Introduction
Lung cancer is a common primary pulmonary malignant
tumor, with high incidence and mortality rates worldwide (1). In recent years, the incidence of lung
cancer has increased annually and exhibited a trend towards those
of younger ages (1). The 5-year
survival rate of lung cancer in China is only 10%, since the
majority of patients have mid-late stage lung cancer or are no
longer eligible for surgery at the time of diagnosis (2). The typical chemotherapeutic approach for
lung cancer is to inhibit DNA replication or metabolic enzymes,
thus interfering with cell division (3); however, the side effects of these
treatments mean that patients do not tolerate them well.
Furthermore, the intermittent period between these treatments is
long, which may lead to disease recurrence and drug resistance
(4). Therefore, it is necessary to
develop novel therapeutic strategies for the treatment of lung
cancer. Previous studies have suggested that a low-dose and high
frequency/long duration of treatment with certain chemotherapeutic
drugs may induce cellular senescence, improving the patient's
quality of life and prolonging survival; this chemotherapy is
called continuous low-dose chemotherapy (LDM) (5,6).
Compared with traditional chemotherapy, LDM has
several advantages, including a lower toxicity, lower cost and
shorter required intermittent period. This may reduce disease
recurrence and drug resistance, in addition to making it easier for
patients to receive and accept long-term treatment, thus improving
their quality of life and prolonging survival (7,8). However,
the mechanism underlying the effects of LDM remains unclear.
Induction of cellular senescence may inhibit the cell cycle in
G1 phase (9).
Cellular senescence refers to the transition of a
cell from an active growing state to an irreversible growth arrest
state (10,11). It was previously demonstrated that
senile cells primarily contained G1 phase DNA,
suggesting that they were arrested in G1 (12). Activation of the retinoblastoma (Rb)
protein is associated with G1 phase cell cycle arrest.
It has previously been revealed that negative feedback regulation
of the E3 ubiquitin-protein ligase Mdm2 (Mdm2)-Rb signaling pathway
serves a role in tumorigenesis (13,14).
Etoposide (VP-16) is as an antitumor drug that specifically targets
the cell cycle (15). The present
study investigated the effect of various concentrations (1–25
µmol/l) of VP-16 on the Mdm2-Rb signaling pathway and cellular
senescence in A549 lung adenocarcinoma cells.
Materials and methods
Cell culture
The A549 lung cancer cell line was obtained from the
Cancer Laboratory of the First Affiliated Hospital of Chengdu
Medical College (Chengdu, China). A549 cells were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C with 5%
CO2 and saturated humidity. Cells in the logarithmic
phase were digested using 0.25% trypsin (Fuzhou Maixin
Biotechnology Development Co., Ltd., Fuzhou, China) for 2–3 min and
then the cell suspension was obtained.
Observation of cell morphology and
cell cycle analysis
A549 cells in the logarithmic phase of growth were
seeded into 6-well plates (2×105/well) and cultured at
37°C for 24 h. Subsequently, 0 (control group), 1 (group 1), 5
(group 2) and 25 (group 3) µmol/l VP-16 (Jiangsu Hengrui Medicine
Co., Ltd., Lianyungang, China) was added and the cells were
cultured for a further 48 h. The cells were then placed under an
inverted microscope. Six fields of view were randomly selected for
observation and determination of cell morphologies. The cell cycle
distribution was detected by flow cytometry using a FACSCalibur
flow cytometer with BD FACStation software (ImagePro-Plus v6.0)
(both BD Biosciences, Franklin Lakes, NJ, USA).
Detection of senescence
Prior to cell seeding, 6-well plates were placed on
a sterilized tray and 1×105 cells were added to each
well. Then the 6-well plates were covered with plastic film, and
the cells were cultured overnight at 37°C. Various concentrations
(0, 1, 5 and 25 µmol/l) of VP-16 were added to the culture medium,
followed by culture at 37°C for 48 h. One ml of
senescence-associated β-galactosidase fixation fluid (Beyotime
Institute of Biotechnology, Haimen, China) was added, followed by
incubation at room temperature for 15 min. The
senescence-associated β-galactosidase was detected using a
previously described method (16) and
was used to calculate the percentage of senescent cells in each
treatment group.
