Introduction
High-grade glioma is the most common type of
intracranial malignant tumor and is characterized by the rapid
proliferation and invasion of the tumor (1). At present, there are certain
conventional therapeutic methods available for patients with
high-grade glioma, including neurosurgical resection,
radiochemotherapy and polychemotherapy. However, the 5-year
survival rate is less than 3% (2).
Thus, the study of the molecular mechanisms of high-grade glioma
and the development of effective methods of diagnosis and treatment
is essential.
Inhibitor of growth (ING) 4 is a vital tumor
suppressor factor. Previous studies found that ING4 performed an
important role in inhibiting tumor growth, promoting tumor
apoptosis, recovering intercellular contact inhibition, affecting
cell cycle progression and inhibiting tumor angiogenesis (3–6). In
vitro experiments revealed that ING4 was abundantly expressed
in normal tissues, but expression was significantly reduced in
various malignant tumors, including melanoma (7), ovarian cancer (8), lung cancer (9) and glioma (10).
Malignant tumor growth and angiogenesis are largely
caused by the generation and rapid growth of new blood vessels,
with angiogenesis reported as the hallmark of malignant tumors
including high-grade glioma (11,12). The
microvessel density (MVD) of human tissues has been reported as an
important indicator in the evaluation of angiogenesis (13). A previous study suggested that ING4
could suppress brain tumor growth and angiogenesis by regulating
the nuclear factor κB cells signaling pathway (10). In addition, Zhao et al
(14) have demonstrated that ING4
expression has an important clinical significance in human brain
astrocytoma. However, the association between ING4 and cellular
proliferation and MVD has not been investigated.
In the present study, semi-quantitative reverse
transcription polymerase chain reaction (RT-PCR) western blotting
and immunohistochemistry (IHC) methods were performed to detect the
expression levels of ING4 in normal brain tissue and glioma tissues
of different pathological grades. The correlations between the
level of ING4 expression and human glioma pathological grading,
cellular proliferation and MVD were then analyzed, aiming to reveal
the mechanism of how ING4 is associated with glioma.
Materials and methods
Patients
The present study recruited a total of 85 patients
with glioma at The First Affiliated Hospital of Harbin Medical
University (Harbin, China) between January 2012 and September 2013.
The patients, including 29 males and 56 females, were aged between
20 and 71 years and had a median age of 41 years, with complete
clinicopathological records. The pathological grading of the glioma
tissues was performed according to The World Health Organization
(WHO) 2008 Classification of Tumours of the Central Nervous System
(15). Among the 85 patients, 11
patients were classified as grade I, 18 as grade II, 41 as grade
III and 15 as grade IV. None of the patients received chemotherapy
or radiotherapy prior to surgery, and the glioma tissue was
collected during surgery. Normal brain tissue adjacent to the brain
tumors was used as the negative control. The present study was
approved by the Ethics Committee of Harbin Medical University
(Harbin, China). Written informed consent was obtained from all
participants.
All specimens were freshly collected using
RNase-free techniques. The specimens were divided into 2 sections:
The first section was immediately stored in liquid nitrogen for
subsequent use in the semi-quantitative RT-PCR and western
blotting, and the other section was fixed in 10% formalin solution
for the IHC.
RNA isolation, reverse transcription
and semi-quantitative RT-PCR
The neuronal tissue was homogenized on ice with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and the RNA was extracted according to the
manufacturers protocol. High-quality RNA was reverse transcribed
into complementary DNA (cDNA) using a reverse transcription kit
(Promega Corporation, Madison, WI, USA). The primers were: ING4
forward, 5′-CACAAGTCCTGAGTATGGGAT-3′ and reverse,
5′-AGGGGATGTGGAAGAAACTGT-3′; GAPDH forward,
5′-CAGGGCTGCTTTTAACTCTG-3′ and reverse,
5′-CTGTTGTCGGAGTTCTAGTAG-3′. The PCR reaction mixes were composed
of 10 ng cDNA, 0.1 µM primers, 1 mM deoxy-ribonucleoside
triphosphate, 5 U Taq DNA Polymerase (Promega Corporation), 2.5 µl
10X buffer, and double distilled water for a final volume of 25 µl.
