Introduction
Lung cancer is a major health problem throughout the
world, particularly in China (1,2). Lung
adenosquamous cancer (ASC) is a subtype of non-small cell lung
cancer (NSCLC) that accounts for between 0.4 and 4% of all lung
malignancies (3–5). ASC is defined as a carcinoma containing
squamous cell carcinoma (SCC) and adenocarcinoma (ADC) components
with each comprising ≥10% of the total tumor (6). Although the symptoms of ASC are similar
to those of other histological subtypes of NSCLC (7), aggressive characteristics including
early metastasis, invasiveness, rapid growth and poor prognosis
have been recognized (8–10). Consequently, valuable prognostic
factors are urgently needed for patients with ASC.
The association between inflammation and cancer
proposed by Virchow in 1863 is now widely accepted (11). Inflammation contributes to the
survival, proliferation and metastasis of cancer cells, as well as
promoting angiogenesis. In addition, inflammation prevents the
adaptive immune response and is able to alter responses to systemic
therapies (11,12). Numerous studies have revealed the
association between inflammatory indexes and prognoses of patients
with NSCLC, including platelet (PLT), fibrinogen (FBG), neutrophil
to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR)
(13–23). The study conducted by Unal et
al (23) first identified the
association between PLR and prognosis in patients with NSCLC, and
suggested that assessing NLR and PLR together may predict the
prognosis of patients with NSCLC. However, to the best of our
knowledge, the prognostic value of these inflammatory markers for
patients with ASC who have undergone surgery has not been reported.
Therefore, the aim of the present study was to assess the
association between NLR, PLR, PLT and FBG with the prognosis of
patients with ASC and further analyze whether the combination of
increased NLR, PLR, FBG and PLT (CO-NPF) was superior to any ratio
alone.
Patients and methods
Study population
Patients who presented at The Cancer Institute and
Hospital of Tianjin Medical University between January 2005 and
December 2013 were enrolled in the present study. Demographic and
clinicopathological characteristics as well as preoperative
laboratory parameters were recorded and retrospectively reviewed.
The study was supported by the Ethics Committee and the
Institutional Review Board of The Cancer Institute and Hospital of
Tianjin Medical University. Prior to enrollment into the present
study, written informed consent was received from all patients or
their families. The inclusion criteria were as follows: i)
Histologically diagnosed with ASC; ii) with stage I–IIIA [7th
Tumor-Node-Metastasis Classification of Malignant Tumors (TNM)
(24)]; and iii) underwent surgery.
Patients were excluded who met any of the following criteria: i)
Presented with other types of carcinoma within 5 years; ii)
succumbed within 1 month of surgery; iii) received adjuvant
chemotherapy or radiotherapy prior to surgery; iv) suffered from
active infection(s) prior to surgery; and v) lost to follow up. A
total of 134 patients with ASC were recruited into the present
study.
Definition of CO-NPF
NLR and PLR were defined as the ratio of neutrophils
to lymphocytes and platelets to lymphocytes, respectively.
According to threshold values that were determined by receiver
operating characteristic (ROC) curve analysis, the NLR, PLR, PLT
and FBG were each divided into two groups: <2.16 and ≥2.16,
<145 and ≥145, <289 and ≥289 and <4 and ≥4, respectively.
CO-NPF was defined as the combination of increased NLR, PLR, PLT
and FBG. CO-NPF were scored between 0 and 4 according to the number
of increased parameters (NLR, PLR, PLT and FBG). For example,
patients with four increased indexes were scored 4, whereas
patients with three, two, one or zero increased indexes were scored
3, 2, 1 or 0, respectively. CO-NPF was then divided into two groups
according to the presence (≥2) or absence (<2) of the
combination of increased inflammatory indexes.
Statistical analysis
Statistical analysis was performed using SPSS
(version 22.0; IBM Corp., Armonk, NY, USA). Continuous data are
presented as the mean ± standard deviation or median (range), and
categorical data are expressed as percentages and frequencies.
Differences between two groups were compared using χ2
test or Fisher's exact test. Overall survival (OS) was defined as
the period from the date of surgery to the date of mortality or
censoring. Disease-free survival (DFS) was calculated from the date
of surgery to the date of relapse or mortality. Kaplan-Meier
estimator survival curves were created and differences between
survival curves were analyzed using the log-rank test. Independent
prognostic factors were identified using a multivariate Cox's
regression model in which all the significant variables that were
identified by univariate analysis were involved in a forward
conditional manner. Hazard ratios (HRs) and 95% confidence
intervals (95% CIs) were obtained from multivariate analysis. Areas
under ROC curves (AUCs) were applied to compare NLR, PLR, PLT, FBG
and CO-NPF. P<0.05 was considered to indicate a statistically
significant difference.
Results
In total, 134 patients with ASC were enrolled in the
present retrospective study. For total patients, the 3-year and
5-year survival rates were 36.1 and 24.8%, respectively. The median
follow-up time was 22 months (range, 2–120 months) and during the
follow-up period 84 (61.8%) patients developed tumor relapse and 96
(70.6%) patients succumbed. Patient baseline characteristics are
summarized in Table I. The median
patient age was 60 years (range, 34–83 years). Of the patient
cohort, 81 (60.5%) were male and 53 (39.5%) were female. The
majority of patients were stage I–II (n=80, 59.7%), with 54 (40.3%)
patients in stage IIIA. The majority of patients underwent
pulmonary lobectomy (n=112, 83.6%), with 12 (8.9%) receiving local
tumor resection and 10 (7.5%) receiving pneumonectomy. Of the total
of 134 patients, 78 (58.2%) received platinum-based chemotherapy
following surgery.
 | Table I.Baseline patient characteristics. |
Table I.