The use of X-Gal as a substrate for the
β-galactosidase enzyme generates deep blue colored products, thus
senile cells were defined as those with blue granules when observed
under a DVM6 optical microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Immunocytochemistry
Prior to cell seeding, the 6-well plates were placed
on a sterilized tray and 1×105 cells were added to each
well. The cells were then cultured overnight at 37°C. Subsequently,
0, 1, 5 and 25 µmol/l VP-16 were added to the culture medium and
the cells were cultured for a further 48 h culture at 37°C. Cells
were incubated overnight at 4°C with the following primary
antibodies: Polyclonal rabbit anti-human Mdm2 antibody (catalogue
number PAB27165; dilution 1:200; Abzoom Biolabs, Inc., Dallas, TX,
USA), polyclonal rabbit anti-human p-Rb antibody (catalogue number
FZ200784; dilution 1:100; Shanghai Fuzhong Biological Science Co.,
Ltd., Shanghai, China). After adding polymer enhancer and PBS
washing, 50 µl of goat anti-rabbit IgG horseradish
peroxidase-labeled secondary antibody (catalogue number 150077;
dilution 1:200; Abcam, Cambridge, USA) was drop wisely added to
each section, followed by incubation at 37°C for 30 min and PBS
washing for 3 times. Following coloration, counterstain and
mounting, the sections were observed using a Q550CW image
acquisition and analysis system (Leica Microsystems GmbH).
Statistical analysis
Results are expressed as the mean ± standard
deviation. One-way analysis of variance was performed using SPSS
software (version 19.0; SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
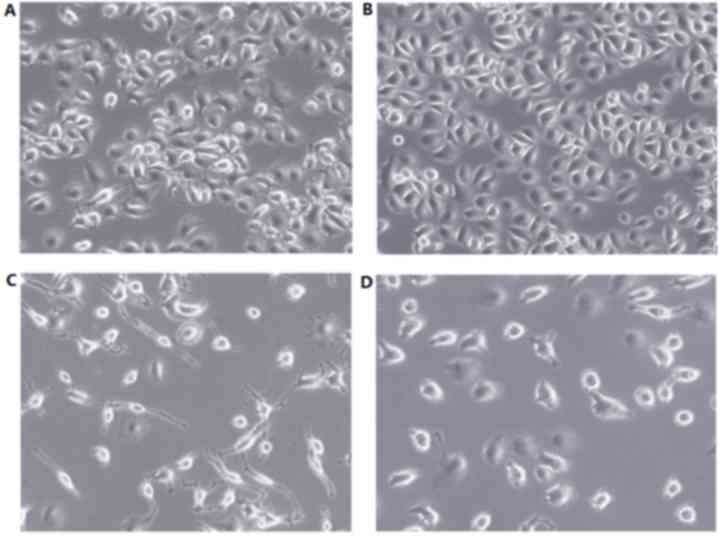
Cell morphology changes
Prior to treatment with VP-16, the number of cells
and cell morphology of the control and experimental groups was
similar. Following a 48 h treatment with VP-16 (Fig. 1), the control group cells grew with
uniform sizes and shapes (Fig. 1A),
whereas group 2 demonstrated significantly decreased cell numbers
and irregular morphology, including increased cell volumes,
pseudopodia, large nuclei, reduced cytoplasm and intracytoplasmic
vacuoles (Fig. 1C). Groups 1 and 3
demonstrated no notable changes in cellular morphology (Fig. 1B and D, respectively).
VP-16 alters the cycle distribution of
A549 cells
The results of flow cytometry analysis demonstrated
that, compared with the control group, in group 2 the percentage of
cells in G1 phase was significantly increased and the
percentage of cells in S phase was significantly decreased (both
P<0.05; Table I). In group 3, the
percentage of cells in the G1 was significantly
decreased and the percentage of cells in S phase was significantly
increased (P<0.05; Table I). There
as no significant differences between the percentage of cells in
G1 or S phases between group 1 and the control group,
and no significant difference in the percentage of cells in
G2 phase was revealed between the four groups (Table I).
 | Table I.Effect of VP-16 on the cell cycle
distribution of A549 cells. |
Table I.
