Introduction
Persistent chronic inflammation has emerged as a
potential risk factor in the development of carcinoma of several
organ types, including liver, large bowel, urinary bladder, gastric
mucosa, lung, colon and pancreas (1–3).
Experimental evidence suggests that longstanding chronic
inflammation promotes prostate carcinogenesis (4,5). In fact,
a positive link between clinical history of prostatitis and
increased relative risk of prostate cancer has been documented
among 70,000 North American men (6).
Chronic inflammation is frequently identified in prostate biopsies,
radical prostatectomy specimens and tissue resected from benign
prostatic hyperplasia (7,8). A number of possible etiological factors
have been proposed in the development of chronic inflammation in
the prostate, including dietary imbalances, exposure to
environmental pollutants, alterations in testosterone:estrogen
ratio, pathogen infection, race or genetic alterations (9–12). The
resulting microenvironment, characterized by the accumulation of
various types of immune cells, generates reactive oxygen and
nitrogen species, inducing oxidative stress in proliferating
epithelium that may interact with DNA to generate permanent genomic
alterations (13). Extensive research
efforts have been undertaken to investigate a possible link between
inflammation and the development of prostate cancer (14,15). We
previously reported the effect of chronic inflammation on prostate
carcinogenesis in a 5-year follow-up study of needle biopsy
specimens from 177 patients with clinical parameters suspicious for
malignancy. In follow-up biopsies over a 5-year period, 20% of
patients with only chronic inflammation on initial biopsy exhibited
a new diagnosis of adenocarcinoma, and an additional 6% exhibited
high-grade prostatic intraepithelial neoplasia (HGPIN) lesions; by
contrast, among patients initially showing no inflammation, only 6%
subsequently developed cancer (16).
In recent years, an injury-and-regeneration model
has been proposed suggesting that repeated bouts of injury to the
prostatic epithelium, presumably as a result of inflammation in
response to pathogens or autoimmune disease and/or dietary factors,
results in proliferation of epithelial cells that possess a
phenotype intermediate between basal cells and mature luminal cells
(17). These alterations may result
in the modulation of a delicate balance between cell proliferation
and cell death. A defect in the normal regulation of programmed
cell death by a subset of cells that are able to survive and
continue to proliferate may favor the acquisition of further
genetic alterations that would promote the development of
malignancy. A current hypothesis is that morphological changes in
acinar or ductal epithelia result from the continuous influence of
inflammation (18). These findings
are characterized as HGPIN, a lesion that is a putative precursor
of prostatic adenocarcinoma. A pathological entity of chronic
inflammation accompanied by focal glandular atrophy and epithelial
cell proliferation, termed proliferative inflammatory atrophy (PIA)
and post-atrophic hyperplasia (PAH), has been recognized (19,20). PIAs
are atrophic lesions frequently observed in the peripheral zone of
the prostate, occasionally adjacent to the foci of HGPIN and cancer
(19). It has been hypothesized that
the atrophic epithelial cells in PIA lesions are the targets of
neoplastic transformation and potentially give rise to carcinoma.
PAH has been shown to be closely associated with persistent chronic
inflammation (21). PAH represents
‘post-inflammatory’ atrophic lesions. It is hypothesized that
proliferative regeneration of secretory cells following disruption
of the epithelium may generate small, hyperplastic glands arranged
in a lobular configuration, which is a typical feature of PAH. PAH
is occasionally confused with carcinoma due to its overlapping
architectural and nuclear features. In particular, these lesions
are predominantly composed of small crowded glands, usually
surrounded by centrally situated and dilated large ducts, irregular
and atrophic-appearing contours lined by flattened-to-cuboidal
epithelial cells, with nuclear enlargement and prominent nucleoli
(21,22). PIA/PAH lesions have been reported to
show increased proliferative activity compared with benign
prostatic epithelium and simple atrophy (SA) (23). The signs as well as other
morphological evidence may suggest the transition from a PIA/PAH
lesion to the development of HGPIN; however, the molecular evidence
for cell survival and proliferation under the influence of chronic
inflammation is lacking. To gain knowledge in this area, the
present study examined the morphology of 106 needle-biopsy
specimens. Immunohistochemistry was used to investigate the
association between the expression of Bcl-2, a cell survival
protein that plays a role in apoptosis by blocking programmed cell
death (24), and the expression of
proliferating cell nuclear antigen (PCNA), a proliferation marker
(25). The results suggest an inverse
correlation between the expression of Bcl-2 and that of PCNA in
epithelial cells under the influence of chronic inflammation.
Since, in our opinion, PAH represents the end result of
longstanding PIA, these morphological lesions were grouped together
and designated as PIA/PAH lesions for the sake of the present
analysis.
Materials and methods
Prostate biopsy material
The study was performed in formalin-fixed,
paraffin-embedded prostate biopsy specimens obtained between August
2001 to June 2003 from male patients aged >40 years who had
serum prostate-specific antigen levels of >4 ng/ml. Needle
biopsies were performed at the University Hospitals Cleveland
Medical Center (Cleveland, OH, USA). Specimens from all ethnic
groups were included. The study was approved by the Institutional
Review Board (08-03-38) at the University Hospitals Cleveland
Medical Center.
Characterization of extent and
intensity of inflammatory findings
All specimens were examined by two expert urological
pathologists (QR and GTM) for the presence and distribution of
chronic inflammatory infiltrate, which was assigned a score of 1
(mild), 2 (moderate), 3 (strong) or 4 (very strong), as previously
reported (16). The degree of
inflammation was assigned according to the approximate percentage
of stroma and glands in the biopsy specimen involved by aggregates
of ≥50 lymphocytes, or by granulomatous inflammation on a scale of
mild inflammation (1–10% involvement), moderate inflammation
(11–50% involvement) and severe/strong inflammation (>50%
involvement). A total of 106 hematoxylin and eosin-stained
needle-biopsy specimens were examined.
Classification of prostatic
lesions
Prostatic needle-biopsy specimens were further
evaluated and categorized for foci of SA, PIA and/or PAH, HGPIN,
and cancer. The morphology of these lesions and the criteria for
their diagnosis are well-established, and readily found in standard
journals of surgical pathology (26,27).
Immunohistochemistry
Immunohistochemical staining for Bcl-2 and PCNA was
performed in 36 needle-biopsy specimens previously categorized in
groups as SA, PIA/PAH, HGPIN and cancer, using standard techniques.
