Introduction
Pleural effusion is a common clinical complication
associated with multiple benign and malignant conditions (1). Congestive heart failure, pneumonia and
tuberculosis are common causes of benign pleural effusion (BPE)
(2). Certain types of malignancy,
including lung, breast, ovarian cancer and lymphoma also cause
pleural effusion (3–5). Among patients with cancer, ~50% develop
malignant pleural effusion (MPE) (6).
The median survival time following MPE presentation is 4 months
(7).
MPE is induced by the interaction of tumor cells,
endothelial cells, myeloid cells and lymphoid cells in the pleural
cavity. The concentration of protein and lactate dehydrogenase
(LDH) in MPE is a prognostically significant biochemical feature
(8). In addition, numerous types of
cytokine, including interleukin (IL)-1β, IL-5, IL-6, IL-8, IL-10,
IL-12, monocyte chemoattractant protein-1 (MCP-1) and tumor
necrosis factor-α (TNF-α) are detected in MPE (9–11). An
increased concentration of vascular endothelial growth factor
(VEGF), which is mainly secreted from endothelial cells is also
detected in MPE (12). Certain
biochemical properties of pleural effusion, including glucose and
total protein concentration, may predict mortality in patients with
MPE (13). Interferon γ (IFNG) and
transforming growth factor β (TGFB) 1 are associated with
tuberculosis pleural effusion, but not MPE (14,15).
However, no cytokines or biochemical features that differentiate
MPE and BPE sensitively and specifically have been identified at
present.
Since clinical features, biomedical features and
numerous cytokines have been reported to be associated with MPE,
and a single parameter may not serve as an optimal biomarker for
predicting MPE (8–15), the present study assessed whether a
combination of biochemical features, clinical features and cytokine
levels in pleural effusion may distinguish between BPE and MPE. The
clinical and biochemical features of pleural effusion were
determined and the concentration of cytokines in collected BPE and
MPE samples was evaluated using cytometric bead arrays.
Materials and methods
Patients
In the present study, 75 patients, including 34 with
BPE (22 males and 12 females; median age, 67.59 years) and 41
patients with MPE (19 males and 22 females; median age, 65.68
years) were enrolled between January 2013 and December 2013 at the
Kaohsiung Medical University Hospital (Kaohsiung, Taiwan). The
Institutional Review Board (IRB) of Kaohsiung Medical University
Hospital approved the present study and all patients provided
written, informed consent in accordance with the Declaration of
Helsinki (IRB no. KMUH-IRB-20120275). Pleural effusion was
subsequently collected. Exudative and transudative BPE was
classified according to Light's criteria (16). The histology of specimens, obtained
using bronchoscopy and lung puncture, or the malignant cells in the
pleural effusion were used for malignancy diagnosis (17,18). MPE
was collected from patients, including those with malignant
tumors.
Cytometric bead array (CBA) to assess
cytokine levels
Aliquots (200 µl) of pleural effusion from the 75
patients were centrifuged for 10 min at 3,000 × g at 4°C and the
supernatants were stored at −80°C. The concentrations of IL-1β,
IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IFNG, colony stimulating
factor 2, MCP-1, TNF-α, TGFB1 and VEGF were measured using a CBA
Flex Set kit (BD Biosciences, Franklin Lakes, NJ, USA) according to
the manufacturer's protocol. Each sample was determined once. Data
were obtained using a BD Accuri C6 flow cytometer and analyzed
using FCAP Array V3.0 software (both from BD Biosciences).