Effect of VP-16 on the cell cycle
distribution of A549 cells.
|
| Percentage of
cells |
|---|
|
|
|
|---|
| Group | G1 | S | G2 |
|---|
| Control | 56.70±1.17 | 32.51±2.52 | 11.01±1.25 |
| Group 1 | 60.91±0.26 | 29.21±1.71 | 9.83±0.78 |
| Group 2 |
77.35±2.32a |
12.31±2.79a | 10.31±0.76 |
| Group 3 |
46.17±2.73a |
43.11±2.28a | 10.71±0.88 |
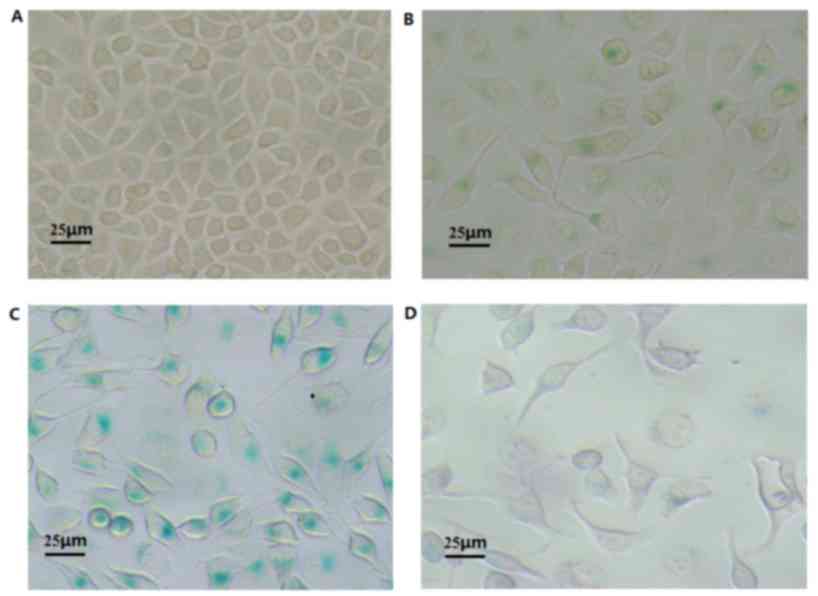
VP-1 effects the senescence of A549
cells
Detection of senescence-associated β-galactosidase
is an important staining method that can be used to effectively
detect senile cells. The lysosome contents of senile cells are
increased, which induces an increased expression of the lysosomal
enzyme β-galactosidase. Following treatment with 1, 5 or 25 µmol/l
VP-16 for 48 h, the cells contained blue particles (Fig. 2), indicating that VP-16 induces the
senescence of A549 cells. Group 2 has the highest level of the
staining, whereas that in groups 1 and 3 was not as high (Table II). The percentage of senescent cells
between the control group and experimental groups, in addition to
the comparisons between the experimental groups, were significantly
different (P<0.05; Table II).
 | Table II.Effect of VP-16 on the senescence of
A549 cells. |
Table II.
Effect of VP-16 on the senescence of
A549 cells.
| Group | Percentage of
senescent cells |
|---|
| Control | 1.41±1.06 |
| Group 1 |
11.03±1.82a |
| Group 2 |
79.11±6.09a,b |
| Group 3 |
5.62±1.16a,b,c |
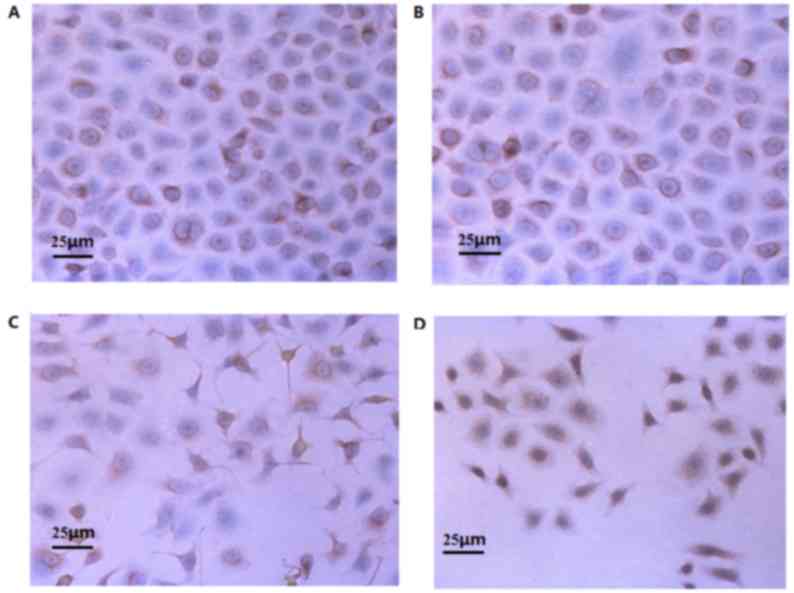
Impact of VP-16 treatment on Mdm2
protein expression
Mdm2 protein was primarily expressed as
brownish-yellow/tan particles in the cytoplasm and nuclei of A549
cells (Fig. 3). Following treatment
with various concentrations of VP-16 (0, 1, 5 and 25 µmol/l) for 48
h, group 2 exhibited significantly decreased expression of Mdm2
protein compared with the control group and groups 1 and 3
(P<0.05; Table III). The
differences in the expression of Mdm2 between the control group and
groups 1 and 3 were not significant, and the difference between
groups 1 and 3 was also not significantly different (Table III).