Baseline patient characteristics.
| Characteristic | Number of patients
(of a total of 134) (%) |
|---|
| Age, years |
|
| Median
(range), 60 (34–83) |
|
| ≥60 | 60 (44.8) |
|
<60 | 74 (55.2) |
| Sex |
|
| Male | 81 (60.5) |
|
Female | 53 (39.5) |
| Smoking index |
|
| ≥400 | 60 (44.8) |
|
<400 | 74 (55.2) |
| Lymph node
metastasis |
|
| Yes | 79 (59.0) |
| No | 55 (41.0) |
| TNM stage |
|
| I–II | 83 (61.9) |
| IIIA | 51 (38.1) |
| Operative
approach |
|
| Local
tumor resection | 12 (8.9) |
| Pulmonary
lobectomy | 112 (83.6) |
|
Pneumonectomy | 10 (7.5) |
| Albumin, g/dl |
|
| Mean ±
SD, 43.56±5.33 |
|
|
≥49.5 | 10 (7.5) |
|
<49.5 | 124 (92.5) |
| WBC count,
×109 cells/l |
|
| Mean ±
SD, 6.98±2.94 |
|
| ≥5.6 | 99 (73.9) |
|
<5.6 | 35 (26.1) |
| Neutrophil count,
×109 cells/l |
|
| Mean ±
SD, 4.23±1.61 |
|
|
Lymphocyte count,
×109 cells/l |
|
| Mean ±
SD, 1.96±0.65 |
|
| NLR |
|
| Mean ±
SD, 2.36±1.13 |
|
|
≥2.16 | 60 (44.8) |
|
<2.16 | 74 (55.2) |
| PLR |
|
| Mean ±
SD, 143.14±65.00 |
|
|
≥145 | 90 (67.2) |
|
<145 | 44 (32.8) |
| PLT count,
×109 cells/l |
|
| Mean ±
SD, 254.12±72.30 |
|
|
≥289 | 36 (26.9) |
|
<289 | 98 (73.1) |
| FBG, g/l |
|
| Mean ±
SD, 3.64±0.89 |
|
| ≥4 | 45 (33.6) |
|
<4 | 89 (66.4) |
| CO-NPF, score |
|
|
<2 | 81 (60.4) |
| ≥2 | 53 (39.6) |
The association between clinical characteristics and
laboratory parameters with CO-NPF is presented in Table II. Significant differences between
CO-NPF and age (P=0.010), TNM stage (P=0.034), neutrophil count
(P=0.002), lymphocyte count (P=0.001), monocyte count (P=0.023),
PLT count (P<0.001), FBG count (P<0.001), NLR (P<0.001)