A reaction system without cDNA served as a negative control. The
PCR program was as follows: 95°C for 3 min, 28 cycles of 95°C for
10 sec, 55°C for 30 sec and 72°C for 30 sec. The PCR products were
separated by 2% agarose gel electrophoresis. Ethidium bromide at a
concentration of 0.5 mg/ml (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) was used to stain the DNA for visualization
using a UV transilluminator. The PCR products were analyzed using
gel analysis software Quantity One version 4.4 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and the data were used to
measure corresponding optical density ratios (ING4 optical density
value/GAPDH optical density value). The experiment was repeated 3
times to calculate the mean value.
Western blotting
The neural tissues were milled and then placed in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) on ice for 30 min. The supernatant
was acquired by centrifugation at 10,000 × g for 15 min at 4°C to
facilitate protein quantitation. The proteins were separated on 10%
SDS-PAGE gel and transferred onto polyvinylidene fluoride
membranes. The membranes were blocked in 5% non-fat milk diluted
with PBS for 1 h. Subsequently, the membranes were incubated
overnight at 4°C with rabbit anti-human ING4 polyclonal antibody
(dilution, 1:200; #ab113425, Abcam, Cambridge, UK) or mouse
anti-human β-actin monoclonal antibody (dilution, 1:200; #ab6276,
Abcam), washed 3 times with PBS, and incubated with goat anti-mouse
or mouse anti-rabbit IgG (H+L)-horseradish peroxidase (HRP;
dilution, 1:200; #ab97240 and #ab99702, Abcam, respectively) for 2
h at room temperature. The proteins were detected using enhanced
chemiluminescence (EMD Millipore, Billerica, MA, USA) and protein
expression was quantified using Quantity One version 4.4 software
(Bio-Rad Laboratories, Inc.). The experiment was repeated 3
times.
Detection of ING4 and Ki-67 expression
by IHC
The specimens were fixed in 10% formalin solution
for 24 h, embedded into paraffin blocks, and then sliced into 4 µm
thick sections. The sections were deparaffinized for 4 h at 65°C
and dehydrated with gradient ethanol. IHC was performed using the
two-step immunohistochemistry detection kit according to the
manufacturers protocol (Harbin Hongbo Biotech Co., Ltd, Harbin,
China). In brief, the sections were incubated with 3%
H2O2 to block the endogenous peroxidase
activity for 10 min at room temperature. An antigen retrieval was
performed in citrate buffer (10 mM, pH 6.0) for 10 min at 95°C. The
sections were then incubated with rabbit anti-human ING4 polyclonal
antibody (dilution, 1:200; #ab113425, Abcam), mouse anti-human
Ki-67 monoclonal antibody (dilution, 1:200; #ab8191, Abcam) and
mouse anti-human tumor necrosis factor receptor superfamily member
8 (CD34) monoclonal antibody (dilution, 1:200; #ab8536, Abcam)
overnight. The sections were washed with PBS, and subsequently
incubated with goat anti-mouse or mouse anti-rabbit IgG(H+L)-HRP
(dilution, 1:200; #ab97240 and #ab99702, Abcam, respectively) at
37°C for 30 min. Finally, the sections were stained with
diaminobenzidine (Harbin Hongbo Biotech Co., Ltd, Harbin, China)
for 5 min and re-stained with hematoxylin for 2 min. Sections of
predetermined positive glioma tissue were used as the positive
control, and PBS was used to replace the primary antibody in the
negative controls.
Cells were considered to be ING4 positive when the
nucleus stained brown-yellowish. A total of 4 fields were randomly
selected and observed at a magnification of ×400, and 100 cells
were counted in each field. The expression of ING4 was assessed by
a subjectively graded scale according to a previous study (16): Absence of positive cells were recorded
as negative (−); ≤25% positive cells were recorded as weakly
positive (+); 25–50% positive cells were recorded as moderately
positive (++); and positive cells >50% were recorded as strongly
positive (+++).