Statistical analysis
Biochemical features and the concentration of
cytokines were compared between BPE and MPE samples using the
Kruskal-Wallis or Mann-Whitney U test. The concentrations of
cytokines and biochemical features for which these tests revealed a
significant difference were used to generate a receiver operating
characteristic (ROC) curve. The survival curves were obtained using
the Kaplan-Meier method. SPSS version 19 (IBM Corp., Armonk, NY,
USA) was used for all statistical analysis and to generate the
graphs. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
Of the 75 patients enrolled in the present study, 41
(19 males and 22 females; median age, 65.68 years) exhibited lung
cancer (adenocarcinoma, squamous cell or bronchogenic carcinoma, 28
patients) or extrathoracic cancer-induced MPE (including breast and
colorectal cancer, 13 patients). The remaining 34 patients (22
males and 12 females; median age, 67.59 years) exhibited transudate
(11 patients) or exudate-induced BPE (23 patients). The causes of
pleural effusion are presented in Table
I.
 | Table I.Causes of pleural effusion in 75
patients. |
Table I.
Causes of pleural effusion in 75
patients.
| A, Patients with
benign pleural effusion (n=34) |
|---|
|
|---|
| Cause of pleural
effusion | No. of patients |
|---|
| Transudates | 11 |
|
Congestive heart failure | 2 |
|
Cardiogenic syncope | 1 |
| Coronary
artery disease | 4 |
| Liver
cirrhosis | 4 |
| Exudates | 23 |
| Bacterial
pneumonia | 13 |
|
Empyema | 2 |
| Pulmonary
tuberculosis | 6 |
| Pleural
tuberculosis | 2 |
|
| B, Patients with
malignant pleural effusion (n=41) |
|
| Cause of pleural
effusion | No. of patients |
|
| Lung cancer | 28 |
|
Adenocarcinoma | 23 |
| Squamous
cell carcinoma | 3 |
|
Bronchogenic carcinoma | 2 |
| Extrathoracic
cancer | 13 |
| Breast
cancer | 5 |
|
Colorectal cancer | 2 |
|
Hepatocellular carcinoma | 1 |
|
Esophageal cancer | 1 |
| Thyroid
cancer | 1 |
| Oral
cancer | 1 |
| Tongue
cancer | 1 |
| Ovarian
cancer | 1 |
Biochemical and clinical features of
MPE and BPE
Patients with BPE were divided into transudate and
exudate groups, while patients with MPE were divided into lung and
extrathoracic cancer groups. The levels of LDH, glucose and protein
and the number of total cells, neutrophils, lymphocytes and
monocytes among four groups (transudate, exudate, lung cancer and
extrathoracic groups) are presented in Table II. A significant difference was
demonstrated in protein concentration and lymphocyte number among
the four groups (P=0.0001 and P=0.040, respectively). However,
protein concentration was the only factor for which a significant
difference between the BPE and MPE groups was demonstrated
(P=0.007). No significant difference was observed between the level
of LDH, glucose, count of total cell, neutrophil, lymphocytes and
monocytes between the entire BPE and entire MPE groups (P=0.310,
0.117, 0.699, 0.840, 0.589 and 0.333, respectively). This result
suggested that protein concentration but not lymphocyte number, may
serve as a predictor for distinguishing between BPE and MPE.
 | Table II.Biochemical and clinical
characteristics of 75 patients with pleural effusion. |
Table II.
Biochemical and clinical
characteristics of 75 patients with pleural effusion.