and PLR (P<0.001) were identified.
 | Table II.Clinical characteristics and
laboratory parameters according to CO-NPF. |
Table II.
Clinical characteristics and
laboratory parameters according to CO-NPF.
| Characteristic | CO-NPF <2, n=81
(%) | CO-NPF ≥2, n=53
(%) | P-value |
|---|
| Age, years |
|
| 0.010 |
|
<60 | 52 (70.3) | 22 (29.7) |
|
|
≥60 | 29 (48.3) | 31 (51.7) |
|
| Sex |
|
| 0.708 |
|
Female | 31 (58.5) | 22 (41.5) |
|
|
Male | 50 (61.7) | 31 (38.3) |
|
| Smoking index |
|
| 0.420 |
|
<400 | 47 (63.5) | 27 (36.5) |
|
|
≥400 | 34 (56.7) | 26 (43.3) |
|
| Lymph node
metastasis |
|
| 0.088 |
|
Yes | 43 (54.4) | 36 (45.6) |
|
| No | 38 (69.1) | 17 (30.9) |
|
| TNM stage |
|
| 0.034 |
|
I–II | 56 (67.5) | 27 (32.5) |
|
|
IIIA | 25 (49.0) | 26 (51.0) |
|
| Operative
approach |
|
| 0.777 |
| Local
tumor resection | 7 (58.3) | 5 (41.7) |
|
|
Pulmonary lobectomy | 69 (61.6) | 43 (38.4) |
|
|
Pneumonectomy | 5 (50.0) | 5 (50.0) |
|
| WBC count,
109 cells/l |
|
| 0.372 |
|
<5.6 | 24 (66.7) | 12 (33.3) |
|
|
≥5.6 | 57 (58.2) | 41 (41.8) |
|
| Neutrophil count,
109 cells/l |
|
| 0.002 |
|
<3.4 | 35 (79.5) | 9 (20.5) |
|
|
≥3.4 | 46 (51.1) | 44 (48.9) |
|
| Lymphocyte count,
109 cells/l |
|
| 0.001 |
|
<2.30 | 52 (52.0) | 48 (48.0) |
|
|
≥2.30 | 29 (85.3) | 5 (14.7) |
|
| Monocyte count,
109 cells/l |
|
| 0.023 |
|
<0.54 | 57 (67.9) | 27 (32.1) |
|
|
≥0.54 | 24 (48.0) | 26 (52.0) |
|
| PLT count,
109 cells/l |
|
| <0.001 |
|
<289 | 74 (75.5) | 24 (24.5) |
|
|
≥289 | 7 (19.4) | 29 (80.6) |
|
| FBG count, g/l |
|
| <0.001 |
|
<4 | 71 (79.8) | 18 (20.2) |
|
| ≥4 | 10 (22.2) | 35 (77.8) |
|