Paraffin-embedded prostate needle-biopsy specimens were cut at a
4-µm thickness and the sections were incubated with target
retrieval solution (cat. no. S2367; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA) in a steamer for 45 min. The sections
were permeabilized in a solution containing 100 mM Tris (pH 7.5),
150 mM NaCl, 0.5% blocking agent, 0.3% Triton X and 0.2% saponin
for 20 min at room temperature. Sections were incubated overnight
in a humid chamber at 4°C with antibodies against Bcl-2 (cat. no.
M0887; Dako; Agilent Technologies, Inc.) at 1:50 dilution. Control
sections were incubated with antisera in the presence of 10-fold
excess of these antibodies or with isotype-matched IgG (cat. no.
sc-516102; Santa Cruz Biotechnology Inc., Dallas, TX) in normal
goat serum (cat. no. 927502; BioLegend, Inc., San Diego, CA) for 30
min at room temperature. Subsequent to washing three times in TBS,
detection was achieved using standard HRP-labeled LSAB2 system
(cat. no. K0609; Dako; Agilent Technologies, Inc.), with
3-3-diaminobenzidine as the chromogen. For PCNA (cat. no. sc-71858;
Santa Cruz Biotechnology, Inc.), the immunoreactive complexes were
detected using AEC+ substrate-chromogen from the Dako EnVision+
System (cat. no. K4002; Dako; Agilent Technologies, Inc.),
consisting of 3-amino-9-ethylcarbazole as the chromogen. Slides
were then counterstained in Mayer's hematoxylin, mounted in crystal
mount media, and dried overnight on a level surface, as previously
described (28).
Scoring of stained cells
The immunostained sections were examined
independently by the pathologist (QR) and co-investigators (MG and
SS) using light microscopy. Sections were viewed under an Olympus
BH2 inverted microscope (Olympus Corporation, Tokyo, Japan) and
images were acquired with Image-Pro Plus v.7 software (Media
Cybernetics Rockville, MD, USA) and digitally stored on a computer.
The intensity of immunohistochemical staining for Bcl-2 was scored
as 0 (negative), 1 (weak), 2 (moderate) or 3 (intense). Bcl-2
staining was heterogeneous, exhibiting areas with intense staining
and other areas with moderate-to-weak staining. Because the
majority of the observed lesions were heterogeneous, the overall
score assigned represented the average for the entire lesion. The
PCNA staining was scored by counting the positively stained cells
and total number of cells, quantified in random microscopic field
with a minimum 500-cell count (magnification, ×40) with the
assistance of a software program (Olympus Microsuite v.5; Olympus
America Inc. Center Valley, PA). A minimum of four screenshots per
specimen, representing individual microscopic fields, were counted
for determining the tissue staining distribution.
Statistical analysis
Statistical analysis was performed using
Kruskal-Wallis test, a nonparametric test based on Wilcoxon scores,
to examine the median differences in Bcl-2 and PCNA among the four
subcategories of tissues (SA, PIA/PAH, HGPIN and cancer), followed
by pair-wise comparison of any two tissue types where the P-value
was not adjusted for multiple comparisons. The association between
various grades of inflammation (mild, moderate, strong and very
strong) with Bcl-2 and PCNA was also estimated using Spearman
correlation coefficient. All tests were two-sided and P<0.05 was
considered to indicate a statistically significant difference.
Results
As shown in Table I,
of the 106 specimens examined, 20% (20/106) showed unremarkable
prostatic tissue, 8% (8/106) of specimens exhibited SA, whereas 42%
(45/106) of cases showed PIA/PAH lesions. HGPIN was observed in 8%