The positive standard of the Ki-67 stain was set as
the appearance of brown particles inside the nuclei of the tumor
cells, 4 representative visions were selected at a magnification of
×400. The proliferation index (PI) of the Ki-67 strain was
calculated as: PI (%)=number of positive cells/cell number
×100.
CD34 is a marker of vascular endothelial cells and
was used to indicate the presence blood vessels. Positive CD34
expression located in the cytoplasm or membrane of vascular
endothelial cells was used for quantifying MVD. Blood vessel
distribution was observed under a light microscope (BX41, Olympus
Corporation, Tokyo, Japan) at a magnification of ×100, and the area
with the highest density of microvessels was selected to count
microvascular numbers at a magnification of ×200 in 4 sections. The
mean of the 4 sections was used as the MVD for this tissue
section.
Statistical analyses
Statistical analyses were performed using SPSS 18.0
statistics software (SPSS Inc., Chicago, IL, USA). Statistical
analyses of the mRNA and protein expression levels of ING4, MVD and
PI in normal tissues and various grades of glioma tissues were
performed by a Wilcoxon rank sum test. Data are expressed as the
mean ± the standard deviation. The ING4 positive expression rate in
normal tissue and tissue with various grades of glioma was analyzed
using a χ2 test. The correlation of ING4 expression
levels with PI and MVD was analyzed by Spearman's rank correlation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Semi-quantitative RT-PCR and western blotting
analysis revealed that the mRNA (P=0.015) and protein (P=0.0123)
expression levels of ING4 in glioma brain tissue were lower than
that of the normal brain tissue (Table
I). IHC with anti-ING4 antibody showed that the expression of
ING4 protein in the nucleus presented as brown-yellowish granules
and indicated that the ING4 positive expression rate significantly
decreased in the glioma tissues compared with the normal brain
tissue (P=0.01; Table I). The mRNA
and protein expression levels of ING4 were compared based on age,
gender, tumor size and pathological grading. The results revealed
that the expression of ING4 was not correlated with age, gender or
tumor size in the glioma tissues (Table
I). However, there was a significant decrease in ING4 mRNA
(P=0.026, Fig. 1A) and protein
(P=0.003, Fig. 1B) expression as the
pathological grade increased (Table
I). The ING4 positive expression rate also decreased as the
pathological grade increased (P=0.015, Fig. 1C; Table
I).
 | Table I.Comparisons of ING4 expressions and