| A, Clinical
characteristics of 75 patients with pleural effusion |
|---|
|
|---|
| Characteristics of
patients | Patients with
transudate-induced BPE (n=11) | Patients with
exudate-induced BPE (n=23) | Patients with lung
cancer-induced MPE (n=28) | Patients with
extrathoracic cancer-induced MPE (n=13) | n | P-value |
|---|
| Age,
yearsa | 66.36 (12.96) | 68.17 (16.85) | 67.04 (13.31) | 62.77 (10.63) | 75 | 0.735 |
| No. of
malesb | 4 (36.40) | 18 (78.30) | 13 (46.40) | 6 (46.20) | 75 | 0.050 |
| No. of
smokersb | 4 (36.40) | 11 (47.80) | 9 (32.10) | 3 (23.10) | 75 | 0.472 |
|
| B, Biochemical
characteristics of 75 patients with pleural effusionc |
|
| Component of
pleural effusion | Patients with
transudate-induced BPE (n=11) | Patients with
exudate-induced BPE (n=23) | Patients with lung
cancer-induced MPE (n=28) | Patients with
extrathoracic cancer-induced MPE (n=13) | n | P-value |
| LDH, IU/l | 6, 297.5
(137.25–440.75) | 13, 158
(135.5–252) | 12, 220
(156.25–308.5) | 9, 223
(180.5–265.5) | 40 | 0.342 |
|
| Glucose, mg/dl | 9, 131
(117.5–157) | 17, 141
(108–168) | 22, 117
(95.5–143.25) | 8, 114
(74.75–191.5) | 56 | 0.482 |
| Protein, g/dl | 10, 1.8
(1.30–2.13) | 22, 3.45
(1.88–4.63) | 26, 4.2
(3.38–4.8) | 10, 4.15
(2.45–4.5) | 68 | 0.001 |
| Cell
count,/cum | 11, 270
(138–990) | 22, 1436.5
(199–2743.75) | 27, 1065.0
(517–1980) | 11, 930
(267–2270) | 71 | 0.194 |
| Neutrophil (%) | 11, 17% (1–45) | 21, 5% (3–16) | 19, 13% (2–46) | 10, 4.5%
(1–20.5) | 61 | 0.788 |
| Lymphocyte (%) | 11, 22%
(11–51) | 21, 68%
(31–83) | 18, 48.5%
(30.5–68.25) | 10, 48%
(20.50–70.75) | 60 | 0.040 |
| Monocyte (%) | 11, 19% (8–38) | 21, 7% (4–21) | 19, 14% (5–19) | 10, 8.5%
(3.75–18.5) | 61 | 0.206 |
Cytokine concentration in MPE and
BPE
The concentration of cytokines was analyzed using a
CBA Flex Set kit (Table III). The
concentration of TNF-α (P=0.035), VEGF (P=0.002) and IFNG (P=0.020)
differed significantly across the four groups. The highest
concentration of IFNG was detected in the exudate group and the
highest concentration of TNF-α was detected in the extrathoracic
cancer group. However, neither IFNG nor TNF-α distinguished BPE and
MPE; there was no significant difference between the BPE and MPE
groups in the concentration of TGFB1 (P=0.865), TNF-α (P=0.589),
CSF-2 (P=0.814), IFNG (P=0.321), IL-1B (P=0.594), IL-4 (P=0.783),
IL-5 (P=0.449), IL-6 (P=0.568), IL-8 (P=0.530), IL-10 (P=0.827),
IL-12 (P=0.371) and MCP-1 (P=0.489). The results of the present
study revealed that VEGF concentration in MPE was increased
compared with that in BPE (P=0.001). Therefore, the present study
suggests that VEGF may potentially distinguish MPE and BPE.
 | Table III.Clinical characteristics of 75
patients with pleural effusion. |
Table III.
Clinical characteristics of 75
patients with pleural effusion.
