Introduction
An imbalance between cell proliferation and
apoptosis is important during the occurrence and development of
oral cancer. This dynamic balance is essential to maintaining
homeostasis, which ensures the balance and stability of the human
body at the cellular level. Previous studies have observed that the
reduction of apoptosis also serves a key role in oral cancer
incidence and development. A previous study demonstrated that
pro-apoptotic factors, including tumor protein 53 and Fas, as well
as anti-apoptotic factors, including the B-cell lymphoma-2 family
and inhibitors of apoptosis protein family (IAPs), serve crucial
roles in the pathogenesis of oral cancer (1). Of the IAPs, survivin is the protein with
the highest apoptosis inhibitory ability, and also regulates the
cell cycle (2). Previous studies
compared the expression profile of survivin in normal oral mucosa,
oral precancerous lesions and oral cancer tissue, revealing that
survivin was not expressed in the normal oral mucosa but was
expressed in the early and precancerous stages of oral cancer
(3–5).
The positive expression rate of survivin in epithelial paraplastic
tissues was ~97 and ~98% in oral cancer tissue (3–5), and its
expression in distinct splice variants was also altered during
tumorigenesis (6). This indicates
that survivin not only serves an important role in the occurrence
of oral cancer, but that the increase in its expression levels are
an early event in the development of this type of cancer. A
previous study also observed that survivin was associated with
angiogenesis, as it was not able not be detected in quiescent
endothelial cells, but was strongly expressed in angiogenic
factor-stimulated endothelial cells (7). In addition, the application of antisense
survivin technology during angiogenesis resulted in the instant
abrogation of the cytoprotective effects of vascular endothelial
growth factor (VEGF), inhibition of blood vessel growth and
apoptosis of endothelial cells (7).
In the present study, immunohistochemistry and cell apoptosis
detection were used to investigate the roles of survivin and
caspase-3 (its downstream target in the apoptosis signaling
pathway) in the incidence and development of oral cancer, and to
explore the association between neovascularization and survivin
expression during this process.
Materials and methods
Case selection
A total of 45 paraffin-embedded tissue specimens
were obtained from the Department of Pathology, Beijing
Stomatological Hospital (Beijing, China) from September 2005 to
August 2007 were selected, including: 16 cases of oral leukoplakia
accompanied by low-moderate epithelial dysplasia (OL-LMED) (age,
61.8±15.0 years old; 9 males and 7 females), 12 cases of oral
leukoplakia accompanied by severe epithelial dysplasia (OL-SED)
(age, 63.6±16.8 years old; 5 males and 7 females) and 17 cases of
high-moderate differentiated oral squamous cell carcinoma (OSCC)
(age, 62.4±14.0 years old; 9 males and 8 females). The patients did
not receive any treatments prior to enrolment in the present study.
All tissue specimens were diagnosed by two experienced pathologists
(Department of Pathology, Oral Medicine, Beijing Stomatological
Hospital) according to the histologic classification criteria of
oral mucosa cancer and precancerous lesions issued by the World
Health Organization in 1996 (8). A
further 10 normal oral mucosa tissue specimens were selected as
controls. This study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of Capital Medical University (Beijing, China). Written informed
consent was obtained from all participants.
Immunohistochemical streptavidin
peroxidase assay
A 3,3′-diaminobenzidine chromogenic reagent kit
(Fuzhou Maixin Biotech Co. Ltd., Fuzhou, China) was used to detect
the expression of survivin (mouse anti-human survivin monoclonal
antibody; dilution 1:400; catalogue number TA301427; OriGene
Technologies, Inc., Rockville, MD, USA), caspase-3 (mouse
anti-human caspase-3 monoclonal antibody; dilution 1:400; catalogue
number 610322; BD Biosciences, Franklin Lakes, NJ, USA) and factor
VIII (rabbit anti-human von Willebrand factor polyclonal antibody;
dilution 1:400; catalogue number MBS1489331; MyBioSource, CA, USA)
in the tissue samples. The incubation was performed at 4°C
overnight. Goat anti-mouse IgG (dilution 1:500; catalogue number
L3031; Signalway Antibody LLC, MA, USA) and goat anti-rabbit IgG
(dilution 1:500; catalogue number L3041; Signalway Antibody) were
used as the secondary antibodies. The incubation was performed at
37°C for 10 min. Other experimental procedures were performed
according to the manufacturer's protocols.