| NLR |
|
| <0.001 |
|
<2.16 | 67 (90.5) | 7 (9.5) |
|
|
≥2.16 | 14 (23.3) | 46 (76.7) |
|
| PLR |
|
| <0.001 |
|
<145 | 78 (86.7) | 12 (13.3) |
|
|
≥145 | 3 (6.8) | 41 (93.2) |
|
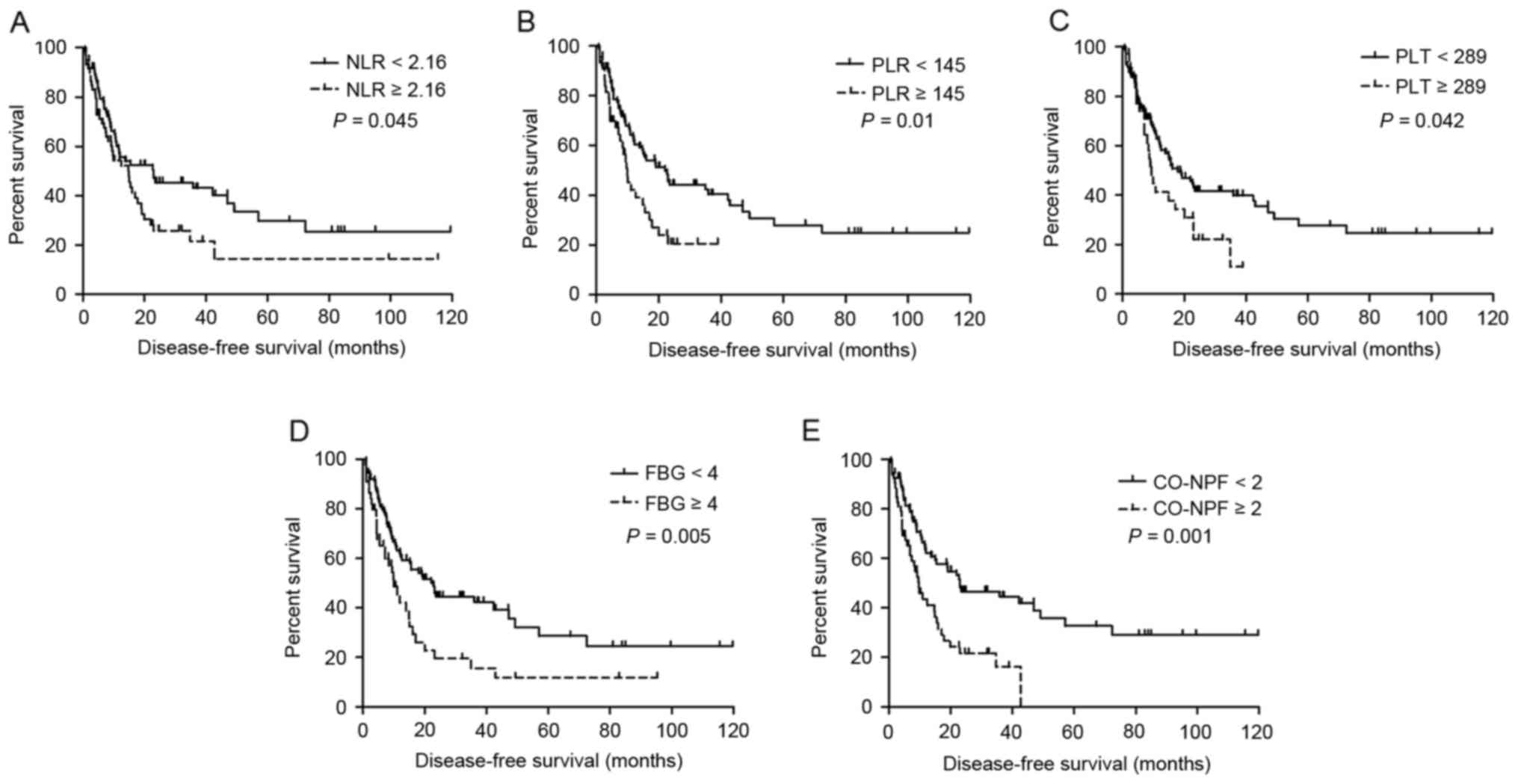
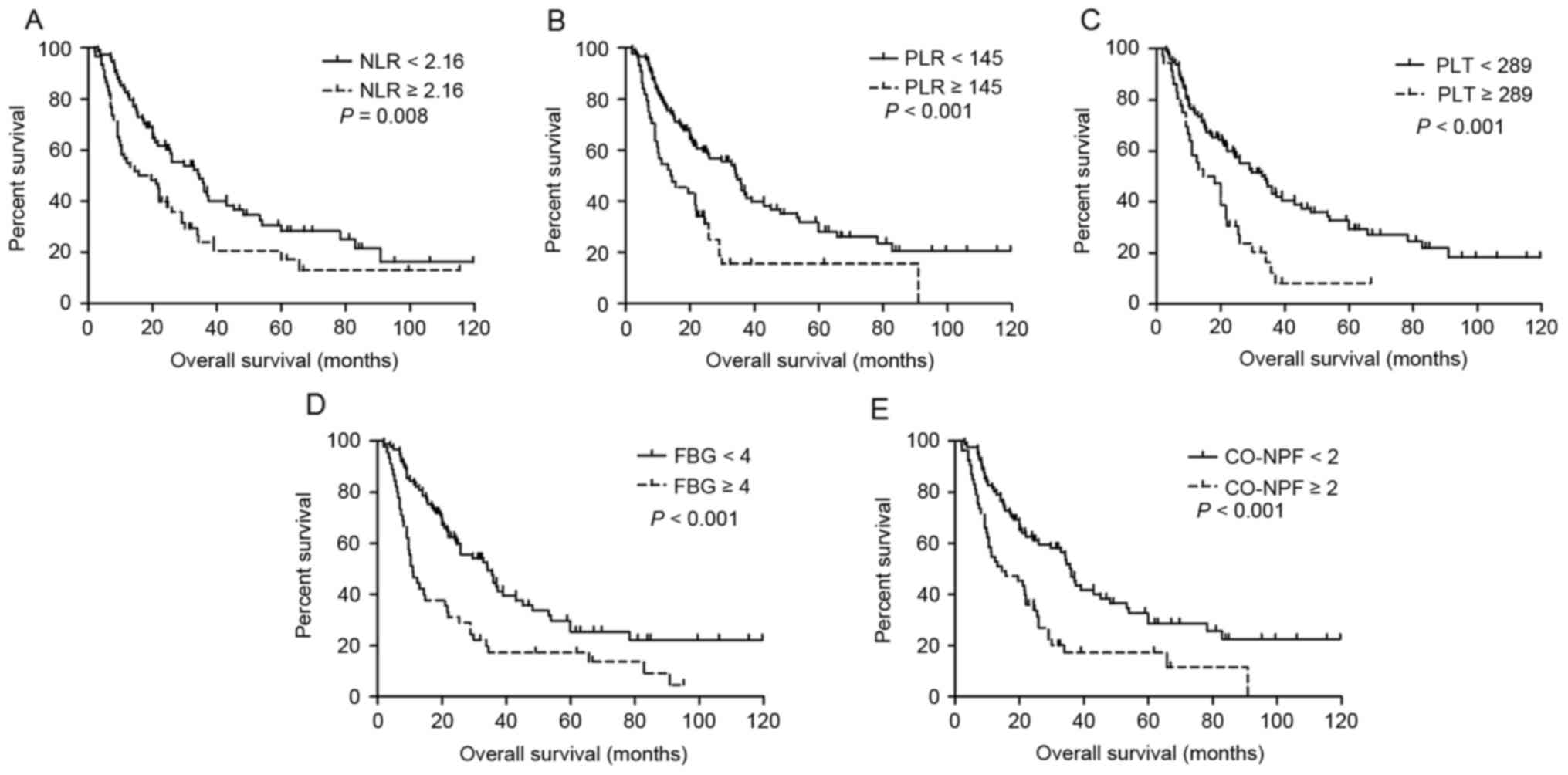
Univariate survival analysis results are summarized
in Table III. Increased NLR
(P=0.045 and P=0.008, respectively), PLR (P=0.010 and P<0.001,
respectively), PLT (P=0.042 and P<0.001, respectively), FBG
(P=0.005 and P<0.001, respectively) and CO-NPF (P=0.001 and
P<0.001, respectively) were significantly associated with
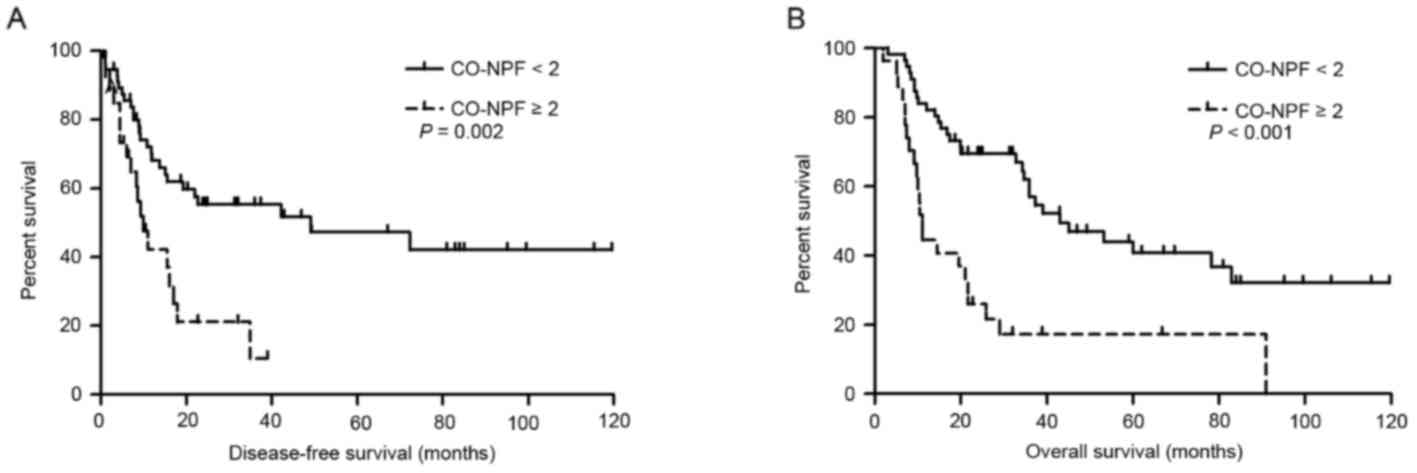
shorter DFS (Fig. 1A-E, respectively)
and OS (Fig. 2A-E, respectively).
Other significant prognostic factors for DFS and OS identified
included lymph node metastasis, TNM stage, age and serum albumin
(all P≤0.05).
 | Figure 1.Kaplan-Meier estimator curves of DFS
for all patients with adenosquamous cancer. DFS rates according to
(A) NLR, (B) PLR, (C) PLT, (D) FBG and (E) CO-NPF. DFS,
disease-free survival; NLR, neutrophil to lymphocyte ratio; PLR,
platelet to lymphocyte ratio; PLT, platelet; FBG, fibrinogen;
CO-NPF, combination of NLR, PLR, FBG and PLT. |
 | Figure 2.Kaplan-Meier estimator curves of OS
for all patients with adenosquamous cancer. OS rates according to
(A) NLR, (B) PLR, (C) PLT, (D) FBG and (E) CO-NPF. OS, overall
survival; NLR, neutrophil to lymphocyte ratio; PLR, platelet to
lymphocyte ratio; PLT, platelet; FBG, fibrinogen; CO-NPF,
combination of NLR, PLR, FBG and PLT. |
 | Table III.Univariate analysis of prognostic
factors influencing OS and DFS. |
Table III.