| Type of
cytokine | Patients with
transudate-induced BPE (n=11) | Patients with
exudate-induced BPE (n=23) | Patients with lung
cancer-induced MPE (n=28) | Patients with
extrathoracic cancer-induced MPE (n=13) | P-value |
|---|
| TGFB1 | 76.18
(53.15–550.04) | 101.55
(38.30–200.43) | 84.19
(53.63–247.94) | 115.80
(49.70–189.76) | 0.990 |
| TNF-α | 0.00
(0.00–0.85) | 0.11
(0.00–4.55) | 0.08
(0.00–1.77) | 1.80
(0.27–10.67) | 0.035 |
| VEGF | 61.94
(20.51–134.80) | 84.9
(24.51–210.24) | 400.16
(40.12–1324.58) | 1146.79
(225.55–4987.09) | 0.002 |
| CSF2 | 0.11
(0.00–0.45) | 0.21
(0.00–0.44) | 0.00
(0.00–0.58) | 0.39
(0.00–1.85) | 0.339 |
| IFNG | 0.30
(0.00–0.67) | 1.05
(0.53–5.51) | 0.58
(0.22–1.95) | 0.65
(0.20–1.12) | 0.020 |
| IL-1B | 0.00
(0.00–0.00) | 0.00
(0.00–0.65) | 0.00
(0.00–0.008) | 0.00
(0.00–0.11) | 0.152 |
| IL-4 | 0.00
(0.00–0.00) | 0.00
(0.00–0.20) | 0.00
(0.00–0.11) | 0.00
(0.00–0.17) | 0.380 |
| IL-5 | 1.30
(0.00–4.03) | 2.51
(0.47–7.14) | 2.935
(0.79–8.45) | 1.45
(0.74–28.20) | 0.526 |
| IL-6 | 5884.94
(2786.79–8844.97) | 7874.02
(2858.57–16297.47) | 6254.9
(2566.33–11590.90) | 14540.13
(3572.61–19640.46) | 0.182 |
| IL-8 | 497.86
(100.66–1089.34) | 311.10
(80.95–1192.00) | 311.46
(105.27–1123.08) | 1129.18
(199.46–4560.84) | 0.343 |