Terminal deoxynucleotidyl transferase
2′-deoxyuridine, 5′-triphosphate nick end labeling (TUNEL)
assay
TUNEL was performed to detect apoptosis using the
apoptosis detection kits (Wuhan Boster Biological Technology, Ltd.,
Wuhan, China), according to manufacturer's protocols.
Double-distilled water was used to replace terminal
deoxynucleotidyl transferase staining (negative control). The
standard positive slice included in the kit was used as the
positive control (Wuhan Boster Biological Technology, Ltd.). The
reaction was terminated when the positive control achieved
coloration, followed by observation with a light microscope and the
quantification of TUNEL-positive cells.
Detection of survivin and
caspase-3
Positive staining of survivin was primarily
localized to the cytoplasm and nucleus, exhibiting uniform
yellow-brown granules. Positive staining of caspase-3 was
principally localized to the cytoplasm, or simultaneously expressed
in the cytoplasm and the nucleus, also exhibiting uniform,
yellow-brown granules. Five high power fields were counted
(magnification, 400x), with a total cell count >1,000 in order
to quantify the number of positive cells. According to the degree
of staining, positive signals were subdivided into three
categories, as follows: weakly positive (pale yellow, or individual
cells were stained yellow-brown), moderately positive (intermediate
staining) and intensely positive (dark brown staining.
Microvessel counting
Microvessel counting was performed using the Weidner
method (9). Using this technique, the
endothelial cells (identified by the positive staining of factor
VIII) or cell clusters that exhibited clear boundaries between
adjacent microvessels and cancerous cells in tumor tissues, and
stained brown or tan were considered as the nascent tumor blood
vessel. The appearance of red blood cells or lumen was not included
in the judgment criteria, and for blood vessels with a thick
muscular layer and a lumen diameter >8, red blood cells were not
counted. For each lesion, ≥3 fields were randomly selected
(magnification, 200x) to determine the number of blood vessels, and
image analysis software (ImagePro Plus version 6; Media Cybernetics
Inc., Rockville, MD, USA) was used to calculate the number of
microvessels (strips/mm2) per unit area. The mean value