(8/106) of specimens, and adenocarcinoma was diagnosed in 11%
(12/106) of specimens. Additionally, 2% (2/106) of the specimens
exhibited atypical small acinar proliferations, suspicious for, but
not diagnostic of, malignancy. Representative images of these
lesions observed from needle biopsies are shown in Fig. 1A-D.
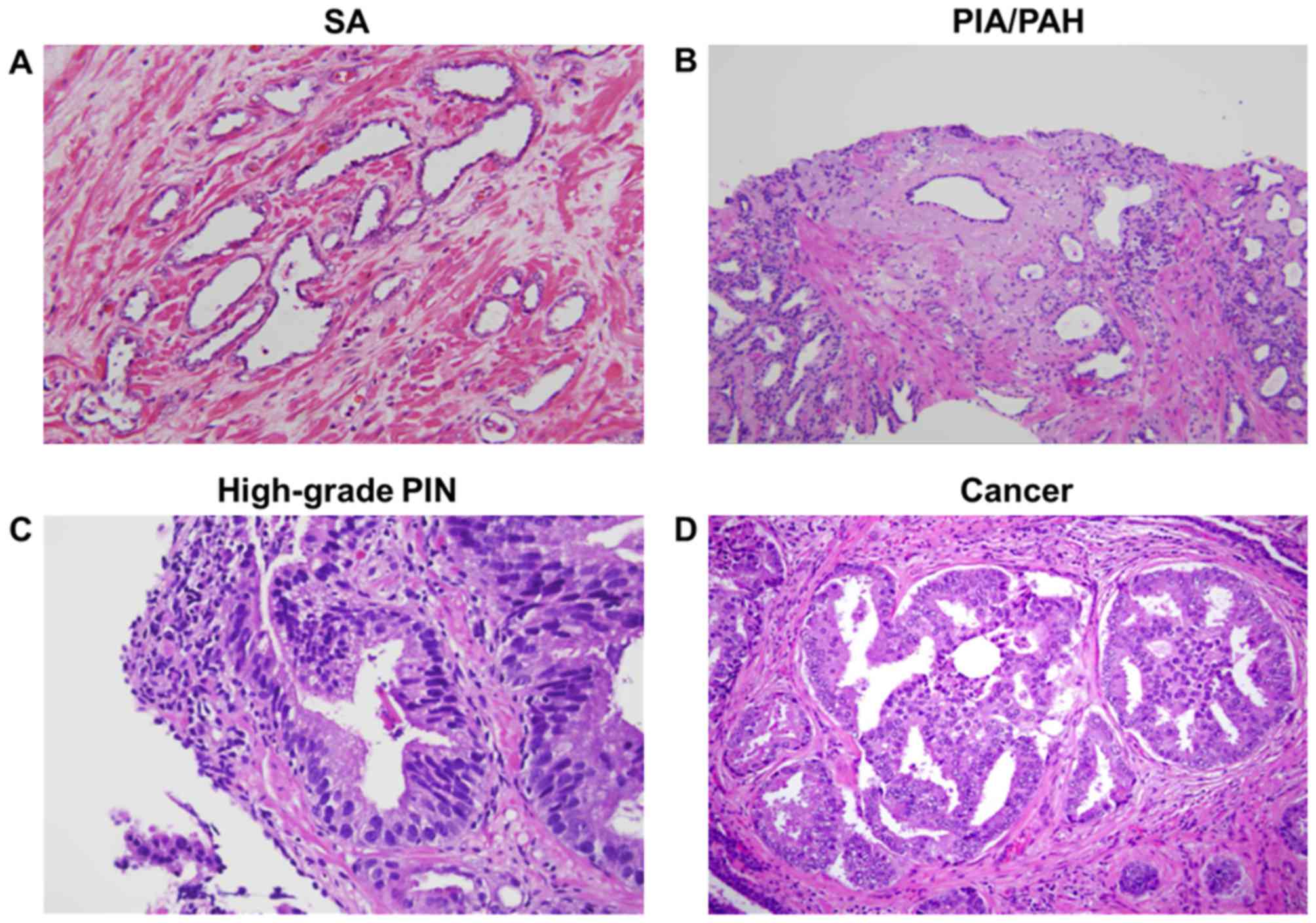 | Figure 1.Pathological findings in
needle-biopsy specimens by hematoxylin and eosin staining. (A)
Prostate tissue with an area of SA, and some small and some dilated
glands. The glands are lined by small cuboidal cells with bland
nuclei and minimal cytoplasm. (B) Prostate tissue with PIA and PAH
lesions. Crowded, irregular, atrophic glands with abundant chronic
inflammatory infiltrate in the surrounding stroma are visible in
the PIA (left), whereas PAH lesions show a dilated central acinus
flanked by numerous small and large atrophic acini in a lobular
pattern, distorted by sclerotic stroma, which exhibits prominent
inflammatory cell infiltrate (right). (C) High-grade PIN involving
a large pre-existing duct. Chronic inflammation is present in the
stroma (center) is concentrated around another large duct (right).
(D) Prostatic adenocarcinoma with cribriform structures.
Magnification, ×20. SA, simple atrophy; PIA, proliferative
inflammatory atrophy; PAH, post-atrophic hyperplasia; PIN,
prostatic intraepithelial neoplasia. |
 | Table I.Characteristics and evaluation of
prostate needle-biopsy specimens (n=106). |
Table I.
Characteristics and evaluation of
prostate needle-biopsy specimens (n=106).
| Type | n | % |
|---|
| No glandular
abnormality | 12 | 11 |
| Atrophy without
inflammation | 8 | 8 |
| Simple atrophy | 19 | 18 |
| PIA/PAH | 45 | 42 |
| HGPIN | 8 | 8 |
| Cancer | 12 | 11 |
| Othersa | 2 | 2 |
Bcl-2 protein expression
A total of 36 needle biopsy specimens that contained
areas of inflammation were randomly selected for equivalent
portions of strong/very strong, moderate or mild/absent
inflammatory infiltrates detected in the biopsy core for various
lesions and stained for Bcl-2 by immunohistochemistry (Fig. 2A-D). The samples were analyzed for the
percentage of cells expressing Bcl-2 as well as intensity of
expression. The average intensity of the stain was graded from 0
(no expression) to 3 (strong expression). In these samples, basal
epithelial cells exhibited prominent Bcl-2 staining, which was
noted to be in close proximity to the inflammatory lesions, with
77.1% of basal epithelial cells showing strong expression. However,
negative-to-weak Bcl-2 expression was noted in the majority of
luminal epithelial cells (>90%), with heterogeneous staining
pattern in the normal/benign areas. Average epithelial cell stain
intensity was in the low-medium range (mean, 1.67). The immune
cells in the specimens also expressed Bcl-2, but only 38.2%
exhibited immunoreactivity to this antigen. The immune cells
expressing Bcl-2 exhibited a higher staining intensity (mean, 2.04)
than the epithelial cells. Cytoplasmic Bcl-2 expression was almost
exclusively observed amongst epithelial cells, whereas immune cells
were observed to have extensive Bcl-2 expression within the
nucleus. Only 3.7% of inflammatory cells and epithelial cells in
areas adjacent to but not involved by inflammation showed Bcl-2
expression, with an average staining intensity of 0.95. Compared to
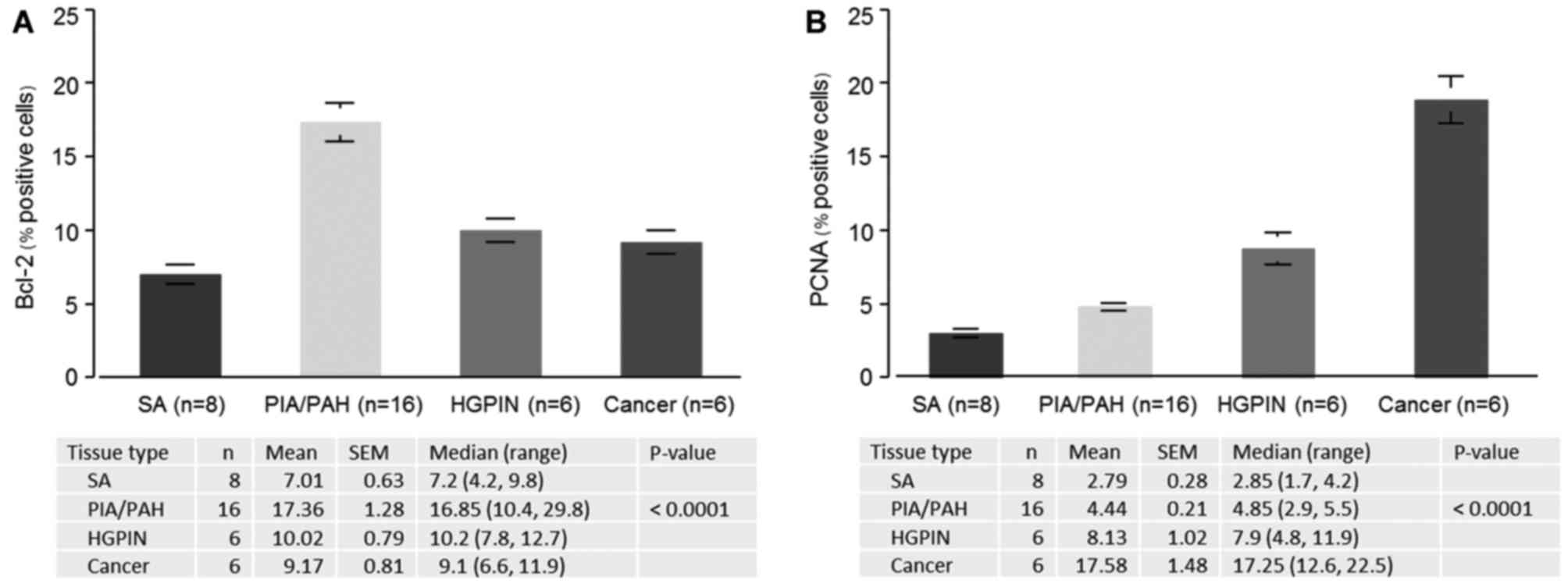
SA (7.0%), the highest percentage (17.4%) of Bcl-2-positive cells
was observed in PIA/PAH lesions (P<0.0001), followed by HGPIN
(10.1%) and cancer lesions (9.2%), respectively (Fig. 3A).