| IL-10 | 24.19
(10.37–62.67) | 23.19
(11.82–38.65) | 24.16
(14.21–34.93) | 20.81
(12.74–57.15) | 0.951 |
| IL-12 | 0.00
(0.00–0.00) | 0.00
(0.00–0.65) | 0.00
(0.00–0.008) | 0.00
(0.00–0.11) | 0.181 |
| MCP-1 | 514.37
(268.95–1852.68) | 615.07
(187.60–1134.39) | 549.50
(154.86–2072.26) | 905.90
(457.38–9980.78) | 0.419 |
Identifying MPE and BPE according to
protein and VEGF concentration
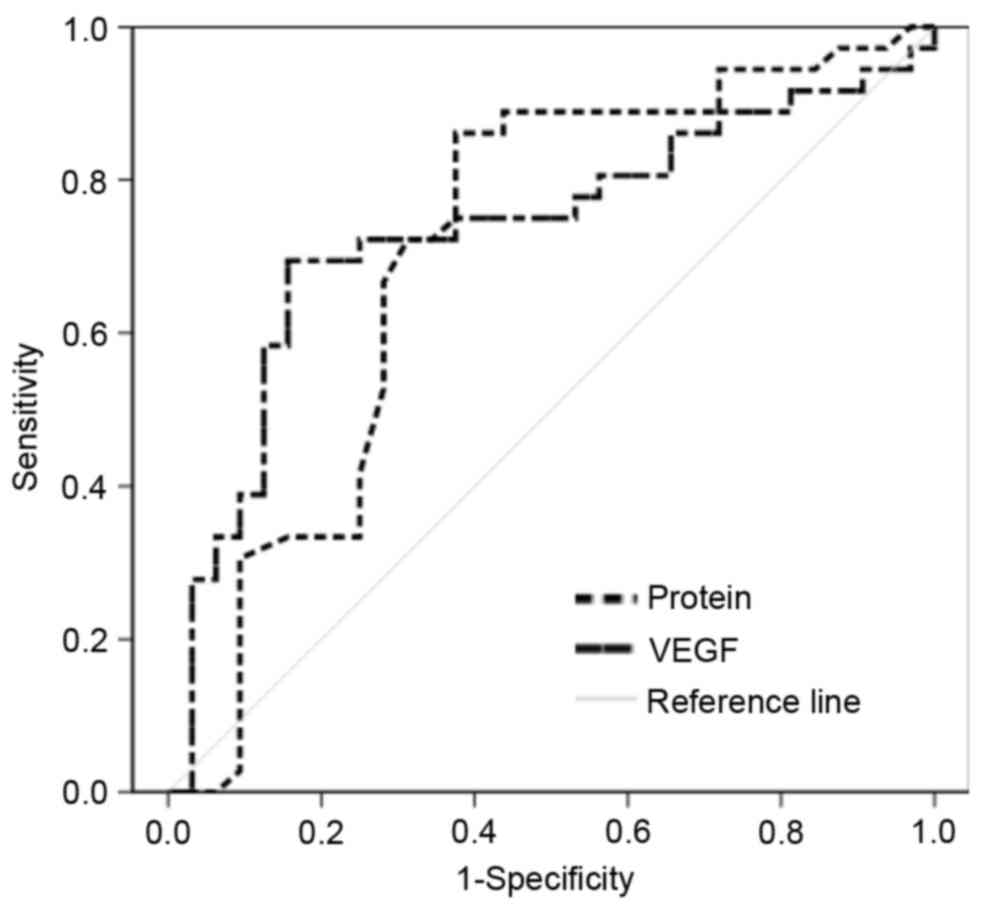
The ROC curve of protein and VEGF concentration was
used to generate the optimal cutoff point for MPE and BPE. The
protein concentration cutoff point [area under the curve (AUC):
0.708] was 3.35 g/dl and the VEGF cutoff point (AUC: 0.728) was 214
pg/ml for predicting MPE (Fig. 1). In
accordance with the cutoff value of VEGF and protein, the
sensitivity, specificity and accuracy of VEGF (>214 pg/ml;
sensitivity, 70.6%; specificity, 82.4%; accuracy, 76.0%), protein
(>3.35 g/dl; sensitivity, 75.6%; specificity, 70.6%; accuracy,
73.3%), and VEGF and protein (VEGF, >214 pg/ml; protein,
>3.35 g/dl; sensitivity, 92.6%; specificity, 61.7%; accuracy,
78.6%) were presented in Table IV.
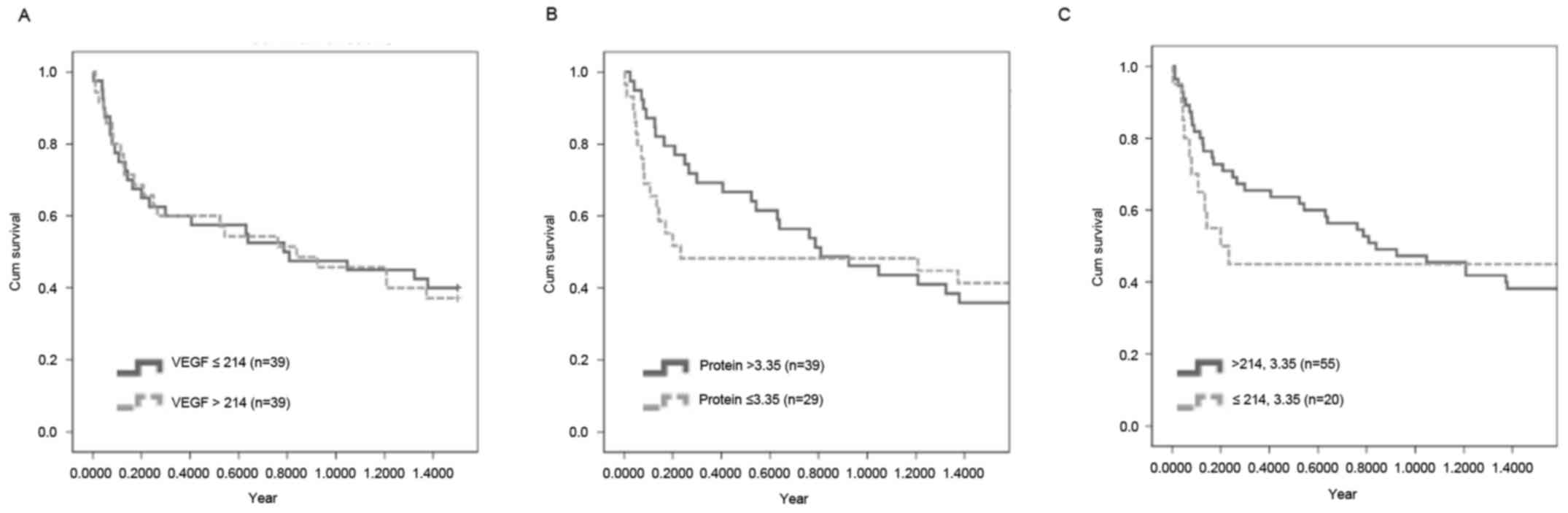
However, the concentration of VEGF and protein was not associated
with a poor survival rate (Fig.