Univariate analysis of prognostic
factors influencing OS and DFS.
|
| OS | DFS |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years (≥60 or
<60) | 1.634 | 1.093–2.444 | 0.016 | 1.038 | 0.675–1.597 | 0.864 |
| Sex (male or
female) | 0.935 | 0.620–1.411 | 0.749 | 0.854 | 0.552–1.321 | 0.477 |
| Smoking index (≥400
or <400) | 1.243 | 0.831–1.858 | 0.288 | 0.894 | 0.579–1.381 | 0.613 |
| Lymph node
metastasis (yes or no) | 1.905 | 1.243–2.918 | 0.003 | 1.620 | 1.035–2.537 | 0.033 |
| TNM stage (I–II or
IIIA) | 1.745 | 1.156–2.635 | 0.008 | 1.878 | 1.215–2.905 | 0.005 |
| Operative
approach | 1.199 | 0.744–1.933 | 0.510 | 1.118 | 0.642–1.946 | 0.511 |
| Albumin (≥49.5 or
<49.5 g/dl) | 2.078 | 1.076–4.010 | 0.026 | 1.978 | 0.952–4.110 | 0.062 |
| WBC count (≥5.6 or
<5.6 ×109 cells/l) | 1.261 | 0.799–1.992 | 0.318 | 1.118 | 0.692–1.808 | 0.647 |
| NLR (≥2.16 or
<2.16) | 1.724 | 1.150–2.584 | 0.008 | 1.551 | 1.005–2.395 | 0.045 |
| PLR (≥145 or
<145) | 2.188 | 1.430–3.349 | <0.001 | 1.818 | 1.146–2.884 | 0.010 |
| PLT (≥289 or
<289×109 cells/l) | 2.222 | 1.426–3.461 | <0.001 | 1.635 | 1.012–2.642 | 0.042 |
| FBG (≥4 or <4
g/l) | 2.131 | 1.417–3.206 | <0.001 | 1.875 | 1.202–2.925 | 0.005 |
| CO-NPF (≥2 or
<2) | 2.187 | 1.451–3.295 | <0.001 | 2.156 | 1.380–3.370 | 0.001 |
Multivariate analysis results are presented in
Table IV. CO-NPF and preoperative
NLR, PLR, PLT and FBG were successively used for a multivariate
Cox's regression model with other significant factors (excluding
node stage) identified by univariate analyses. Increased CO-NPF
(P=0.001 and P<0.001, respectively), PLR (P=0.011 and P=0.001,
respectively) and FBG (P=0.001 and P<0.001, respectively) were
identified to be independently and significantly associated with
shorter DFS and OS. Other independent prognostic factors for OS
were NLR (P=0.006), PLT (P=0.001), age (P=0.033) and serum albumin
(P=0.003). TNM stage was identified to be independently associated
with DFS (P<0.05), as presented in Table IV.
 | Table IV.Multivariate analysis of prognostic
factors influencing OS and DFS. |
Table IV.
Multivariate analysis of prognostic
factors influencing OS and DFS.
|
| OS | DFS |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Model 1 |
|
|
|
|
|
|
| Age,
years (≥60 or <60) | 1.586 | 1.039–2.423 | 0.033 |
|
|
|
| Albumin
(≥49.5 or <49.5 g/dl) | 3.037 | 1.528–6.033 | 0.003 |
|
|
|
| TNM stage (I–II or
IIIA) |
|
|
| 1.602 | 1.019–2.517 | 0.041 |
| CO-NPF
(≥2 or <2) | 2.177 | 1.420–3.337 | <0.001 | 1.904 | 1.210–3.020 | 0.006 |
| Model 2 |
|
|
|
|
|
|
| Age,
years (≥60 or <60) | 1.814 | 1.196–2.752 | 0.005 |
|
|
|
| Albumin
(≥49.5 or <49.5 g/dl) | 2.731 | 1.357–5.494 | 0.005 |
|
|
|
| TNM
stage (I–II or IIIA) | 1.621 | 1.069–2.460 | 0.023 | 1.878 | 1.215–2.905 | 0.005 |
| NLR
(≥2.16 or <2.16) | 1.782 | 1.176–2.700 | 0.006 |
|
|
|
| Model 3 |
|
|
|
|
|
|
| Age,
years (≥60 or <60) | 1.738 | 1.142–2.643 | 0.010 |
|
|
|
| Albumin
(≥49.5 or <49.5 g/dl) | 2.615 | 1.313–5.211 | 0.006 |
|
|
|
| TNM
stage (I–II or IIIA) | 1.538 | 1.006–2.351 | 0.047 | 1.715 | 1.097–2.681 | 0.018 |
| PLR
(≥145 or <145) | 2.005 | 1.294–3.106 | 0.002 | 1.609 | 1.004–2.577 | 0.048 |
| Model 4 |
|
|
|
|
|
|
| Age,
years (≥60 or <60) | 1.956 | 1.284–2.980 | 0.002 |
|
|
|
| Albumin
(≥49.5 or <49.5 g/dl) | 2.099 | 1.046–4.214 | 0.037 |
|
|
|
| TNM stage (I–II or
IIIA) |
|
|
| 1.878 | 1.215–2.905 | 0.005 |
| PLT
(≥289 or <289 ×109 cells/l) | 2.276 | 1.432–3.617 | 0.001 |
|
|
|
| Model 5 |
|
|
|
|
|
|
| Age,
years (≥60 or <60) | 1.553 | 1.009–2.389 | 0.045 |
|
|
|
| Albumin
(≥49.5 or <49.5 g/dl) | 2.437 | 1.224–4.852 | 0.011 |
|
|
|
| TNM
stage (I–II or IIIA) | 1.716 | 1.131–2.604 | 0.011 | 1.871 | 1.209–2.894 | 0.005 |
| FBG (≥4
or <4 g/l) | 2.045 | 1.332–3.141 | 0.001 | 1.865 | 1.196–2.910 | 0.006 |
Subgroup analysis demonstrated that patients with
CO-NPF <2 exhibited an improved prognosis compared with those
with CO-NPF ≥2. Patients with CO-NPF <2 exhibited a 3-year OS
rate of 46.9% and a 5-year OS rate of 28.6%, whereas those with
CO-NPF ≥2 exhibited a 3-year OS rate of 17.4% and a 5-year OS rate
of 11.6%. The median survival times of the two groups were 35.9 and
14.4 months, respectively (P<0.001; Fig. 2E). The prognostic impact of CO-NPF
according to TNM stage (I–II or IIIA) was then analyzed and
increased CO-NPF was identified to be significantly associated with
shorter DFS and OS in the stage I–II subgroup (HR, 2.638; 95% CI,
1.422–4.894; P=0.002; and HR, 3.045; 95% CI, 1.742–5.324;
P<0.001, respectively; Fig.