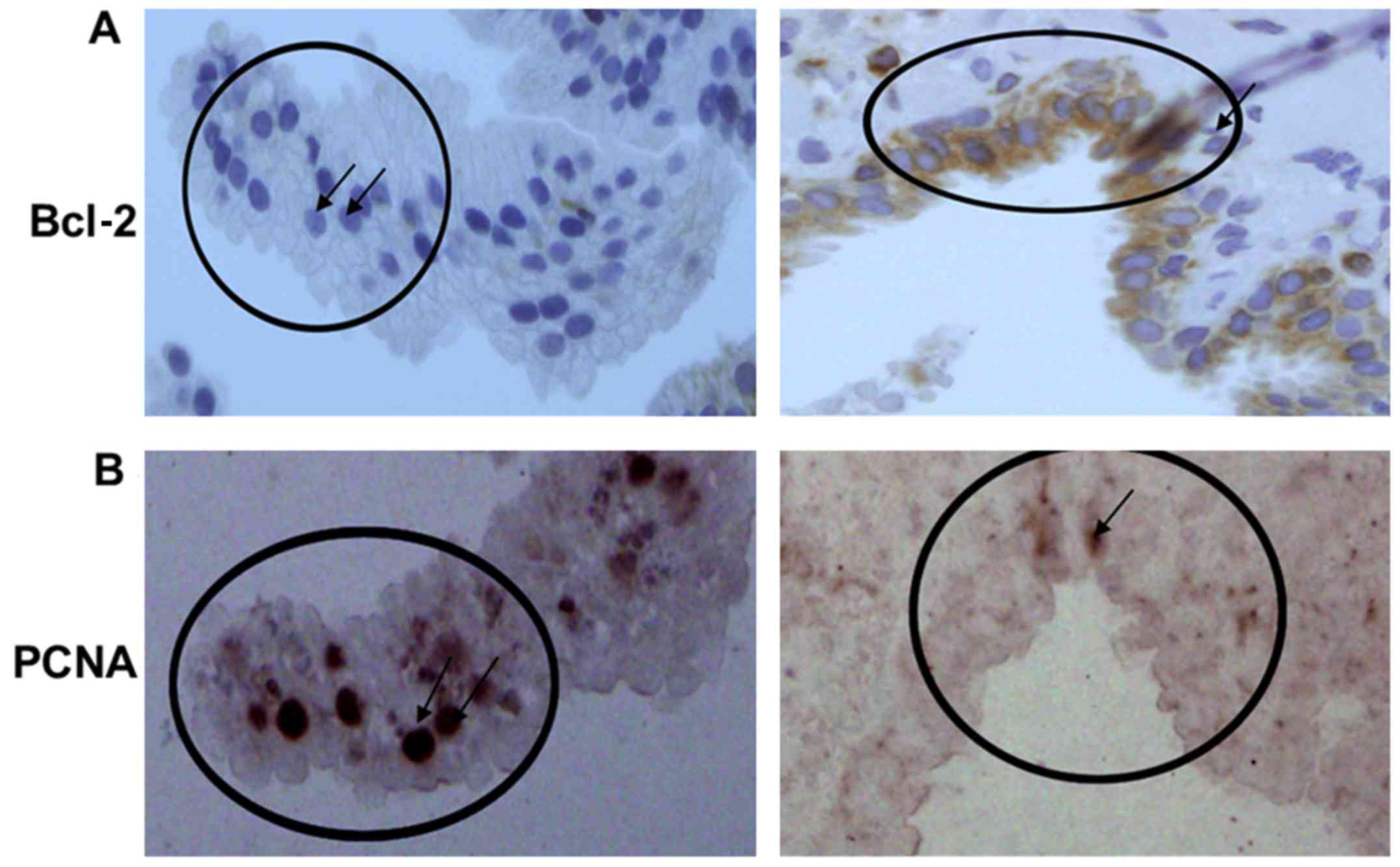
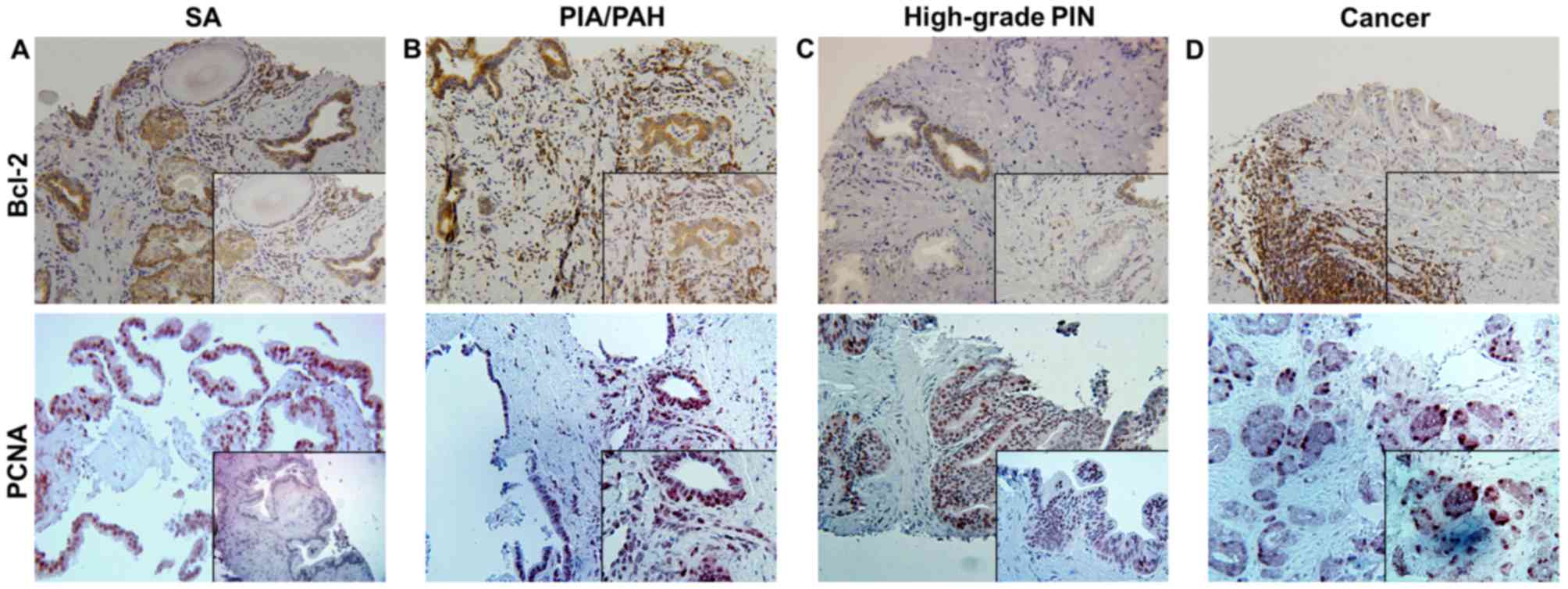 | Figure 2.Immunohistochemical staining for
Bcl-2 and PCNA in needle-biopsy specimens within (A) SA, (B)
PIA/PAH lesions, (C) high-grade PIN and (D) cancer. Bcl-2 is widely
expressed in inflammatory and epithelial tissues of SA and PIA/PAH,
with more intense staining in the basal epithelial cells near the
areas of chronic inflammation, as well as predominantly in
infiltrating immune cells. High expression of PCNA is observed in
the proliferating epithelium in high-grade PIN and cancer.
Magnification, ×20 (inset, ×40). PCNA, proliferating cell nuclear
antigen; SA, simple atrophy; PIA, proliferative inflammatory
atrophy; PAH, post-atrophic hyperplasia; PIN, prostatic
intraepithelial neoplasia. |
PCNA protein expression
The staining for the proliferative marker PCNA was
also examined in the prostate needle-biopsy specimens (Fig. 2A-D). In normal/benign glands, PCNA
expression was more often noted in the basal epithelial cells, than
in the luminal epithelial cells. The proliferation index analyzed
from numerous microscopic fields revealed a progressive increase in
proliferation index in epithelial cells from SA to cancer. Compared
to SA (2.8%), a significant increase in PCNA was observed in
PIA/PAH (4.4%), HGPIN (8.1%) and cancer (17.6%) (P<0.0001),
respectively (Fig. 3B).
Relationship between Bcl-2 and PCNA