MVD in age, gender, tumor size and various grades of glioma
tissues. |
Table I.
Comparisons of ING4 expressions and
MVD in age, gender, tumor size and various grades of glioma
tissues.
|
|
|
| ING4 positive
expression rate |
|
|---|
|
|
|
|
|
|
|---|
|
|
|
| Expression degree of
ING4 protein, (%) |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Category | Relative ING4 mRNA
expression, % | Relative ING4 protein
expression, % | − | + | ++ | +++ | Total | MVD, band |
|---|
| Sample |
|
|
|
|
|
|
|
|
| Normal
brain tissue | 1.59±0.10 | 24.04±2.21 | 0 | 0 | 4 (25) | 12 (75) | 16 (100) | 3.77±1.08 |
| Human
glioma tissue | 0.65±0.30 | 16.27±4.68 | 33 (38.9) | 36 (42.3) | 16 (18.8) | 0 | 52 (61.1) | 17.56±4.08 |
| Age |
|
|
|
|
|
|
|
|
| ≤40 years
old | 0.62±0.32 | 15.02±4.22 | 17 (37) | 20 (43.4) | 9 (19.6) | 0 | 29 (63.0) | 17.80±3.88 |
| >40
years old | 0.69±0.28 | 17.75±4.90 | 16 (41) | 16 (41.0) | 7 (18.0) | 0 | 23 (59.0) | 16.32±4.53 |
| Gender |
|
|
|
|
|
|
|
|
| Male | 0.66±0.39 | 15.47±4.92 | 12 (41.4) | 11 (38.0) | 6 (20.6) | 0 | 17 (58.6) | 17.81±3.99 |
|
Female | 0.66±0.25 | 16.66±4.53 | 21 (37.5) | 25 (44.6) | 10 (17.9) | 0 | 35 (62.5) | 17.13±4.12 |
| Tumor size |
|
|
|
|
|
|
|
|
| ≤3
cm | 0.67±0.27 | 16.35±4.65 | 24 (40.7) | 23 (39.0) | 12 (20.3) | 0 | 35 (59.3) | 17.19±4.18 |
| >3
cm | 0.63±0.41 | 16.04±4.59 | 9 (34.6) | 13 (50.0) | 4 (15.4) | 0 | 17 (65.4) | 18.25±3.01 |
| Pathological
grading |
|
|
|
|
|
|
|
|
| I | 1.14±0.28 | 21.29±1.68 | 1 (9.1) | 3 (27.2) | 7 (63.7) | 0 | 10 (90.9) | 10.97±1.50 |
| II | 0.75±0.08 | 18.79±3.71 | 4 (22.2) | 9 (50.0) | 5 (27.8) | 0 | 14 (77.8) | 16.59±1.59 |
|
III | 0.45±0.08 | 14.06±2.86 | 17 (41.5) | 20 (48.8) | 4 (9.7) | 0 | 24 (58.5) | 19.43±1.73 |
| IV | 0.29±0.04 | 8.18±0.64 | 11 (73.3) | 4 (26.7) | 0 | 0 | 4 (26.7) | 23.93±2.67 |
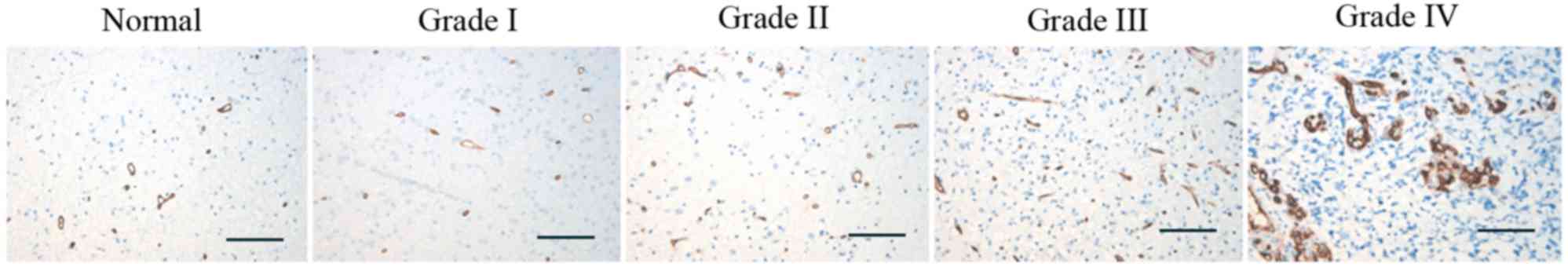
IHC with anti-CD34 antibody showed that CD34-labeled
microvessels existed in the normal brain and glioma tissues, with
the level of MVD higher in the glioma brain tissues compared with
the normal brain tissues (P=0.011, Table
I). The level of MVD was not correlated with age, gender or
tumor size in the glioma tissues, however, MVD level significantly
increased as the pathological grades increased (P=0.0018; Fig. 2; Table
I). The level of MVD was lower in the ING4 protein positively
expressed (ING4+) glioma tissues (17.35±4.12) compared
with the ING4 protein negatively expressed (ING4−)
glioma tissues (22.95±1.88; P≤0.001; Table II).
 | Table II.MVD and PI in ING4 positively and
negatively expressed glioma tissues. |
Table II.