of three fields of vision was used as the final microvascular
density (MVD) for each patient tissue sample.
Detection of apoptosis
A cell with brown granules in the nucleus was
considered to be positive and, therefore, an apoptotic cell. For
each tissue section, the five most positively stained high-power
fields (magnification, 400x) were randomly selected to count
>500 cells and calculate the apoptotic index (AI), which was
defined as the numbers of apoptotic cells per 100 cells.
Statistical analysis
Data were presented as the mean ± standard
deviation. Comparisons of survivin expression and caspase-3
expression, and the associations between AI and tissue type were
analyzed using the non-parametric Kruskal-Wallis test. Fisher's
least significant difference method was used for intergroup
comparison. The immunohistochemical staining of factor VIII was
analyzed using SPSS statistical software version 11.5 for Windows
(SPSS, Inc., Chicago, IL, USA). Analysis of variance was used to
analyze normally distributed data, whilst non-normal distributions
were analyzed using the rank sum test. Intergroup paired comparison
was then performed to examine any statistically significant
differences. P<0.05 was considered to indicate a statistically
significant difference.
Results
Immunohistochemical assay
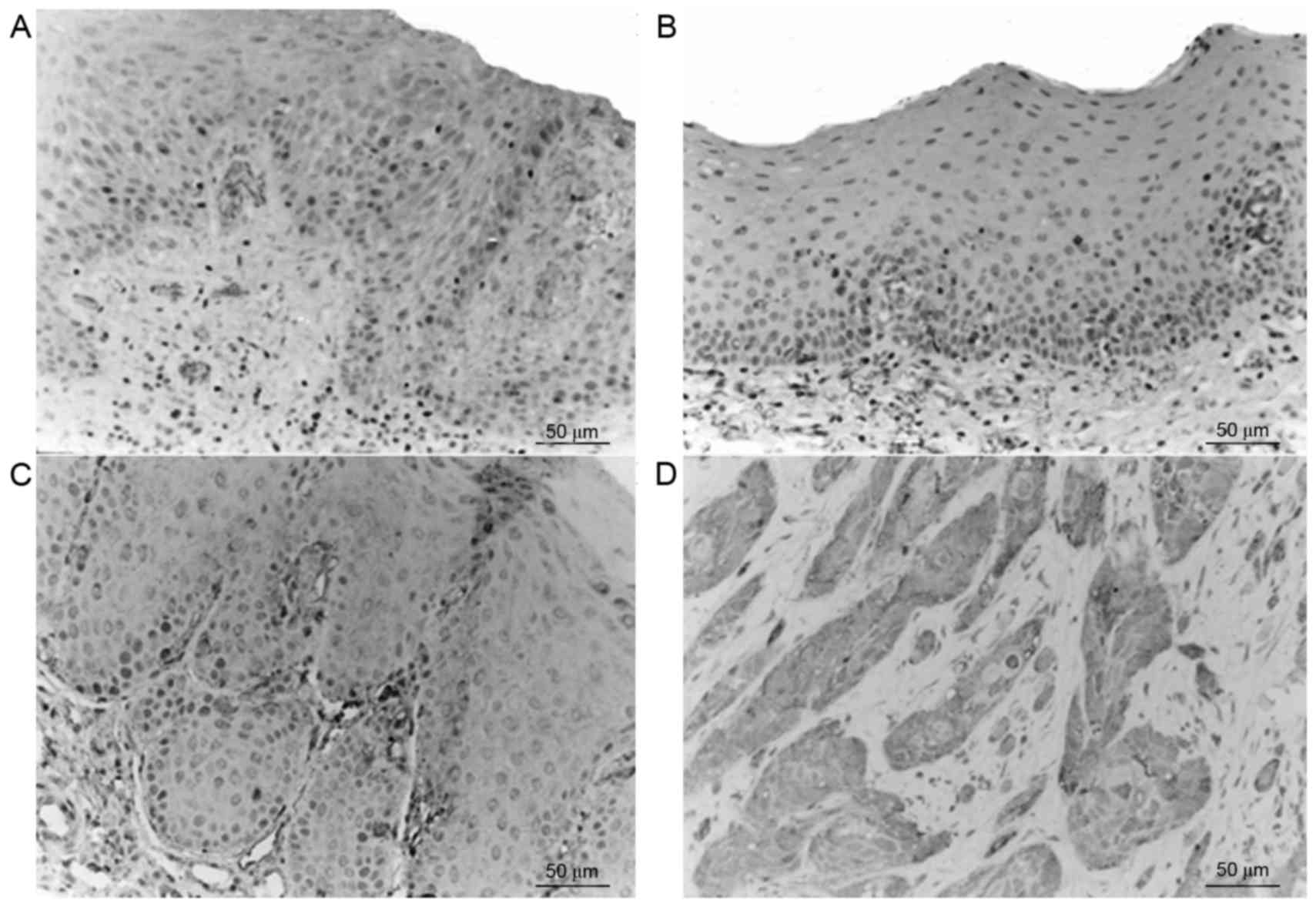
Survivin was primarily expressed in the cytoplasm,
nucleus and membrane of the epithelial cells, as well as in the
cytoplasm of inflammatory stromal cells. The majority of normal
tissues exhibited no significant survivin expression; however, in
accordance with the progression from epithelial dysplasia to oral
cancer the expression levels of survivin gradually increased
(Fig. 1), and the expression levels
in the tumor edge were greater, as compared with those in the tumor
center. Staining of survivin exhibited heterogeneity, and the
staining intensity and staining area varied between tissues and
regions. The expression of survivin in the OSCC group was
significantly increased compared with the OL-LMED, OL-SED and
normal control group (P<0.05; Table
I).
 | Table I.Expression of survivin in various
groups (mean ± standard deviation). |
Table I.
Expression of survivin in various
groups (mean ± standard deviation).