expression
A negative correlation was observed within the
serial specimens stained for PCNA and Bcl-2. Cells expressing Bcl-2
were observed to have low levels or an absence of PCNA expression.
A similar association was observed in samples that exhibited high
PCNA expression, wherein little-to-no Bcl-2 expression was
detected. PCNA stainings was observed within the nucleus among
epithelial cells, as compared with the predominant cytoplasmic
staining observed in epithelial cells stained for Bcl-2. Immune
infiltrate cells did not express high levels of PCNA as compared
with the Bcl-2 expression (Fig. 4A and
B).
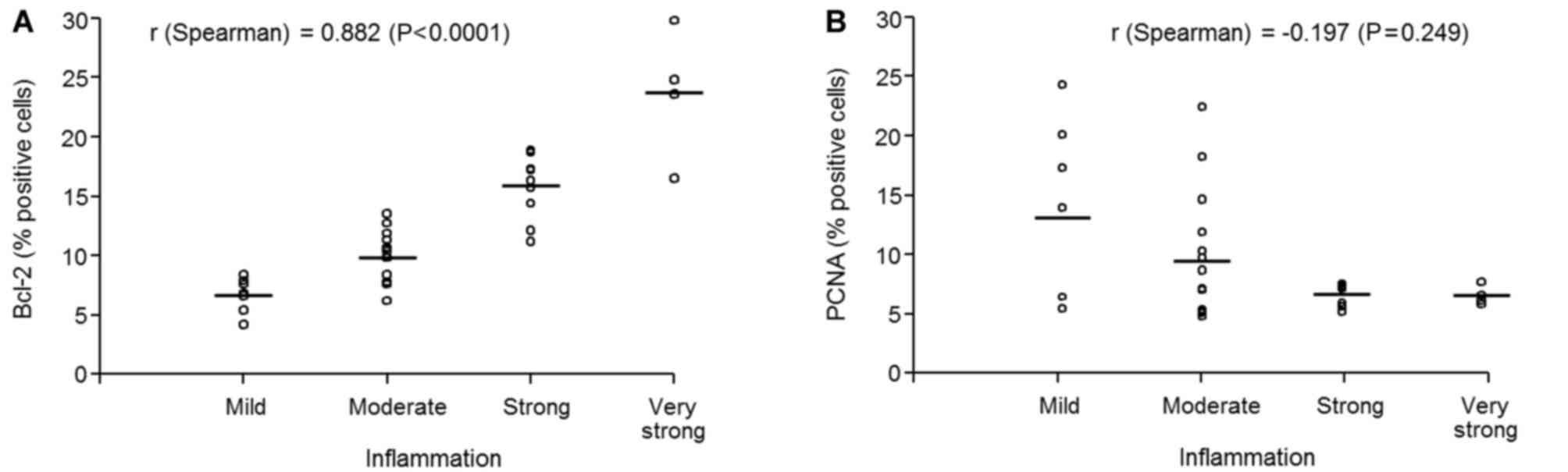
Relationship between inflammation,
cell survival and neoplastic progression
Subsequently, the correlation between inflammation,
cell survival and neoplastic progression was assessed with regard
to the Bcl-2 and PCNA scoring by a pathologist. (Fig. 5). As shown in Fig. 5A, a strong positive association
between inflammation and Bcl-2 expression was observed (r=0.88;
P<0.0001) suggesting that cell survival increases with the
increasing inflammation. A negative association (r=−0.197; P=0.249)
between inflammation and proliferation was identified (Fig. 5B), suggesting that prolonged
inflammation facilitates the initiation of normal prostate
epithelial cells through increased survival, which then gain
independence to proliferate and progress to malignancy.