2).
 | Table IV.Accuracy of predictors in PE from 75
patients. |
Table IV.
Accuracy of predictors in PE from 75
patients.
| PE component and
concentration | TPR (%) | FPR (%) | SPC (%) | ACC (%) | PPV (%) | NPV (%) |
|---|
| VEGF, 214
pg/ml | 70.6 | 17.1 | 82.4 | 76.0 | 82.8 | 70.0 |
| PE total protein,
3.35 g/dl | 75.6 | 29.4 | 70.6 | 73.3 | 75.6 | 70.6 |
| VEGF, 214 pg/ml and
PE total protein, 3.35g/dl | 92.6 | 25.5 | 61.7 | 78.6 | 74.5 | 87.5 |
Discussion
The clinical and biochemical features of pleural
effusion, including the level of procalcitonin, adenosine
deaminase, C-reactive protein, carcinoembryonic antigen, and LDH
have been demonstrated to represent diagnostic markers in
differentiating MPE from tuberculosis pleural effusion (19,20).
However, the present study demonstrated no significant difference
in the level of LDH between BPE and MPE groups. BPE samples were
collected in the present study from patients with different
diseases, including 8 patients with tuberculosis, and this may have
decreased the sensitivity of LDH. A previous study suggested that
the ratio of serum LDH to adenosine deaminase in pleural fluid
enhanced the sensitivity and specificity for identifying MPE
(21), a result that requires further
study. Furthermore, protein, glucose concentration, total cell,
neutrophil, monocyte and lymphocyte counts represent MPE-associated
features (22,23). However, protein concentration was the
only parameter to reveal a significant difference between BPE and
MPE groups in the present study. Therefore, protein concentration
may potentially serve to distinguish between MPE and BPE.
Although numerous types of cytokine may be detected
in BPE and MPE, the pattern of cytokines may not differentiate MPE
and BPE (24,25). The present study demonstrated no
significant difference in the concentration of cytokines between
the MPE and BPE groups, except for TNF-α, IFNG and VEGF. However,
while increased concentrations of TNF-α and IFNG were observed in
the extrathoracic cancer and exudates groups, respectively, there
was no significant difference between MPE and BPE overall. VEGF was
used as a biomarker in the present study (optimal cutoff value=214
pg/ml). Duysinx et al (26)
suggested that the optimal value of VEGF for differentiating MPE
from BPE is 382 pg/ml and Fiorelli et al (27) demonstrated that sensitivity and
specificity were 63 and 83%, respectively, when VEGF is >652
pg/ml. These cutoff values may differ from that of the present
study due to the use of different experimental designs and sample
sizes.
VEGF induces vascular permeability and is a critical
mediator of pleural effusion formation (28), suggesting that blocking VEGF
potentially represents a strategy for suppressing the formation of
pleural effusion (29). A previous
study demonstrated that the level of VEGF in pleural effusion was
associated with lymph node and distant metastasis and that the IL-8
level in pleural effusion was associated with lymph node metastasis
(30). Due to the limitation of a
small sample size, patients with MPE were not divided into patients
with primary and metastatic tumors in the present study. The
association between VEGF and IL-8 and metastasis as described by
this previous study was not observed in MPE samples in the present
study.