| Group | Cases, n | Average proportion of
positive cells, % |
|---|
| Normal control | 10 |
1.05±1.21a |
| LMED | 16 |
6.06±4.87a |
| SED | 12 |
12.49±8.41a |
| OSCC | 17 |
21.89±10.45 |
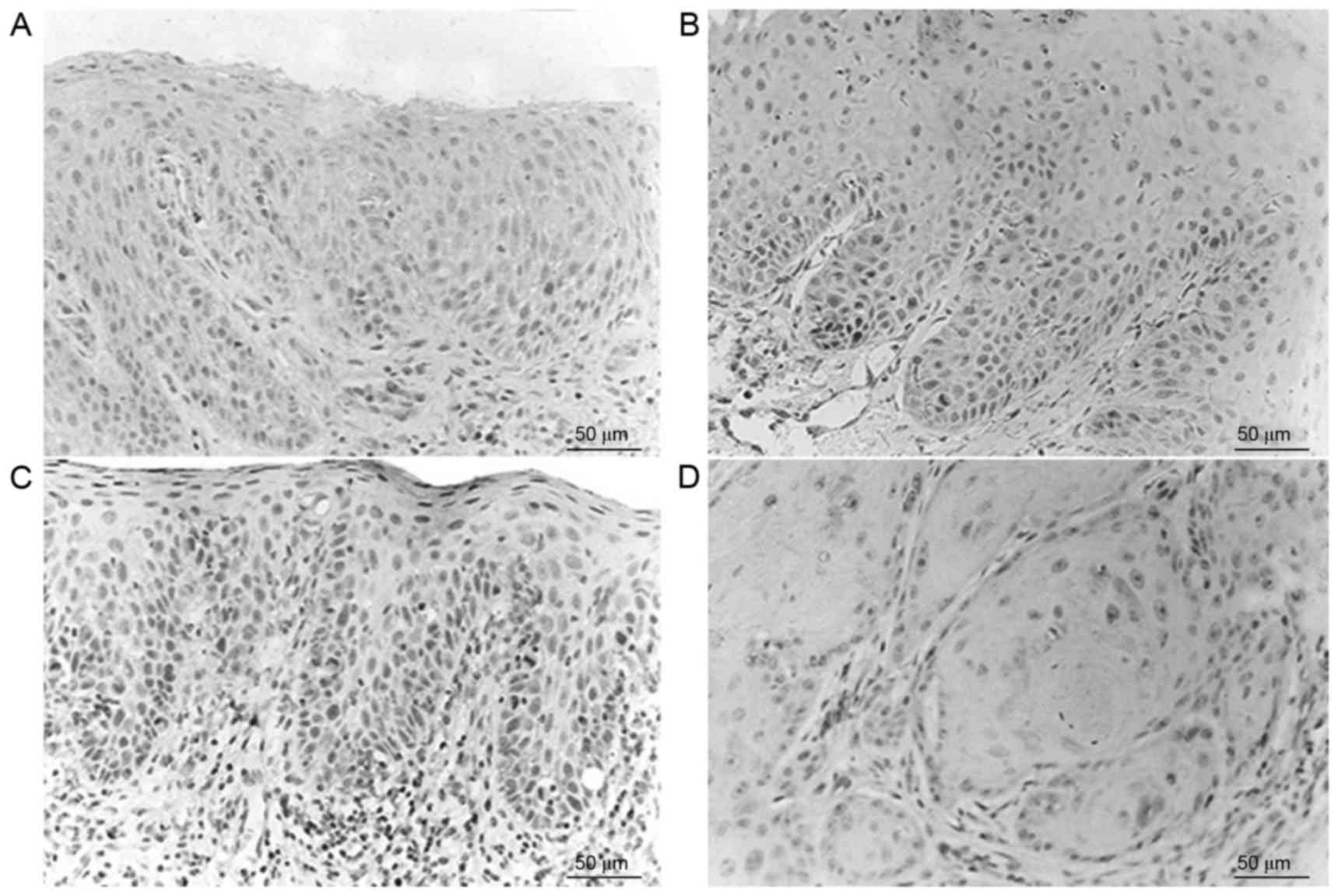
Caspase-3 positive staining was observed as brown
particles, primarily either in the cytoplasm of epithelial cells or
in the nucleus, and rarely on the nuclear membrane. The normal
control group exhibited positive caspase-3 expression (10/10), with
the majority exhibiting intense positive staining (Fig. 2). Corresponding to the progression
from epithelial dysplasia to oral cancer, the expression levels of
caspase-3 were gradually decreased. Immunostaining exhibited
heterogeneity and the staining intensity and staining area changed
in various tissues and regions. The expression of caspase-3 in the
normal control group, OL-LMED group and OL-SED group were
significantly increased compared with in the OSCC group (P<0.05;
Table II).
 | Table II.Expressions of caspase-3 in various
groups (mean ± standard deviation). |
Table II.
Expressions of caspase-3 in various
groups (mean ± standard deviation).