3).
Finally, by comparing the AUC, the association
between NLR, PLR, PLT, FBG and CO-NPF with 3-year OS rates was
assessed (Fig. 4). CO-NPF exhibited
greater predictive significance (AUC, 0.652; P=0.008; 95% CI,
0.551–0.752) compared with NLR (AUC, 0.629; P=0.024; 95% CI,
0.524–0.734), PLR (AUC, 0.645; P=0.011; 95% CI, 0.546–0.744), PLT
(AUC, 0.624; P=0.030; 95% CI, 0.524–0.724) or FBG (AUC, 0.592;
P=0.107; 95% CI, 0.486–0.697).
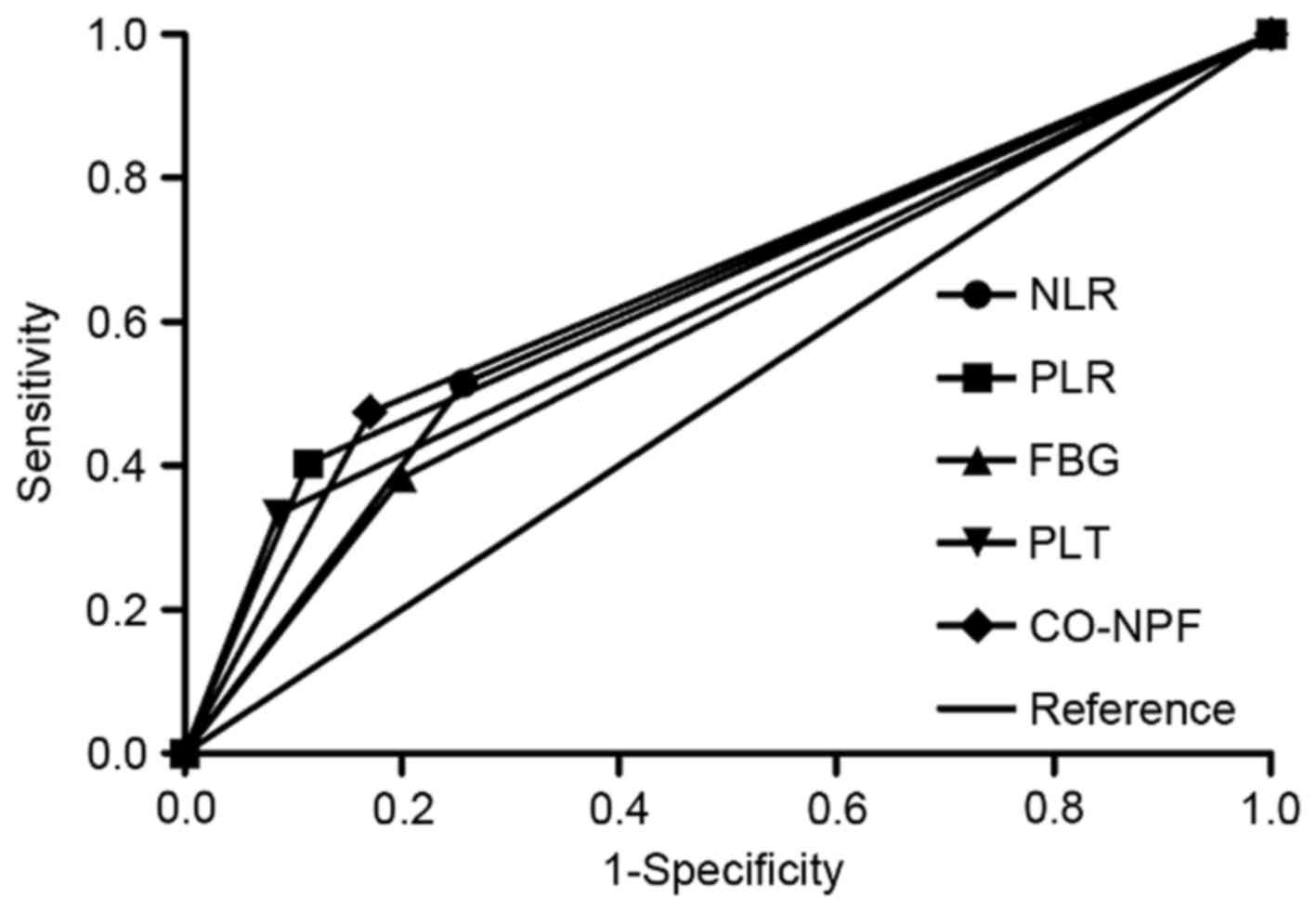 | Figure 4.Area under the curve of NLR, PLR, PLT,
FBG and CO-NPF. Comparison of receiver operating characteristic
curves based on NLR, PLR, PLT, FBG and CO-NPF in the 3-year overall
survival rate. NLR, neutrophil to lymphocyte ratio; PLR, platelet
to lymphocyte ratio; PLT, platelet; FBG, fibrinogen; CO-NPF,
combination of NLR, PLR, FBG and PLT. |
Discussion
The present retrospective study assessed the
association between preoperative systemic inflammatory indexes and
a novel inflammation-based marker (CO-NPF) with the postoperative
prognosis of 134 patients with ASC. The results revealed that
CO-NPF, NLR, PLR, PLT and FBG were independently and significantly
associated with decreased OS rates. Furthermore, CO-NPF, PLR and
FBG were all independent prognostic factors for DFS. CO-NPF was
superior to NLR, PLR, PLT and FBG individually in predicting 3-year
survival rates.