 | Table III.Effect of VP-16 on the expression of
Mdm2 protein in A549 cells. |
Table III.
Effect of VP-16 on the expression of
Mdm2 protein in A549 cells.
| Group | Percentage of Mdm2
protein-positive cells |
|---|
| Group | 90.18±2.38 |
| Group 1 | 87.03±3.86 |
| Group 2 |
65.60±6.81a,b,c |
| Group 3 | 86.50±4.01 |
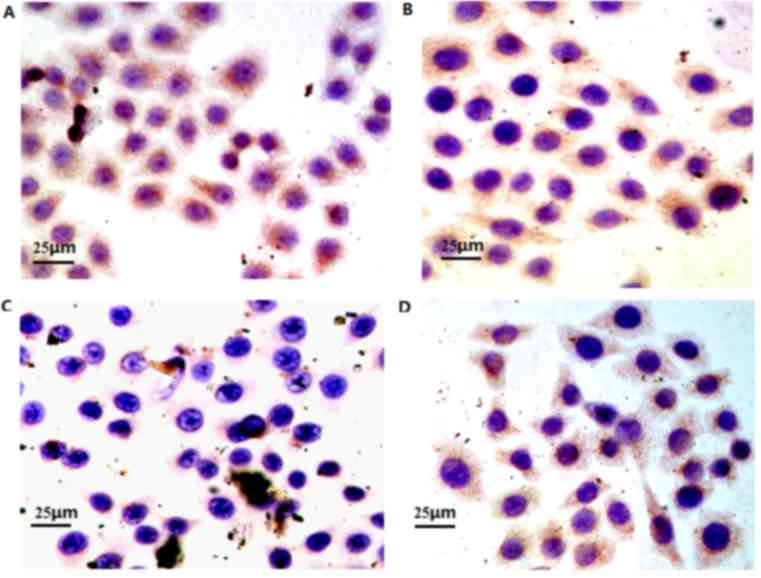
Effect of VP-16 on the expression of
p-Rb protein
The Rb protein was primarily identified as a nuclear
phosphoprotein inside the nuclei of the A549 cells (Fig. 4). Following treatment with various
concentrations of VP-16 (0, 1, 5 and 25 µmol/l) for 48 h, group 2
exhibited a significantly decreased expression p-Rb protein
compared with the control group and groups 1 and 3 (P<0.05),
whereas the differences between the control group and experimental
groups 1 and 3 was not significantly different (Table IV). The difference between groups 1
and 3 was also not significantly different (Table IV).
 | Table IV.Effect of VP-16 on the expression of
phosphorylated Rb protein in A549 cells. |
Table IV.
Effect of VP-16 on the expression of
phosphorylated Rb protein in A549 cells.
| Group | Percentage of
phosphorylated Rb protein-positive cells |
|---|
| Control | 90.23±2.24 |
| Group 1 | 86.51±3.43 |
| Group 2 |
59.12±7.66a,b,c |
| Group 3 |
86.15±13.51c |
Discussion
During the 1960 s, Hayflick et al (17) demonstrated in a fibroblast culture
that normal diploid cells would enter a state of senescence when
proliferated for the 50–70th generation in vitro. No further
subculture could continue while the cells remained in senescence;
this phenomenon was named the ‘Hayflick limit’. A previous study
(18) revealed that cellular
senescence is the third most important cancer prevention process,
following cell DNA repairing and apoptosis; therefore, it is
closely associated with the occurrence, development and treatment
strategy for tumors.
It has been demonstrated that senile cells primarily
contain G1 phase DNA, thus it was considered that the
senile cells were arrested in phase G1 and were not able
to enter phase S successfully (19).