| Group | Cases, n | Average proportion of
positive cells, % |
|---|
| Normal control | 10 |
12.37±5.48a |
| LMED | 16 |
19.51±13.15a |
| SED | 12 |
9.76±7.83a |
| OSCC | 17 |
6.08±6.91 |
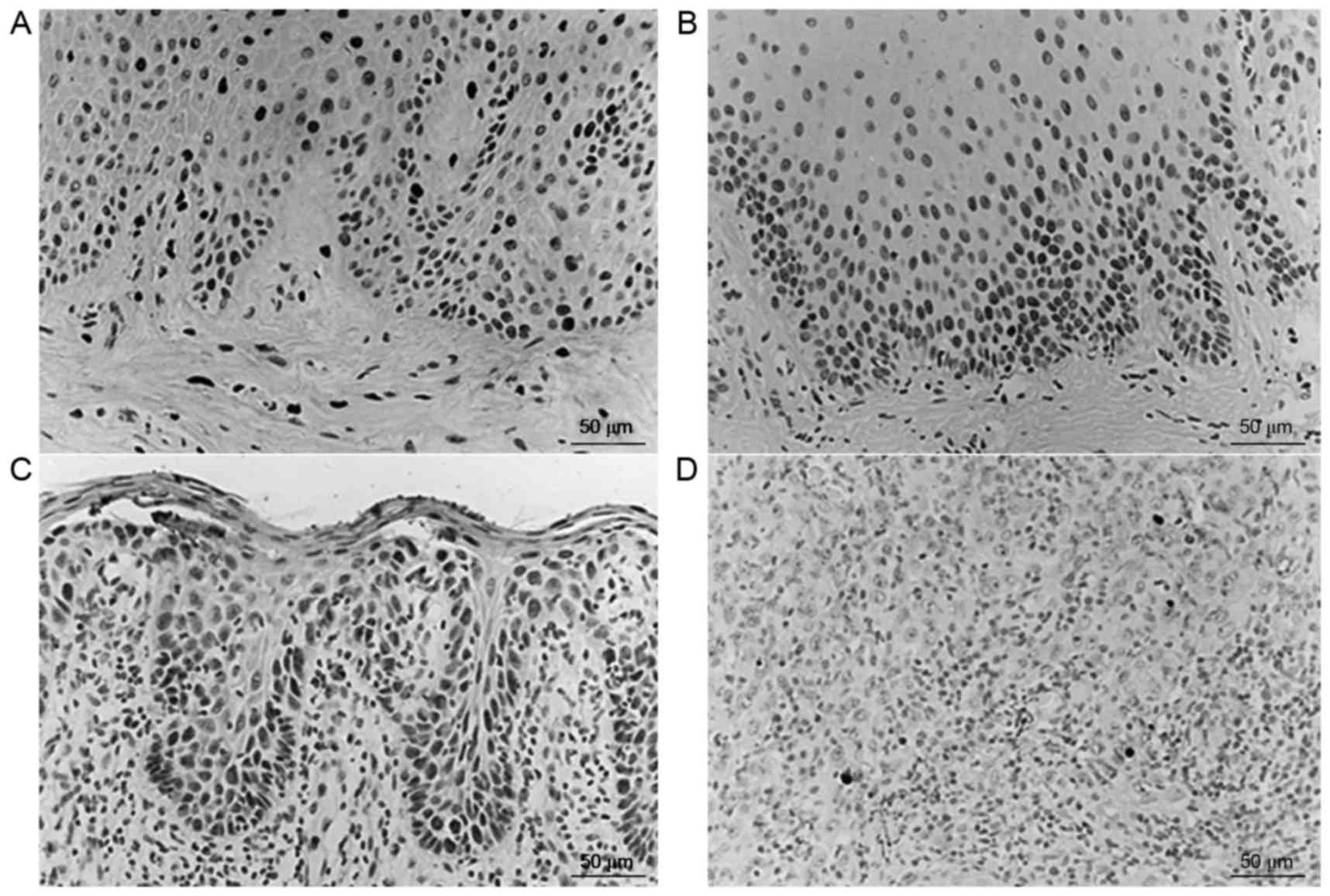
MVD in the normal control group, OL-LMED group,
OL-SED group and OSCC group was 28.49±11.87, 47.92±25.58,
49.73±23.76 and 91.98±40.20 strips/mm2, respectively. A
gradual increase in MVD was observed from the normal control group
to the OL-LMED group, then the OL-SED group, with the highest MVD
in the OSCC group (Table III).
Comparisons between the normal control group and OSCC group,
between the OL-LMED group and OSCC group, and between the OL-SED
group and OSCC group all exhibited statistically significant
differences (P<0.05; Fig. 3).
 | Table III.MVD in different groups (mean ±
standard deviation). |
Table III.
MVD in different groups (mean ±
standard deviation).
| Group | Cases, n | MVD,
strips/mm2 |
|---|
| Normal control | 10 |
28.49±11.87a |
| LMED | 16 |
47.92±25.58a |
| SED | 12 |
49.73±23.76a |
| OSCC | 17 |
94.97±40.32 |
AI
Apoptotic cells were stained brownish yellow using
TUNEL and exhibited pyknosis. Cells were round and oval, and a
number were plate-shaped or crescent-shaped. The majority of
apoptotic cells were scattered and had no surrounding inflammation.