The combination of VEGF and protein for
differentiating BPE and MPE increased the sensitivity and accuracy
but decreased the specificity compared with using a single
parameter. In addition, a poor survival rate was not significantly
associated with VEGF, protein or the combination of the two. To the
best of our knowledge, the present study is the first to use a
combination of pleural effusion VEGF and protein levels to predict
whether pleural effusion from patients was malignant. To conclude,
this novel combination may represent a tool for predicting MPE and
facilitating early diagnosis, but not for predicting the survival
rate of patients with MPE and BPE.
Acknowledgements
The present study was supported by the Ministry of
Science and Technology (grant no. MOST 104-2320-B-037-014-MY3) and
the Kaohsiung Medical University Hospital Research Foundation
(grant nos. KMUH104-4R08 and KMUH101-1M65).
References
|
1
|
Medford AR and Maskell N: Pleural
effusion. Postgrad Med J. 81:702–710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomas R and Lee YC: Causes and management
of common benign pleural effusions. Thorac Surg Clin. 23:25–42.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
American Thoracic Society, . Management of
malignant pleural effusions. Am J Respir Crit Care Med.
162:1987–2001. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henschke CI, Yankelevitz DF and Davis SD:
Pleural diseases: Multimodality imaging and clinical management.
Curr Probl Diagn Radiol. 20:155–181. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martínez-Moragón E, Aparicio J, Sanchis J,
Menéndez R, Cruz Rogado M and Sanchis F: Malignant pleural
effusion: Prognostic factors for survival and response to chemical
pleurodesis in a series of 120 cases. Respiration. 65:108–113.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heffner JE: Diagnosis and management of
malignant pleural effusions. Respirology. 13:5–20. 2008.PubMed/NCBI
|
|
7
|
Heffner JE, Nietert PJ and Barbieri C:
Pleural fluid pH as a predictor of survival for patients with
malignant pleural effusions. Chest. 117:79–86. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bielsa S, Salud A, Martinez M, Esquerda A,
Martín A, Rodríguez-Panadero F and Porcel JM: Prognostic
significance of pleural fluid data in patients with malignant
effusion. Eur J Intern Med. 19:334–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chung CL, Chen YC and Chang SC: Effect of
repeated thoracenteses on fluid characteristics, cytokines, and
fibrinolytic activity in malignant pleural effusion. Chest.
123:1188–1195. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stathopoulos GT and Kalomenidis I:
Malignant pleural effusion: Tumor-host interactions unleashed. Am J
Respir Crit Care Med. 186:487–492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stathopoulos GT, Psallidas I, Moustaki A,
Moschos C, Kollintza A, Karabela S, Porfyridis I, Vassiliou S,
Karatza M, Zhou Z, et al: A central role for tumor-derived monocyte
chemoattractant protein-1 in malignant pleural effusion. J Natl
Cancer Inst. 100:1464–1476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hamed EA, El-Noweihi AM, Mohamed AZ and
Mahmoud A: Vasoactive mediators (VEGF and TNF-alpha) in patients
with malignant and tuberculous pleural effusions. Respirology.