The Mdm2-Rb signaling pathway may induce the arrest the cell in
G1, which can result in cellular senescence (20). A low-concentration of VP-16 may also
induce cellular senescence. A low-concentration VP-16 acts on the
Mdm2-Rb signaling pathway, increasing the expression of Rb protein,
decreasing the phosphorylation of Rb and decreasing the expression
of Mdm2, thus promoting the senescence of tumor cells (21). Cellular senescence has various
characteristics, including the generation of long pseudopodia and
increased β-galactosidase activity (22,23).
If normal cells did not undergo senescence, it would
result in the development of tumors; therefore, inducing tumor cell
senescence has become a focus for studies investigating treatments
for cancer. Previous studies have demonstrated that appropriate
concentrations of DNA replication inhibitor agents, including
Adriamycin (24–26), aphidicolin and cisplatin (27) may induce the phenotypes of cellular
senescence. Furthermore, ionizing radiation, cytarabine, etoposide,
paclitaxel, vincristine, hydroxyurea (28,29),
camptothecin (30) and
bromodeoxyuridine (31,32) may also be able to induce senescence.
The most extensively researched agent is Adriamycin, a
topoisomerase inhibitor. Elmore et al (33) reported that among 14 cell lines
derived from solid human tumors, adriamycin induced senescent
phenotypes in 11 cell lines, and it was confirmed that the
drug-induced senescent phenotypes were not associated with the
shortening of telomeres, so could not be inhibited by the
overexpression of telomerase. However, in certain cell lines,
Adriamycin was not able to induce the characteristics of cellular
senescence; therefore, it was suggested that the cellular
senescence of tumor cells may occur spontaneously or be associated
with the cellular microenvironment (24). Drug-induced cellular senescence has
been identified to be associated with p21, p16 and cellular tumor
antigen p53 (p53) (34).
VP-16 is a cell cycle-specific antitumor drug that
inhibits cells in the S/G2 phase. VP-16 acts on DNA
topoisomerase II, forming a stable drug-enzyme-DNA complex, thus
interfering with DNA repairing (35).
Therefore, the present study used 1, 5 and 25 µmol/l of VP-16 for
48 h treatments. The results demonstrated that 5 µmol/l VP-16
significantly induced cellular senescence of A549 cells, 1 µmol/l
only slightly induced cellular senescence and 25 µmol/l VP-16 did
not significantly induce cellular senescence. This suggests that a
very specific concentration of VP-16 is required to induce cellular
senescence in A549 cells. Therefore, the optimal concentration of
VP-16 that induced cellular senescence in A549 cells was 5
µmol/l.
When treated with 5 µmol/l VP-16, p-Rb and Mdm2
protein expression was significantly reduced in the A549 cells,
whereas no obvious change was observed when 1 and 25 µmol/l
treatments were administered. This indicates that low-concentration
treatments of VP-16 reduced the levels of p-Rb protein, resulting
in the release of transcription factor E2F, which prevents cell
cycle progression and downregulates the expression of Mdm2, and
thus its binding to p53 and Rb.
In conclusion, low-concentration treatments of VP-16
may effect the Mdm2-Rb signaling pathway, reducing the expression
of Mdm2 protein and thus its binding to Rb. Therefore,
non-phosphorylated Rb protein expression was increased, whereas the
p-Rb protein expression was reduced, increasing the amount of
functional Rb and resulting in the induction of cellular
senescence. However, as this is a preliminary study, future
investigations are required to further reveal the mechanisms
underlying the effects of VP-16.
References
|
1
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang J, Zhu J, Zhang YH, Chen YS, Ding LL,
Kensler TW and Chen JG: Lung cancer in a rural area of china: Rapid
rise in incidence and poor improvement in survival. Asian Pac J
Cancer Prev. 16:7295–7302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosell R, Pifarré A, Monzó M, Astudillo J,
López-Cabrerizo MP, Calvo R, Moreno I, Sanchez-Céspedes M, Font A
and Navas-Palacios JJ: Reduced survival in patients with stage-I
non-small-cell lung cancer associated with DNA-replication errors.
Int J Cancer. 74:330–334. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crane EJ and Simon G: Adjuvant
chemotherapy for non-small cell lung cancer. Curr Treat Options
Oncol. 7:51–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non small-cell lung cancer. N Eng J Med.