Systemic inflammatory responses may exert dual
effects on cancer, promoting tumor proliferation, while inhibiting
other oncogenic processes. The tumor microenvironment may be
heavily infiltrated by inflammatory cells and increased
pro-inflammatory cytokines released by these cells subsequently
promote tumor growth (11). For
example, neutrophils are able to significantly promote angiogenesis
by secreting vascular endothelial growth factor (VEGF) and tumor
progression by suppressing the cytotoxic activity of lymphocytes
(11). Release of growth factors,
including platelet factor 4, platelet-derived growth factor,
thrombospondin and VEGF by platelets promotes hematogenous cancer
spread, cancer cell adhesion, invasion, angiogenesis as well as
tumor progression (25–27). In addition, fibrinogen may protect
tumor cells from natural killer cytotoxicity (28). Conversely, lymphocytes suppress tumor
growth and invasion through their cytolytic activity (29). Increased NLR may represent increased
neutrophil counts, decreased lymphocyte counts, or both. The same
is true for PLR with respect to the platelet and lymphocyte
association. The prognostic significance of NLR, PLR, PLT and FBG
has been suggested in numerous types of cancer including NSCLC.
Although the prognostic role of these indexes in NSCLC remains
controversial, the results of the present study are in line with a
clinical prognostic value. Several studies have identified that
increased pretreatment NLR or PLR predicts shorter progression-free
survival (PFS), DFS and OS in patients with NSCLC as well as poor
response to first-line platinum-based treatment (16,18,20).
Furthermore, other reports suggest that increased pretreatment PLT
and FBG are significantly associated with unfavorable DFS, PFS or
OS in patients with NSCLC (13–15,19,21,22).
Although the prognostic value of NLR, PLR, PLT and FBG in NSCLC is
consistent with that in ASC, the exact threshold values of these
indexes have not been defined. As a result, ROC curves were used in
the present study to define the optimal threshold values.
NLR, PLR, PLT and FBG all represent systemic
inflammation and serve important roles in cancer. CO-NPF may serve
as a promising prognostic factor defining the association between
inflammation and cancer in a more comprehensive manner. Indeed,
CO-NPF was superior to NLR, PLR, PLT and FBG in predicting 3-year
survival rates. Notably, subgroup analysis revealed that CO-NPF was
significantly associated with decreased DFS and OS rates of
patients in the stage I–II subgroup, but not of those in the stage
IIIA subgroup (data not shown). This implies that the prognostic
value of inflammatory indexes may be influenced by the TNM stage.
By analyzing the association of clinical characteristics and
laboratory parameters with CO-NPF, it was revealed that there were
significant differences between the two CO-NPF groups in age, TNM
stage, neutrophil count, lymphocyte count, monocyte count, PLT
count, FBG count, NLR and PLR. However, CO-NPF was not
significantly associated with lymph node metastasis, implying that
inflammation is more likely to contribute to hematogenous
metastasis, which is consistent with a previous study (30).
All patients recruited in the present study were
from a single center which may limit the results, thus these
results require further confirmation by a multi-center study. In
addition, as a retrospective study, certain useful parameters which
were not routinely measured prior to surgery may have been missed.
For example, C-reactive protein measurement (30) and Glasgow Prognostic Score (31) may be used to confirm the results in a
prospective study. Wu et al (32) and Kwon et al (33), respectively, demonstrated that
phosphorylated insulin-like growth factor-1 receptor (pIGF1R) and
mucin 4 expression were associated with the prognosis of patients
with pulmonary adenocarcinoma. However, the optimum prognostic
factor among CO-NPF, mucin 4 and plGF1R requires further
confirmation by future studies. However, to the best of our
knowledge, the present study is the first to investigate the
prognostic role of PLT, FBG, NLR and PLR in patients with ASC, and
the results of the present study further demonstrated that CO-NPF
has improved predictive power compared with NLR, PLR, PLT and FBG
individually. These inflammatory indexes are cost-effective,
readily available and effective prognostic factors, with the
results of the present study suggesting that assessing them
together is superior to their use independently.
Acknowledgements
The authors are grateful to Dr Douglas E. Linn
(Brigham & Women's Hospital, Boston, MA, USA) for a critical
reading of the paper. The present study was supported by grants
from the National Natural Science Foundation of China (grant nos.
81372517 and 81000899), the Tianjin Municipal Science and
Technology Commission Key Application Research Projects (grant no.
11JCZDJC18900), the National Natural Science Foundation of China
(grant no. 81501983), the National Science and Technology Major
Project (grant no. 2013ZX) and the Tianjin Municipal Science and
Technology Commission Projects (grant nos. 11JCYBJC11300 and
12ZCDZSY15600).
References
|
1
|
Chang S, Dai M, Ren JS, Chen YH and Guo
LW: Estimates and prediction on incidence, mortality and prevalence
of lung cancer in China in 2008. Zhonghua Liu Xing Bing Xue Za Zhi.
33:391–394. 2012.(In Chinese). PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. In J Cancer. 127:2893–2917. 2010.
|
|
3
|
Fitzgibbons PL and Kern WH: Adenosquamous
carcinoma of the lung: A clinical and pathologic study of seven
cases. Hum Pathol. 16:463–466. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishida T, Kaneko S, Yokoyama H, Inoue T,
Sugio K and Sugimachi K: Adenosquamous carcinoma of the lung.
Clinicopathologic and immunohistochemical features. Am J Clin
Pathol. 97:678–685. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimizu J, Oda M, Hayashi Y, Nonomura A
and Watanabe Y: A clinicopathologic study of resected cases of
adenosquamous carcinoma of the lung. Chest. 109:989–994. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gibbs AR and Thunnissen FB: Histological
typing of lung and pleural tumours: Third edition. J Clin Pathol.
54:498–499. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sridhar KS, Bounassi MJ, Raub W Jr and
Richman SP: Clinical features of adenosquamous lung carcinoma in
127 patients. Am Rev Respir Dis. 142:19–23. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee Y, Chung JH, Kim SE, Kim TJ and Lee
KW: Adenosquamous carcinoma of the lung: CT, FDG PET, and
clinicopathologic findings. Clin Nucl Med. 39:107–112.