Discussion
The purpose of the present study was to determine
the association between epithelial cell survival and proliferation
under the influence of chronic inflammation, and whether molecular
changes may be an indicator of neoplastic progression. Prostate
carcinogenesis is a continuous process with distinct stages of
atrophy, hyperplasia, prostatic intraepithelial neoplasia and
carcinoma, which can be identified on the basis of cellular
morphology and topographical features (18–23).
During the initiation of prostate cancer, most of the proliferation
occurs in the luminal compartment rather than in the basal
compartment (18–20). This shift is present in most of the
carcinoma precursor lesions, including HGPIN. It has long been
hypothesized that PIA may be a precursor of prostate cancer, as
several studies have reported a morphological transition from PIA
to HGPIN (18,21). This suggestion is based on the
following findings: i) Morphological overlap between PIA and HGPIN;
ii) the phenotypes of many of the cells in PIA are most consistent
with that of immature secretory-type cells, similar to that of the
cells of HGPIN; and iii) PIA, HGPIN and carcinoma all occur with
high prevalence in the peripheral zone and low prevalence in the
central zone of the prostate. In the present study on needle-biopsy
specimens, a close topographic association between PIA/PAH and
HGPIN was observed, in agreement with the findings in previous
studies (18,21).
As PIA is predominantly associated with
inflammation, we speculated whether chronic inflammation may lead
to neoplastic progression. It has been postulated that, under the
influence of chronic inflammation, the proliferating epithelial
cells of the prostate tend to adapt to the altered microenvironment
by altering the delicate balance between cell death and
proliferation (29). Chronic
inflammation with the accumulation of lymphocyte infiltrate in the
prostate may lead to repeated tissue damage and regeneration, with
release or increased expression of cytokines, growth factors, and
oncogenes, leading to a highly neoplastic state (30,31). This
has been observed by a marked increase in the Bcl-2 protein, a
marker of cell survival, in PIA/PAH lesions by our group and
several other investigators (32,33). Based
on immunohistochemical staining, the present study revealed that
Bcl-2 is upregulated in epithelial cells in areas of chronic
inflammation. The Bcl-2 protein is a product of the BCL2
gene; it is a powerful inhibitor of apoptotic cell death and may
lead to malignant transformation and tumor formation (34,35). Bcl-2
expression in prostate cancer is associated with the
androgen-independent phenotype (36).
High expression of Bcl-2 was also noted in the infiltrating immune
cells in the present study. Further studies by De Marzo et
al (37) have shown that
protection from repeated oxidative or electrophilic DNA damage is
achieved in PIA/PAH lesions by increased expression of glutathione
S-transferase pi (GSTP1), which catalyzes the detoxification of
reactive electrophiles and oxidants and therefore can have a
protective effect against neoplastic transformation; by contrast,
this does not occur in HGPIN and carcinoma (37). Further recent studies have shown that
GSTP1 CpG island hypermethylation changes occur in a proportion of
PIA/PAH lesions, providing evidence that at least some of these
lesions harbor cells already initiated to progress to HGPIN and/or
prostate adenocarcinoma (38,39).
The rate of proliferation observed in the PIA lesion
in the present study is comparatively less than that of HGPIN and
cancer, suggesting that the majority of the cells have not achieved
a neoplastic state. Therefore, it was investigated whether chronic
inflammation may influence the development of cancer through the
precursor HGPIN or PIA by rendering the cells to achieve a
neoplastic state. Previous studies have shown that persistent
chronic inflammation adjacent to PIA lesions may drive the
epithelial cells to achieve increased survival (18–21). These
may be regarded as initiated cells, and acquisition of further
genetic alterations under the influence of continuous inflammation
may drive the cells towards malignancy. It has been postulated that
these initiated cells, over a period of time, may or may not be
further influenced by inflammation. An independent ability to
proliferate in an altered microenvironment may lead to cancer
progression (18–21). This is evident from the increased
proliferation rate, as demonstrated by intense PCNA staining, where
the majority of the neoplastic/malignant cells exhibited a high
proliferation index along with Bcl-2 staining. This suggestion is
consistent with the previous findings, wherein some PIA/PAH lesions
have been shown to acquire somatic chromosome 8 abnormalities and
mutations in the tumor suppressor gene TP53 (genetic alterations
that are associated with prostate cancer and HGPIN lesions)
(40). Aberrant expression of these
two molecules may lead to extended survival of neoplastic cells and
increased likelihood of mutational aberrations, which are
responsible for proliferation and aggressiveness.