MVD and PI in ING4 positively and
negatively expressed glioma tissues.
|
| ING4 negatively
expressed tissues (n=33) | ING4 positively
expressed tissues (n=52) | t | P-value |
|---|
| MVD, band | 22.95±1.88 | 17.35±4.12 | 14.623 | 0.001 |
| PI, % | 48.46±7.26 | 25.98±4.67 | 2.968 | 0.01 |
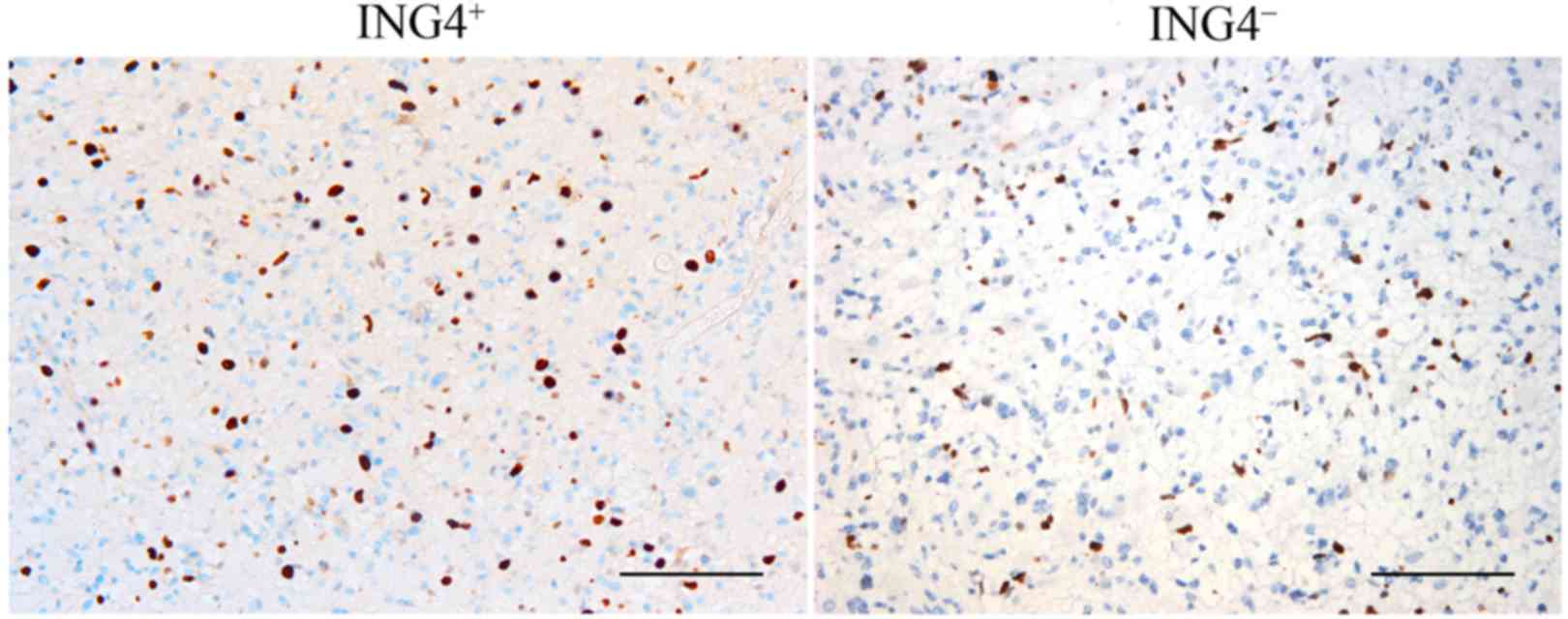
In addition, IHC with anti-Ki-67 antibody revealed
that Ki-67 was expressed in the nucleus. PI decreased in the
ING4+ glioma tissues (25.98±4.67) compared with the
ING4− glioma tissues (48.46±7.26) (P=0.01 Fig. 3, Table
II). Furthermore, the Spearman's rank correlation test
demonstrated that ING4 protein expression was negatively correlated
with PI (r=−0.614, P=0.035). The positive expression rate of the
ING4 gene in different pathologically graded glioma tissues was
negatively correlated with MVD (r=−0.825, P=0.004) while PI and MVD
in the glioma tissue were positively correlated (r=0.364,
P=0.023).