2014.PubMed/NCBI
|
|
9
|
Maeda H, Matsumura A, Kawabata T, Suito T,
Kawashima O, Watanabe T, Okabayashi K and Kubota I: Japan National
Hospital Organization Study Group for Lung Cancer: Adenosquamous
carcinoma of the lung: Surgical results as compared with squamous
cell and adenocarcinoma cases. Eur J Cardiothorac Surg. 41:357–361.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mordant P, Grand B, Cazes A, Foucault C,
Dujon A, Le Pimpec Barthes F and Riquet M: Adenosquamous carcinoma
of the lung: Surgical management, pathologic characteristics, and
prognostic implications. Ann Thorac Surg. 95:1189–1195. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ji Y, Sheng L, Du X, Qiu G and Su D:
Elevated platelet count is a strong predictor of poor prognosis in
stage I non-small cell lung cancer patients. Platelets. 26:138–142.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang HG, Li J, Shi SB, Chen P, Ge LP,
Jiang Q and Tang XP: Value of fibrinogen and D-dimer in predicting
recurrence and metastasis after radical surgery for non-small cell
lung cancer. Med Oncol. 31:222014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim KH, Park TY, Lee JY, Lee SM, Yim JJ,
Yoo CG, Kim YW, Han SK and Yang SC: Prognostic significance of
initial platelet counts and fibrinogen level in advanced non-small
cell lung cancer. J Korean Med Sci. 29:507–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kos M, Hocazade C, Kos FT, Uncu D, Karakas
E, Dogan M, Uncu HG, Yildirim N and Zengin N: Prognostic role of
pretreatment platelet/lymphocyte ratio in patients with non-small
cell lung cancer. Wien Klin Wochenschr. 128:635–640. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwon HC, Kim SH, Oh SY, Lee S and Lee JH,
Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ and Lee JH: Clinical
significance of preoperative neutrophil-lymphocyte versus
platelet-lymphocyte ratio in patients with operable colorectal
cancer. Biomarkers. 17:216–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pinato DJ, Shiner RJ, Seckl MJ, Stebbing
J, Sharma R and Mauri FA: Prognostic performance of
inflammation-based prognostic indices in primary operable non-small
cell lung cancer. Br J Cancer. 110:1930–1935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sheng L, Luo M, Sun X, Lin N, Mao W and Su
D: Serum fibrinogen is an independent prognostic factor in operable
nonsmall cell lung cancer. Int J Cancer. 133:2720–2725.
2013.PubMed/NCBI
|
|
20
|
Yao Y, Yuan D, Liu H, Gu X and Song Y:
Pretreatment neutrophil to lymphocyte ratio is associated with
response to therapy and prognosis of advanced non-small cell lung
cancer patients treated with first-line platinum-based
chemotherapy. Cancer Immunol Immunother. 62:471–479. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu D, Liu B, Zhang L and Du K: Platelet
count predicts prognosis in operable non-small cell lung cancer.
Exp Ther Med. 5:1351–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu JF, Cai L, Zhang XW, Wen YS, Su XD,
Rong TH and Zhang LJ: High plasma fibrinogen concentration and
platelet count unfavorably impact survival in non-small cell lung
cancer patients with brain metastases. Chin J Cancer. 33:96–104.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Unal D, Eroglu C, Kurtul N, Oguz A and
Tasdemir A: Are neutrophil/lymphocyte and platelet/lymphocyte rates
in patients with non-small cell lung cancer associated with
treatment response and prognosis? Asian Pac J Cancer Prev.
14:5237–5242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vallières E, Shepherd FA, Crowley J, Van
Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z and Goldstraw
P: International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals regarding the
relevance of TNM in the pathologic staging of small cell lung
cancer in the forthcoming (seventh) edition of the TNM
classification for lung cancer. J Thorac Oncol. 4:1049–1059. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dubernard V, Arbeille BB, Lemesle MB and
Legrand C: Evidence for an alpha-granular pool of the cytoskeletal
protein alpha-actinin in human platelets that redistributes with
the adhesive glycoprotein thrombospondin-1 during the exocytotic
process. Arterioscler Thromb Vasc Biol. 17:2293–2305. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaplan KL, Broekman MJ, Chernoff A,
Lesznik GR and Drillings M: Platelet alpha-granule proteins:
Studies on release and subcellular localization. Blood. 53:604–618.
1979.PubMed/NCBI
|
|
27
|
Qian X and Tuszynski GP: Expression of
thrombospondin-1 in cancer: A role in tumor progression. Proc Soc
Exp Biol Med. 212:199–207. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng S, Shen J, Jiao Y, Liu Y, Zhang C,
Wei M, Hao S and Zeng X: Platelets and fibrinogen facilitate each
other in protecting tumor cells from natural killer cytotoxicity.
Cancer Sci. 100:859–865. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Huang SH, Li H, Li Y, Chen XL,
Zhang WQ, Chen HG and Gu LJ: Preoperative lymphocyte count is a
favorable prognostic factor of disease-free survival in
non-small-cell lung cancer. Med Oncol. 30:3522013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang H, Zhang L, Zhu K, Shi B, Yin Y, Zhu
J, Yue D, Zhang B and Wang C: Prognostic significance of
combination of preoperative platelet count and
neutrophil-lymphocyte ratio (COP-NLR) in patients with non-small
cell lung cancer: Based on a large cohort study. PLoS One.
10:e01264962015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McMillan DC: The systemic
inflammation-based Glasgow prognostic score: A decade of experience
in patients with cancer. Cancer Treat Rev. 39:534–540. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu PF, Huang WC, Yang JC, Lu YS, Shih JY,
Wu SG, Lin CH and Cheng AL: Phosphorylated insulin-like growth
factor-1 receptor (pIGF1R) is a poor prognostic factor in brain
metastases from lung adenocarcinomas. J Neurooncol. 115:61–70.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kwon KY, Ro JY, Singhal N, Killen DE,
Sienko A, Allen TC, Zander DS, Barrios R, Haque A and Cagle PT:
MUC4 expression in non-small cell lung carcinomas: Relationship to
tumor histology and patient survival. Arch Pathol Lab Med.
131:593–598. 2007.PubMed/NCBI
|