Genetic alterations in the number of tumor
suppressor genes, oncogenes, proliferation, apoptosis, and cellular
stress response genes have been reported to be associated with
HGPIN and prostate cancer (41,42). In
the present study, a strong association of PIA/PAH lesions with
chronic inflammation was demonstrated, which seems to predispose
cells to neoplastic development in the prostate. Furthermore, an
inverse correlation between Bcl-2 and PCNA in the glandular
epithelial cells seems to indicate that extended survival and
apoptosis inhibition by Bcl-2, and low proliferation rate under the
influence of low-grade persistent inflammation, may promote
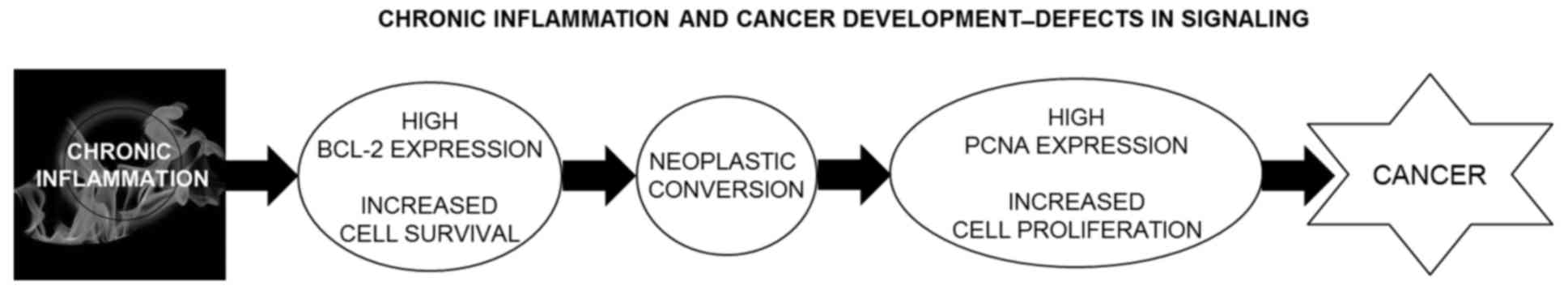
malignant transformation. Fig. 6
illustrates this hypothetical model. However, broader studies of
the apoptosis-regulatory proteins and proliferative proteins should
be performed in an effort to elucidate the relationship between
inflammation and neoplastic progression. In the present study, we
attempted to minimize the pitfalls by categorizing specimens into
four different subcategories (SA, PIA/PAH, HGPIN and cancer)
associated with uniformly high chronic inflammation. Taken
together, the findings of the present study suggest that persistent
chronic inflammation is a common distinct entity resulting in
increased survival of cells, and facilitates neoplastic development
leading to a state of increased proliferation.
In conclusion, the present study identifies chronic
inflammation as a contributor to neoplastic progression of prostate
epithelial cells. Additional studies are required to illustrate the
molecular/genetic alterations that occur during the events of
inflammation that could lead to disease progression.
Acknowledgements
The original study from the authors' laboratory
outlined in this review was supported by VA Merit Review
(1I01BX002494), United States Public Health Service Grants
(RO1CA108512, R21CA193080 and R03CA186179) and a Department of
Defense grant (W81XWH-15-1-0558 awarded to S.G).
Glossary
Abbreviations
Abbreviations:
|
PCNA
|
proliferating cell nuclear antigen
|
|
PIA
|
proliferative inflammatory atrophy
|
|
PAH
|
post-atrophic hyperplasia
|
|
HGPIN
|
high-grade prostatic intraepithelial
neoplasia
|
|
GSTP1
|
glutathione S-transferase pi
|
References
|
1
|
Wang K and Karin M: Tumor-Elicited
Inflammation and colorectal cancer. Adv Cancer Res. 128:173–196.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berasain C, Castillo J, Perugorria MJ,
Latasa MU, Prieto J and Avila MA: Inflammation and liver cancer:
New molecular links. Ann N Y Acad Sci. 1155:206–221. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kundu JK and Surh YJ: Inflammation:
Gearing the journey to cancer. Mutat Res. 659:15–30. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Umbehr MH, Gurel B, Murtola TJ, Sutcliffe
S, Peskoe SB, Tangen CM, Goodman PJ, Thompson IM, Lippman SM, Lucia
MS, et al: Intraprostatic inflammation is positively associated
with serum PSA in men with PSA <4 ng ml(−1), normal DRE and
negative for prostate cancer. Prostate Cancer Prostatic Dis.
18:264–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sfanos KS, Hempel HA and De Marzo AM: The
role of inflammation in prostate cancer. Adv Exp Med Biol.
816:153–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng I, Witte JS, Jacobsen SJ, Haque R,
Quinn VP, Quesenberry CP, Caan BJ and Van Den Eeden SK:
Prostatitis, sexually transmitted diseases, and prostate cancer:
The California Men's Health Study. PLoS One. 5:e87362010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Porcaro AB, Rubilotta E, Petrozziello A,
Ghimenton C, Migliorini F, Zecchini Antoniolli S, Lacola V, Monaco
C, Curti P, Cavalleri S, et al: Chronic inflammation of the
prostate type IV with respect to risk of prostate cancer. Arch Ital
Urol Androl. 86:208–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adamczyk P, Wolski Z, Butkiewicz R,
Nussbeutel J and Drewa T: Inflammatory changes in biopsy specimens
from patients with suspected prostate cancer. Cent European J Urol.
66:256–262. 2013.PubMed/NCBI
|
|
9
|
Vandersluis AD, Guy DE, Klotz LH, Fleshner
NE, Kiss A, Parker C and Venkateswaran V: The role of lifestyle
characteristics on prostate cancer progression in two active
surveillance cohorts. Prostate Cancer Prostatic Dis. 19:305–310.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Discacciati A and Wolk A: Lifestyle and
dietary factors in prostate cancer prevention. Recent Results
Cancer Res. 202:27–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gonzalez CA and Riboli E: Diet and cancer
prevention: Contributions from the European Prospective
Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer.
46:2555–2562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rybicki BA, Kryvenko ON, Wang Y, Jankowski
M, Trudeau S, Chitale DA, Gupta NS, Rundle A and Tang D: Racial
differences in the relationship between clinical prostatitis,
presence of inflammation in benign prostate and subsequent risk of
prostate cancer. Prostate Cancer Prostatic Dis. 19:145–150. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mian OY, Khattab MH, Hedayati M, Coulter
J, Abubaker-Sharif B, Schwaninger JM, Veeraswamy RK, Brooks JD,
Hopkins L, Shinohara DB, et al: GSTP1 Loss results in accumulation
of oxidative DNA base damage and promotes prostate cancer cell
survival following exposure to protracted oxidative stress.
Prostate. 76:199–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vasto S, Carruba G, Candore G, Italiano E,
Di Bona D and Caruso C: Inflammation and prostate cancer. Future
Oncol. 4:637–645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nguyen DP, Li J, Yadav SS and Tewari AK:
Recent insights into NF-κB signaling pathways and the link between
inflammation and prostate cancer. BJU Int. 114:168–176. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
MacLennan GT, Eisenberg R, Fleshman RL,
Taylor JM, Fu P, Resnick MI and Gupta S: The influence of chronic
inflammation in prostatic carcinogenesis: A 5-year followup study.