The AI of the normal control group, OL-LMED group, OL-SED group and
OSCC group was 0.89±0.46, 1.29±0.63, 0.65±0.40 and 0.21±0.12,
respectively (mean ± standard deviation; Fig. 4). The AI was significantly decreased
in the OSCC group, with apoptotic cells predominantly distributed
around the tumor core and rarely observed in the tumor frontier. AI
in normal control group, OL-LMED group and OL-SED group were
significantly higher compared with in the OSCC group (P<0.05;
Table IV).
 | Table IV.AI in various groups (mean ± standard
deviation) |
Table IV.
AI in various groups (mean ± standard
deviation)
| Group | Cases, n | AI, % |
|---|
| Normal control | 10 |
0.89±0.46a |
| LMED | 16 |
1.29±0.63a,b |
| SED | 12 |
0.65±0.40a |
| OSCC | 17 |
0.21±0.12 |
Discussion
The occurrence and development of oral cancer is a
multi-stage process regulated by numerous genes, and includes the
accumulation of mutations and imbalances in accumulation and cell
cycle regulation (10). These changes
may be able to be detected in the precancerous lesion stage,
potentially aiding early prediction and prevention of oral
cancer.
Survivin is considered to be the most potent
apoptosis inhibitor ever discovered. A previous study demonstrated
that no, or low, expression of survivin is able to be observed in
normal tissues, whereas this protein it is specifically and highly
expressed in tumor tissues, and this expression is tumor
cell-dependent (11). In the present
study, survivin was also specifically expressed in tumor cells of
the oral mucosa. Previous studies used immunohistochemical assays
to detect the expression of survivin in oral mucosae, and observed
that its expression in precancerous lesions that had not yet
progressed towards malignancy and had progressed to complete
squamous cancer were 33% (10/30) and 94% (15/16), respectively
(12,13). Previous studies revealed that survivin
gene expression was also present in various precancerous damaged
tissues, including oral leukoplakia, colon polyps, mastitis or
keratotic dermatitis (14,15). The present study obtained similar
results, as the expression levels of survivin were gradually
increased during the progression from abnormal cell proliferation
to malignant transformation.
Caspase-3 is expressed in normal tissues and its
expression level in gastric cancer is significantly reduced, which
is negatively correlated with survivin expression level (16). Previous studies detected survivin and
caspase-3 mRNA expression in tongue cancer, demonstrating that the
overexpressed survivin mRNA inhibited the expression of caspase-3
mRNA (17,18). The expression of the two were
negatively correlated and associated with the occurrence and
progression of tumors (17,18). The results of the present study were
concordant with these findings, in that as survivin expression
levels increased, the expression levels of caspase-3 gradually
decreased.
Survivin was specifically highly expressed during
tumorigenesis, particularly in early-stage cancer, and its
expression was regulated by a variety of factors (19,20).
Therefore, it is possible that during the formation of oral cancer,
a variety of oncogene signaling pathways may induce increased
expression levels of survivin in tumor tissues.
Furthermore, the AI in the OL-LMED group was
increased from that observed in normal oral tissues, which may
occur as part of the tissue stress response towards the lesions. In
oral precancerous lesions, the cells exhibited rapid growth, while
apoptosis was increased in order to balance the resulting
proliferation-apoptosis disorder. Furthermore, during the
carcinogenesis of the oral mucosa the underlying mechanisms of
apoptosis, including p53 mutations and the downregulation of
caspase-3, were inhibited. The inhibition of apoptosis
simultaneously promoted the occurrence of severe epithelial
dysplasia and canceration, concordant with the results of previous
studies (21,22).
Of the angiogenesis-promoting factors, VEGF is
highly expressed in the majority of human and animal tumors. VEGF
may significantly increase the expression levels of survivin, which
may then inhibit the caspase-mediated apoptosis signaling pathway,
thus protecting the nascent immature tumor vascular endothelial
cells, allowing them to evade apoptosis and subsequently produce
more VEGF (23,24). It was hypothesized that highly
expressed survivin may promote uncontrolled cell cycle progression,
bypassing the G2/M DNA damage checkpoint. Therefore, the
cells may lose the restriction of the apoptosis switch during
normal cell proliferation, inducing malignant transformation and a
proliferation-apoptosis imbalance in cells. Survivin may also
promote the proliferation of endothelial cells, and silencing
survivin may be able to inhibit the expression of VEGF (25). The import of survivin-targeting
antisense oligonucleotides into cultured endothelial cells may
promote the apoptosis of these endothelial cells and inhibit
angiogenesis (26,27). A previous study has demonstrated that
basic fibroblast growth factor and survivin also produce
synergistic effects, with positively associated expression patterns
in lung cancer and OSCC (23). This
may promote the proliferation of tumor and endothelial cells, and
enhance angiogenesis (28).