Discussion
High-grade glioma is the most common of the primary
brain tumors in adults, with a high incidence rate and poor
prognosis (2). The present study
demonstrated that the expression level of ING4 was lower in glioma
brain tissue compared with normal brain tissue. Furthermore, there
was a significant decrease in the levels of ING4 mRNA and protein
expression as the pathological grade of the glioma increased,
suggesting that ING4 may perform an important role in the
occurrence and progression of the tumor. In addition, PI and MVD
were lower in the ING4+ glioma tissues compared with the
ING4− glioma tissues, which demonstrated that ING4
protein expression was negatively correlated with PI and MVD in
human glioma.
Previous studies revealed that the downregulation of
ING4 was involved in the progression of hepatocellular carcinoma
tissue (17), head and neck squamous
cell carcinoma (18), human
astrocytomas (19) and colorectal
cancer (20). Consistent with the
results of the present study, Gong et al (21) suggested that the expression of ING4 in
glioma tissue was inhibited, resulting in the inhibition of tumor
cell growth and apoptosis by autophagy. In addition, Colla et
al (22) reported that ING4
exhibits 4 spliceosomes, of which 3 spliceosomes had no complete
nuclear localization signals and were more frequently located in
the nucleus. The results of the IHC of the present study also
demonstrated that the ING4 protein was mainly expressed inside the
nucleus. Additionally, the present study found that the ING4
positive rate decreased as the pathological grading increased, but
was independent from gender, age and tumor size, indicating that
ING4 protein expression in glioma tissue was associated with the
degree of malignancy of the glioma, which are similar to the
results of a study examining the role of ING4 in colon cancer
(23).
Furthermore, as a result of the present study it was
hypothesized that ING4 inhibits glioma proliferation. Zhang et
al (24) found that ING4 could
negatively regulate cell growth by arresting the cell cycle during
the G2/M phase and increasing the sensitivity of HepG2 cells to
certain DNA-damaging agents. In addition, a different study
indicated that the overexpression of ING4 may negatively regulate
U251 cell proliferation by enhancing the binding of the cells to
p53 and increasing levels of p21 expression to induce cell cycle
arrest (25). In addition,
angiogenesis was a feature of a number of tumors and involved with
the growth, invasion, and metastasis of cancer cells (12). The level of MVD has been used as the
gold standard in terms of evaluating the state of angiogenesis of
solid tumors (26). The number of
vessels was significantly higher in human glioma compared with
normal brain tissue (27). The
present study suggested that the suppression of ING4 expression in
glioma tissue was associated with the formation of new blood
vessels. A previous study also showed that the level of MVD was
significantly higher in glioma cells transfected with antisense
ING4 compared with control cells (10). In addition, ING4 inhibited
angiogenesis via interleukin-8 in human glioblastoma (28). The present study also revealed that
the expression level of ING4 was negatively correlated with PI and
MVD. Additionally, it was demonstrated that PI was positively
correlated with the level of MVD in glioma tissue, indicating that
when the expression of ING4 was suppressed in the glioma tissue,
tumor cell proliferation and the formation of tumor microvessels
was promoted and ING4 was negatively associated with the degree of
malignancy of the glioma. Similar to the results of the present
study, Lou et al (23)
demonstrated that there was a negative correlation between the
level of MVD and the level of ING4 expression in patients with
colorectal carcinoma, and a higher tumor node metastasis stage was
correlated with a lower level of ING4 expression and a higher level
of MVD. A limitation of the present study was that the mechanism
underlying ING4-mediated regulation of cell proliferation and
angiogenesis in high-grade glioma is not fully understood. Future
studies are required to investigate this mechanism.
In conclusion, the occurrence and development of
glioma is a multi-stage process and involves multiple gene changes.
