOP expression
was positively associated with tumor differentiation and clinical
stages in the patients with NSCLC, suggesting that SPOP mutations
are be incapable of serving as a tumor suppressor gene and were
early events in NSCLC tumorigenesis. Furthermore, the low
expression level of SPOP was associated with poor postsurgical
survival. Combined with univariate and multivariate analyses on
overall survival, there are sufficient reasons to believe that SPOP
may serve as a novel independent candidate of prognostic marker for
NSCLC.
In summary, SPOP expression in NSCLC tissues and its
prognostic significance was confirmed, which will support further
research on NSCLC development. It was also proposed that it may be
a significant indicator for unfavorable progression in patients
with NSCLC. However, further studies are required to expound the
molecular mechanisms underlying the regulatory role of SPOP in
NSCLC pathogenesis for the development of novel cancer
therapeutics.
Acknowledgements
This study was supported by the Clinical Medicine
Center of Suzhou (grant no. Szzx201502) and the Jiangsu Provincial
Key Medical Discipline (Laboratory)(grant no. ZDXKB2016007).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang T, Zhang DM, Zhao D, Hou XM, Liu XJ,
Ling XL and Ma SC: The prognostic value of osteopontin expression
in non-small cell lung cancer: A meta-analysis. J Mol Histol.
45:533–540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Esposito L, Conti D, Ailavajhala R, Khalil
N and Giordano A: Lung cancer: Are we up to the challenge? Curr
Genomics. 11:513–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagai Y, Kojima T, Muro Y, Hachiya T,
Nishizawa Y, Wakabayashi T and Hagiwara M: Identification of a
novel nuclear speckle-type protein, SPOP. FEBS Lett. 418:23–26.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Marchis L, Cropp C, Sheng ZM, Bargo S
and Callahan R: Candidate target genes for loss of heterozygosity
on human chromosome 17q21. Br J Cancer. 90:2384–2389. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hernández-Muñoz I, Lund AH, van der Stoop
P, Boutsma E, Muijrers I, Verhoeven E, Nusinow DA, Panning B,
Marahrens Y and van Lohuizen M: Stable X chromosome inactivation
involves the PRC1 polycomb complex and requires histone MACROH2A1
and the CULLIN3/SPOP ubiquitin E3 ligase. Proc Natl Acad Sci USA.
102:7635–7640. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kwon JE, La M, Oh KH, Oh YM, Kim GR, Seol
JH, Baek SH, Chiba T, Tanaka K, Bang OS, et al: BTB
domain-containing speckle-type POZ protein (SPOP) serves as an
adaptor of Daxx for ubiquitination by Cul3-based ubiquitin ligase.
J Biol Chem. 281:12664–12672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ande SR, Chen J and Maddika S: The
ubiquitin pathway: An emerging drug target in cancer therapy. Eur J
Pharmacol. 625:199–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kan Z, Jaiswal BS, Stinson J, Janakiraman
V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J, et al:
Diverse somatic mutation patterns and pathway alterations in human
cancers. Nature. 466:869–873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berger MF, Lawrence MS, Demichelis F,
Drier Y, Cibulskis K, Sivachenko AY, Sboner A, Esgueva R, Pflueger
D, Sougnez C, et al: The genomic complexity of primary human
prostate cancer. Nature. 470:214–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barbieri CE, Baca SC, Lawrence MS,
Demichelis F, Blattner M, Theurillat JP, White TA, Stojanov P, Van
Allen E, Stransky N, et al: Exome sequencing identifies recurrent
SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet.
44:685–689. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le GM, O'Hara AJ, Rudd ML, Urick ME,
Hansen NF, O'Neil NJ, Price JC, Zhang S, England BM, Godwin AK, et
al: Exome sequencing of serous endometrial tumors identifies
recurrent somatic mutations in chromatin-remodeling and ubiquitin
ligase complex genes. Nat Genet. 44:1310–1315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li C, Ao J, Fu J, Lee DF, Xu J, Lonard D
and O'Malley BW: Tumor-suppressor role for the SPOP ubiquitin
ligase in signal-dependent proteolysis of the oncogenic
co-activator SRC-3/AIB1. Oncogene. 30:4350–4364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim MS, Je EM, Oh JE, Yoo NJ and Lee SH:
Mutational and expressional analyses of SPOP, a candidate tumor
suppressor gene, in prostate, gastric and colorectal cancers.
APMIS. 121:626–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JH, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 World Health Organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mani RS: The emerging role of speckle-type
POZ protein (SPOP) in cancer development. Drug Discov Today.
19:1498–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding D, Song T, Jun W, Tan Z and Fang J:
Decreased expression of the SPOP gene is associated with poor
prognosis in glioma. Int J Oncol. 46:333–341. 2015.PubMed/NCBI
|
|
20
|
Grasso CS, Wu YM, Robinson DR, Cao X,
Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC,
et al: The mutational landscape of lethal castration-resistant
prostate cancer. Nature. 487:239–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Varshavsky A: Discovery of the biology of
the ubiquitin system. JAMA. 311:1969–1970. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pei XH, Bai F, Li Z, Smith MD, Whitewolf
G, Jin R and Xiong Y: Cytoplasmic CUL9/PARC ubiquitin ligase is a
tumor suppressor and promotes p53-dependent apoptosis. Cancer Res.
71:2969–2977. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoeller D and Dikic I: Targeting the
ubiquitin system in cancer therapy. Nature. 458:438–444. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cohen P and Tcherpakov M: Will the
ubiquitin system furnish as many drug targets as protein kinases?
Cell. 143:686–693. 2010. View Article : Google Scholar : PubMed/NCBI
|