Through analyzing the expression profile of survivin
and MVD in oral leukoplakia and oral cancer tissues, similar
conclusions may be obtained. These are namely that, corresponding
with the increased expression levels of survivin in oral cancer
tissues, MVD was also increased and exhibited a marked increasing
trend, indicating that survivin was involved in tumor angiogenesis,
which may serve an important role in the occurrence and development
of oral cancer.
In conclusion, the present study demonstrated that
survivin and caspase-3 served important roles in the occurrence and
development of oral cancer and, therefore, may be of potential use
in monitoring the progression of oral cancer. As survivin was
differentially expressed in tumor tissues and normal tissues,
highly expressed in tumor cells and the vascular endothelium and
angiogenesis was critically dependent on the viability of the
endothelial cells, any survivin-targeting treatment may be able to
promote the apoptosis and degeneration of the vessels, and
indirectly inhibit tumor growth. Therefore, survivin has the
potential to become a novel therapeutic target for certain types of
cancer. Further in-depth understanding regarding the structures,
properties and physiological effects of survivin and caspase-3 may
facilitate the full elucidation of their roles in apoptosis,
therefore providing more options for the prevention of cancer, and
for the development of novel agents to target tumors and numerous
other chronic diseases. It is speculated that this approach may
provide new treatments, with reduced adverse effects on normal
tissues, whilst effectively inhibiting the proliferation of tumor
cells and tumor growth.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 30500559),
Postdoctoral Science Foundation of China (grant no. 20060400486)
and The New Star Projects of Beijing Municipal Science and
Technology (grant no. 2005B51).
References
|
1
|
Sugerman PB, Joseph BK and Savage NW:
Review article: The role of oncogenes, tumour suppressor genes and
growth factors in oral squamous cell carcinoma: A case of apoptosis
versus proliferation. Oral Dis. 1:172–188. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nassar A, Lawson D, Cotsonis G and Cohen
C: Survivin and caspase-3 expression in breast cancer: Correlation
with prognostic parameters, proliferation, angiogenesis, and
outcome. Appl Immunohistochem Mol Morphol. 16:113–120. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin CY, Hung HC, Kuo RC, Chiang CP and Kuo
MY: Survivin expression predicts poorer prognosis in patients with
areca quid chewing-related oral squamous cell carcinoma in Taiwan.
Oral Oncol. 41:645–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lauxen IS, Oliverira MG, Rados PV, Lingen
MW, Nör JE and Sant'ana Filho M: Immunoprofiling of oral squamous
cell carcinomas reveals high p63 and survivin expression. Oral Dis.
20:e76–e80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cai Y, Ma W, Huang X, Cao L, Li H, Jiang
Y, Lu N and Yin Y: Effect of survivin on tumor growth of colorectal
cancer in vivo. Int J Clin Exp Pathol. 8:13267–13272.
2015.PubMed/NCBI
|
|
6
|
Mishra R, Palve V, Kannan S, Pawar S and
Teni T: High expression of survivin and its splice variants
survivin ΔEx3 and survivin 2 B in oral cancers. Oral Surg Oral Med
Oral Pathol Oral Radiol. 120:497–507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Altieri DC: The molecular basis and
potential role of survivin in cancer diagnosis and therapy. Trends
Mol Med. 7:542–547. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Axéll T, Pindborg JJ, Smith CJ and van der
Waal I: Oral white lesions with special reference to precancerous
and tobacco-related lesions: Conclusions of an international
symposium held in Uppsala, Sweden, May 18–21 1994. International
collaborative group on oral white lesions. J Oral Pathol Med.
25:49–54. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng J, Kloosterbooer F, Xia W and Hung
MC: The NH(2)-terminal and conserved region 2 domains of adenovirus
E1A mediate two distinct mechanisms of tumor suppression. Cancer
Res. 62:346–350. 2002.PubMed/NCBI
|
|
10
|
Rowley H, Sherrington P, Helliwell TR,
Kinsella A and Jones AS: p53 expression and p53 gene mutation in
oral cancer and dysplasia. Otolaryngol Head Neck Surg. 118:115–123.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duffy MJ, O'Donovan N, Brennan DJ,
Gallagher WM and Ryan BM: Survivin: A promising tumor biomarker.