J Urol. 176:1012–1016. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kulac I, Gumuskaya B, Drake CG, Gonzalez
B, Arnold KB, Goodman PJ, Kristal AR, Lucia MS, Thompson IM, Isaacs
WB, et al: Peripheral zone inflammation is not strongly associated
with lower urinary tract symptom incidence and progression in the
placebo arm of the prostate cancer prevention trial. Prostate.
76:1399–1408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Bergh A and Damber JE:
Morphological transition of proliferative inflammatory atrophy to
high-grade intraepithelial neoplasia and cancer in human prostate.
Prostate. 69:1378–1386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Woenckhaus J and Fenic I: Proliferative
inflammatory atrophy: A background lesion of prostate cancer?
Andrologia. 40:134–137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chrisofos M, Papatsoris AG, Lazaris A and
Deliveliotis C: Precursor lesions of prostate cancer. Crit Rev Clin
Lab Sci. 44:243–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vral A, Magri V, Montanari E, Gazzano G,
Gourvas V, Marras E and Perletti G: Topographic and quantitative
relationship between prostate inflammation, proliferative
inflammatory atrophy and low-grade prostate intraepithelial
neoplasia: A biopsy study in chronic prostatitis patients. Int J
Oncol. 41:1950–1958. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Postma R, Schröder FH and van der Kwast
TH: Atrophy in prostate needle biopsy cores and its relationship to
prostate cancer incidence in screened men. Urology. 65:745–749.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Servian P, Celma A, Planas J, Placer J, de
Torres IM, Olivan M and Morote J: Clinical significance of
proliferative inflammatory atrophy finding in prostatic biopsies.
Prostate. 75:1669–1675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kelly PN and Strasser A: The role of Bcl-2
and its pro-survival relatives in tumourigenesis and cancer
therapy. Cell Death Differ. 18:1414–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Strzalka W and Ziemienowicz A:
Proliferating cell nuclear antigen (PCNA): A key factor in DNA
replication and cell cycle regulation. Ann Bot. 107:1127–1140.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bostwick DG and Cheng L: Precursors of
prostate cancer. Histopathology. 60:4–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng L, Montironi R, Bostwick DG,
Lopez-Beltran A and Berney DM: Staging of prostate cancer.
Histopathology. 60:87–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shukla S, MacLennan GT, Fu P, Patel J,
Marengo SR, Resnick MI and Gupta S: Nuclear factor-kappaB/p65 (Rel
A) is constitutively activated in human prostate adenocarcinoma and
correlates with disease progression. Neoplasia. 6:390–400. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Henrique R, Jerónimo C, Teixeira MR, Hoque
MO, Carvalho AL, Pais I, Ribeiro FR, Oliveira J, Lopes C and
Sidransky D: Epigenetic heterogeneity of high-grade prostatic
intraepithelial neoplasia: Clues for clonal progression in prostate
carcinogenesis. Mol Cancer Res. 4:1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nguyen DP, Li J and Tewari AK:
Inflammation and prostate cancer: The role of interleukin 6 (IL-6).
BJU Int. 113:986–992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taverna G, Pedretti E, Di Caro G, Borroni
EM, Marchesi F and Grizzi F: Inflammation and prostate cancer:
Friends or foe? Inflamm Res. 64:275–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang W, Bergh A and Damber JE: Chronic
inflammation in benign prostate hyperplasia is associated with
focal upregulation of cyclooxygenase-2, Bcl-2 and cell
proliferation in the glandular epithelium. Prostate. 61:60–72.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gerstenbluth RE, Seftel AD, MacLennan GT,
Rao RN, Corty EW, Ferguson K and Resnick MI: Distribution of
chronic prostatitis in radical prostatectomy specimens with
up-regulation of bcl-2 in areas of inflammation. J Urol.
167:2267–2270. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Azad N, Iyer A, Vallyathan V, Wang L,
Castranova V, Stehlik C and Rojanasakul Y: Role of
oxidative/nitrosative stress-mediated Bcl-2 regulation in apoptosis
and malignant transformation. Ann N Y Acad Sci. 1203:1–6. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Medan D, Luanpitpong S, Azad N, Wang L,
Jiang BH, Davis ME, Barnett JB, Guo L and Rojanasakul Y:
Multifunctional role of Bcl-2 in malignant transformation and
tumorigenesis of Cr(VI)-transformed lung cells. PLoS One.
7:e370452012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van Soest RJ, van Royen ME, de Morrée ES,
Moll JM, Teubel W, Wiemer EA, Mathijssen RH, de Wit R and van
Weerden WM: Cross-resistance between taxanes and new hormonal
agents abiraterone and enzalutamide may affect drug sequence
choices in metastatic castration-resistant prostate cancer. Eur J
Cancer. 49:3821–3830. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
De Marzo AM, Marchi VL, Epstein JI and
Nelson WG: Proliferative inflammatory atrophy of the prostate:
Implications for prostatic carcinogenesis. Am J Pathol.
155:1985–1992. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kanwal R, Pandey M, Bhaskaran N, Maclennan
GT, Fu P, Ponsky LE and Gupta S: Protection against oxidative DNA
damage and stress in human prostate by glutathione S-transferase
P1. Mol Carcinog. 53:8–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schnekenburger M, Karius T and Diederich
M: Regulation of epigenetic traits of the glutathione S-transferase
P1 gene: From detoxification toward cancer prevention and
diagnosis. Front Pharmacol. 5:1702014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Crundwell MC, Chughtai S, Knowles M, Takle
L, Luscombe M, Neoptolemos JP, Morton DG and Phillips SM: Allelic
loss on chromosomes 8p, 22q and 18q (DCC) in human prostate cancer.
Int J Cancer. 69:295–300. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tapia-Laliena MA, Korzeniewski N,
Hohenfellner M and Duensing S: High-risk prostate cancer: A disease
of genomic instability. Urol Oncol. 32:1101–1107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schrecengost R and Knudsen KE: Molecular
pathogenesis and progression of prostate cancer. Semin Oncol.
40:244–258. 2013. View Article : Google Scholar : PubMed/NCBI
|