ING4 inhibited the proliferation and angiogenesis of glioma. The
expression of ING4 was associated with the pathological grading of
the glioma tissue and negatively correlated with cell proliferation
and angiogenesis, while tumor proliferation and angiogenesis were
positively correlated in the glioma tissues.
Acknowledgements
The present study was supported by Heilongjiang
Provincial Health Department Research Projects (grant no.
2007-534).
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohgaki H and Kleihues P: Epidemiology and
etiology of gliomas. Acta Neuropathol. 109:93–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aggarwal R, Lu J, Kanji S, Das M, Joseph
M, Lustberg MB, Ray A, Pompili VJ, Shapiro CL and Das H: Human
Vγ2V∆2 T cells limit breast cancer growth by modulating cell
survival-, apoptosis-related molecules and microenvironment in
tumors. Int J Cancer. 133:2133–2144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hou Y, Zhang Z, Xu Q, Wang H, Xu Y and
Chen K: Inhibitor of growth 4 induces NFκB/p65 ubiquitin-dependent
degradation. Oncogene. 33:1997–2003. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Culurgioni S, Muñoz IG, Moreno A, Palacios
A, Villate M, Palmero I, Montoya G and Blanco FJ: Crystal structure
of inhibitor of growth 4 (ING4) dimerization domain reveals
functional organization of ING family of chromatin-binding
proteins. J Biol Chem. 287:10876–10884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Li Z, Sheng W, Miao J and Yang J:
Adenovirus-mediated ING4/IL-24 double tumor suppressor gene
co-transfer enhances antitumor activity in human breast cancer
cells. Oncol Rep. 28:1315–1324. 2012.PubMed/NCBI
|
|
7
|
Tang Y, Cheng Y, Martinka M, Ong CJ and Li
G: Prognostic significance of KAI1/CD82 in human melanoma and its
role in cell migration and invasion through the regulation of ING4.
Carcinogenesis. 35:86–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Yu L, Wang Y, Zhang Y, Wang Y and
Zhang G: Expression of tumor suppressor gene ING4 in ovarian
carcinoma is correlated with microvessel density. J Cancer Res Clin
Oncol. 138:647–655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ling C, Xie Y, Zhao D, Zhu Y, Xiang J and
Yang J: Enhanced radiosensitivity of non-small-cell lung cancer
(NSCLC) by adenovirus-mediated ING4 gene therapy. Cancer Gene Ther.
19:697–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garkavtsev I, Kozin SV, Chernova O, Xu L,
Winkler F, Brown E, Barnett GH and Jain RK: The candidate tumour
suppressor protein ING4 regulates brain tumour growth and
angiogenesis. Nature. 428:328–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhattacharya A, Turowski SG, San Martin
ID, Rajput A, Rustum YM, Hoffman RM and Seshadri M: Magnetic
resonance and fluorescence-protein imaging of the anti-angiogenic
and anti-tumor efficacy of selenium in an orthotopic model of human
colon cancer. Anticancer Res. 31:387–393. 2011.PubMed/NCBI
|
|
12
|
Folkman J: How is blood vessel growth
regulated in normal and neoplastic tissue? G.H.A. clowes memorial
award lecture. Cancer Res. 46:467–473. 1986.PubMed/NCBI
|
|
13
|
Rubatt JM, Darcy KM, Hutson A, Bean SM,
Havrilesky LJ, Grace LA, Berchuck A and Secord AA: Independent
prognostic relevance of microvessel density in advanced epithelial
ovarian cancer and associations between CD31, CD105, p53status, and
angiogenic marker expression: A Gynecologic Oncology Group study.
Gynecol Oncol. 112:469–474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao S, Jin C, Zhao X, Jin B, Hui L, Zhou
W, Niu G and Tao S: Expression and clinical significance of ING4
and HIF-1 alpha in brain astrocytoma. Zhonghua Yi Xue Za Zhi.