9:81–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozyurtkan MO, Balci AE and Cakmak M:
Predictors of mortality within three months in the patients with
malignant pleural effusion. Eur J Intern Med. 21:30–34. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu YC, Lee Shin-Jung S, Chen YS, Tu HZ,
Chen BC and Huang TS: Differential diagnosis of tuberculous and
malignant pleurisy using pleural fluid adenosine deaminase and
interferon gamma in Taiwan. J Microbiol Immunol Infect. 44:88–94.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seiscento M, Vargas FS, Antonangelo L,
Acencio MM, Bombarda S, Capelozzi VL and Teixeira LR: Transforming
growth factor beta-1 as a predictor of fibrosis in tuberculous
pleurisy. Respirology. 12:660–663. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Light RW: Clinical practice. Pleural
effusion. N Engl J Med. 346:1971–1977. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsu IL, Su WC, Yan JJ, Chang JM and Lai
WW: Angiogenetic biomarkers in non-small cell lung cancer with
malignant pleural effusion: Correlations with patient survival and
pleural effusion control. Lung Cancer. 65:371–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hung TL, Chen FF, Liu JM, Lai WW, Hsiao
AL, Huang WT, Chen HH and Su WC: Clinical evaluation of HER-2/neu
protein in malignant pleural effusion-associated lung
adenocarcinoma and as a tumor marker in pleural effusion diagnosis.
Clin Cancer Res. 9:2605–2612. 2003.PubMed/NCBI
|
|
19
|
Lee SH, Lee EJ, Min KH, Hur GY and Lee SY,
Kim JH, Shin C, Shim JJ, In KH, Kang KH and Lee SY: Procalcitonin
as a diagnostic marker in differentiating parapneumonic effusion
from tuberculous pleurisy or malignant effusion. Clin Biochem.
46:1484–1488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Valdés L, San-José E, Ferreiro L, Golpe A,
González-Barcala FJ, Toubes ME, Rodríguez-Álvarez MX,
Álvarez-Dobaño JM, Rodríguez-Núñez N, Rábade C and Gude F:
Predicting malignant and tuberculous pleural effusions through
demographics and pleural fluid analysis of patients. Clin Respir J.
9:203–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verma A, Abisheganaden J and Light RW:
Identifying malignant pleural effusion by a cancer ratio (serum
LDH: Pleural fluid ADA ratio). Lung. 194:147–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas R, Cheah HM, Creaney J, Turlach BA
and Lee YC: Longitudinal measurement of pleural fluid biochemistry
and cytokines in malignant pleural effusions. Chest. 149:1494–1500.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bielsa S, Salud A, Martínez M, Esquerda A,
Martín A, Rodríguez-Panadero F and Porcel JM: Prognostic
significance of pleural fluid data in patients with malignant
effusion. Eur J Intern Med. 19:334–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghayumi MA, Mojtahedi Z and Fattahi MJ:
Th1 and Th2 cytokine profiles in malignant pleural effusion. Iran J
Immunol. 8:195–200. 2011.PubMed/NCBI
|
|
25
|
Chen YM, Yang WK, Whang-Peng J, Tsai CM
and Perng RP: An analysis of cytokine status in the serum and
effusions of patients with tuberculous and lung cancer. Lung
Cancer. 31:25–30. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duysinx BC, Corhay JL, Hubin L, Nguyen D,
Henket M and Louis R: Diagnostic value of interleukine-6,
transforming growth factor-beta 1 and vascular endothelial growth
factor in malignant pleural effusions. Respir Med. 102:1708–1714.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fiorelli A, Vicidomini G, Di Domenico M,
Napolitano F, Messina G, Morgillo F, Ciardiello F and Santini M:
Vascular endothelial growth factor in pleural fluid for
differential diagnosis of benign and malignant origin and its
clinical applications. Interact Cardiovasc Thorac Surg. 12:420–424.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grove CS and Lee YC: Vascular endothelial
growth factor: The key mediator in pleural effusion formation. Curr
Opin Pulm Med. 8:294–301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bradshaw M, Mansfield A and Peikert T: The
role of vascular endothelial growth factor in the pathogenesis,
diagnosis and treatment of malignant pleural effusion. Curr Oncol
Rep. 15:207–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng D, Kong H and Li Y: Prognostic
values of VEGF and IL-8 in malignant pleural effusion in patients
with lung cancer. Biomarkers. 18:386–390. 2013. View Article : Google Scholar : PubMed/NCBI
|