Cancer Lett. 249:49–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lo Muzio L, Pannone G, Leonardi R,
Staibano S, Mignogna MD, De Rosa G, Kudo Y, Takata T and Altieri
DC: Survivin, a potential early predictor of tumor progression in
the oral mucosa. J Dent Res. 82:923–928. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poomsawat S, Punyasingh J and Vejchapipat
P: Overexpression of survivin and caspase 3 in oral carcinogenesis.
Appl Immunohistochem Mol Morphol. 22:65–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grossman D, McNiff JM, Li F and Altieri
DC: Expression of the apoptosis inhibitor, survivin, in nonmelanoma
skin cancer and gene targeting in a keratinoyte cell line. J Lab
Invest. 79:1121–1126. 1999.
|
|
15
|
Li SX, Chai L, Cai ZG, Jin LJ, Chen Y, Wu
HR and Sun Z: Expression of survivin and caspase 3 in oral squamous
cell carcinoma and peritumoral tissue. Asian Pac J Cancer Prev.
13:5027–5031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kania J, Konturek SJ, Marlicz K, Hahn EG
and Konturek PC: Expression of survivin and caspase-3 in gastric
cancer. Dig Dis Sci. 48:266–271. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang ZM, Zhao ZG, Guan XM, Liu F and Zhang
LP: Expression of Survivin mRNA and Caspase-3 mRNA and their
correlation in tongue squamous cell carcinoma. Shanghai Kou Qiang
Yi Xue. 16:582–586. 2007.(In Chinese). PubMed/NCBI
|
|
18
|
Xu JH, Wang AX, Huang HZ, Wang JG, Pan CB
and Zhang B: Survivin shRNA induces caspase-3-dependent apoptosis
and enhances cisplatin sensitivity in squamous cell carcinoma of
the tongue. Oncol Res. 18:377–385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holdenrieder S and Stieber P: Circulating
apoptotic markers in the management of non-small cell lung cancer.
Cancer Biomark. 6:197–210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang JY, Casiano CA, Peng XX, Koziol JA,
Chan EK and Tan EM: Enhancement of antibody detection in cancer
using panel of recombinant tumor-associated antigens. Cancer
Epidemiol Biomarkers Prev. 12:136–143. 2003.PubMed/NCBI
|
|
21
|
Srinivasprasad V, Dineshshankar J,
Sathiyajeeva J, Karthikeyan M, Sunitha J and Ragunathan R: Liaison
between micro-organisms and oral cancer. J Pharm Bioallied Sci.
7:(Suppl 2). S354–S360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hills SA and Diffley JF: DNA replication
and oncogene-induced replicative stress. Curr Biol. 24:R435–R444.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang S, Xu J and Zhang Q: Clinical
significance of survivin and vascular endothelial growth factor
mRNA detection in the peripheral whole blood of breast cancer
patients. Neoplasma. 63:133–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao J, Huang Y, Li M, Zhao H, Zhao Y, Li
R, Yan J, Yu Y and Qiao J: Effect of local basic fibroblast growth
factor and vascular endothelial growth factor on subcutaneously
allotransplanted ovarian tissue in ovariectomized mice. PLoS One.
10:e01340352015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ji ZJ, Wang JL and Chen L: Inhibition of
skin squamous cell carcinoma proliferation and promote apoptosis by
dual silencing of NET-1 and survivin. Oncol Rep. 34:811–822.
2015.PubMed/NCBI
|
|
26
|
Coma S, Noe V, Lavarino C, Adán J, Rivas
M, López-Matas M, Pagan R, Mitjans F, Vilaró S, Piulats J and
Ciudad CJ: Use of siRNAs an d antisense oligonucleotides against
survivin RNA to inhibit steps leading to tumor angiogenesis.
Oligonucleotides. 14:100–113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tu SP, Cui JT, Liston P, Huajiang X, Xu R,
Lin MC, Zhu YB, Zou B, Ng SS, Jiang SH, et al: Gene therapy for
colon cancer by adeno-associated viral vector-mediated transfer of
surviving Cys84Ala mutant. Gastroenterology. 128:361–375. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cosgrave N, Hill AD and Young LS: Growth
factor-dependent regulation of survivin by c-myc in human breast
cancer. J Mol Endocrinol. 37:377–390. 2006. View Article : Google Scholar : PubMed/NCBI
|