95:3533–3536. 2015.(In Chinese). PubMed/NCBI
|
|
15
|
Fuller GN: The WHO classification of
tumours of the central nervous system, 4th edition. Arch Pathol Lab
Med. 132:9062008.PubMed/NCBI
|
|
16
|
Liu H, Wan D, Pan Z, Cao L, Wu X, Lu Z and
Kang T: Expression and biological significance of leptin, leptin
receptor, VEGF, and CD34 in colorectal carcinoma. Cell Biochem
Biophys. 60:241–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang F, Luo LB, Tao YM, Wu F and Yang LY:
Decreased expression of inhibitor of growth 4 correlated with poor
prognosis of hepatocellular carcinoma. Cancer Epidemiol Biomarkers
Prev. 18:409–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li XH, Kikuchi K, Zheng Y, Noguchi A,
Takahashi H, Nishida T, Masuda S, Yang XH and Takano Y:
Downregulation and translocation of nuclear ING4 is correlated with
tumorigenesis and progression of head and neck squamous cell
carcinoma. Oral Oncol. 47:217–223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klironomos G, Bravou V, Papachristou DJ,
Gatzounis G, Varakis J, Parassi E, Repanti M and Papadaki H: Loss
of inhibitor of growth (ING-4) is implicated in the pathogenesis
and progression of human astrocytomas. Brain Pathol. 20:490–497.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
You Q, Wang XS, Fu SB and Jin XM:
Downregulated expression of inhibitor of growth 4 (ING4) in
advanced colorectal cancers: A non-randomized experimental study.
Pathol Oncol Res. 17:473–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong A, Ye S, Xiong E, Guo W, Zhang Y,
Peng W, Shao G, Jin J, Zhang Z, Yang J and Gao J: Autophagy
contributes to ING4-induced glioma cell death. Exp Cell Res.
319:1714–1723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colla S, Tagliaferri S, Morandi F, Lunghi
P, Donofrio G, Martorana D, Mancini C, Lazzaretti M, Mazzera L,
Ravanetti L, et al: The new tumor-suppressor gene inhibitor of
growth family member 4 (ING4) regulates the production of
proangiogenic molecules by myeloma cells and suppresses
hypoxia-inducible factor-1 alpha (HIF-1alpha) activity: Involvement
in myeloma-induced angiogenesis. Blood. 110:4464–4475. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lou C, Jiang S, Guo X and Dong XS: ING4 is
negatively correlated with microvessel density in colon cancer.
Tumor Biol. 33:2357–2364. 2012. View Article : Google Scholar
|
|
24
|
Zhang X, Xu LS, Wang ZQ, Wang KS, Li N,
Cheng ZH, Huang SZ, Wei DZ and Han ZG: ING4 induces G2/M cell cycle
arrest and enhances the chemosensitivity to DNA-damage agents in
HepG2 cells. FEBS Lett. 570:7–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu E, Wu J, Cao W, Zhang J, Liu W, Jiang
X and Zhang X: Curcumin induces G2/M cell cycle arrest in a
p53-dependent manner and upregulates ING4 expression in human
glioma. J Neurooncol. 85:263–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goodheart MJ, Ritchie JM, Rose SL,
Fruehauf JP, De Young BR and Buller RE: The relationship of
molecular markers of p53 function and angiogenesis to prognosis of
stage I epithelial ovarian cancer. Clin Cancer Res. 11:3733–3742.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo Y, Jiang F, Cole TB, Hradil VP, Reuter
D, Chakravartty A, Albert DH, Davidsen SK, Cox BF, McKeegan EM and
Fox GB: A novel multi-targeted tyrosine kinase inhibitor, linifanib
(ABT-869), produces functional and structural changes in tumor
vasculature in an orthotopic rat glioma model. Cancer Chemother
Pharmacol. 69:911–921. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brat DJ, Bellail AC and Van Meir EG: The
role of interleukin-8 and its receptors in gliomagenesis and
tumoral angiogenesis. Neuro Oncol. 7:122–133. 2005. View Article : Google Scholar : PubMed/NCBI
|