346:92–98. 2002. View Article : Google Scholar
|
|
6
|
Loven D, Hasnis E, Bertolini F and Shaked
Y: Low-dose metronomic chemotherapy: From past experience to new
paradigms in the treatment of cancer. Drug Discov Today.
18:193–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seng JE and Peterson BA: Low dose
chemotherapy for myelodysplastic syndromes. Leuk Res. 22:481–484.
1998.PubMed/NCBI
|
|
8
|
Bello L, Carrabba G, Giussani C, Lucini V,
Cerutti F, Scaglione F, Landré J, Pluderi M, Tomei G, Villani R, et
al: Low-dose chemotherapy combined with an antiangiogenic drug
reduces human glioma growth in vivo. Cancer Res. 61:7501–7506.
2001.PubMed/NCBI
|
|
9
|
Di Micco R, Fumagalli M, Cicalese A,
Piccinin S, Gasparini P, Luise C, Schurra C, Garre' M, Nuciforo PG,
Bensimon A, et al: Oncogene-in-duced senescence is a DNA damage
response triggered by DNA hyperreplication. Nature. 444:638–642.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Banito A and Lowe SW: A new development in
senescence. Cell. 155:977–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Collado M, Blasco MA and Serrano M:
Cellular senescence in cancer and aging. Cell. 130:223–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hornsby PJ: Senescence as an anticancer
mechanism. J Clin Oncol. 25:1852–1857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryan BM, Calhoun KM, Pine SR, Bowman ED,
Robles AI, Ambs S and Harris CC: MDM2 SNP285 does not antagonize
the effect of SNP309 in lung cancer. Int J Cancer. 131:2710–2716.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang YA, Lin RK, Tsai YT, Hsu HS, Yang YC,
Chen CY and Wang YC: MDM2 overexpression deregulates the
transcriptional control of RB/E2F leading to DNA methyltransferase
3A overexpression in lung cancer. Clin Cancer Res. 18:4325–4333.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan J and Tang D: Prostate cancer
stem-like cells proliferate slowly and resist etoposide-induced
cytotoxicity via enhancing DNA damage response. Exp Cell Res.
328:132–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Itahana K, Itahana Y and Dimri GP:
Colorimetric detection of senescence-associated β galactosidase.
Methods Mol Biol. 965:143–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayflick L and Moorhead PS: The serial
cultivation of human diploid cell strains. Exp Cell Res.
25:585–621. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tchkonia T, Zhu Y, van Deursen J, Campisi
J and Kirkland JL: Cellular senescence and the senescent secretory
phenotype: Therapeutic opportunities. J Clin Invest. 123:966–972.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mao Z, Ke Z, Gorbunova V and Seluanov A:
Replicatively senescent cells are arrested in G1 and G2 phases.
Aging (Albany NY). 4:431–435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huna A, Salmina K, Erenpreisa J,
Vazquez-Martin A, Krigerts J, Inashkina I, Gerashchenko BI,
Townsend PA, Cragg MS and Jackson TR: Role of stress-activated
OCT4A in the cell fate decisions of embryonal carcinoma cells
treated with etoposide. Cell Cycle. 14:2969–2984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yap DB, Hsieh JK, Chan FS and Lu X: Mdm2:
A bridge over the two tumour suppressors, p53 and Rb. Oncogene.
18:7681–7689. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seviour EG, Sehgal V, Lu Y, Luo Z, Moss T,
Zhang F, Hill SM, Liu W, Maiti SN, Cooper L, et al: Functional
proteomics identifies miRNAs to target a p27/Myc/phospho-Rb
signature in breast and ovarian cancer. Oncogene. 35:691–701. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carrillo AM, Hicks M, Khabele D and
Eischen CM: Pharmacologically increasing Mdm2 inhibits DNA repair
and cooperates with genotoxic agents to Kill p53-inactivated
ovarian cancer cells. Mol Cancer Res. 13:1197–1205. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dimri GP, Lee X, Basile G, Acosta M, Scott
G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O,
et al: A biomarker that identifies senescent human cells in culture
and in aging skin in vivo. Proc Natl Acad Sci USA. 92:9363–9367.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmitt CA: In vivo Complexities of
Cellular Senescence in Tumor Development and Therapy. AACR
Education Book. 499–504. 2008. View Article : Google Scholar
|
|
26
|
Roninson IB: Tumor cellular senescence in
cancer treatment. Cancer Res. 63:2705–2715. 2003.PubMed/NCBI
|
|
27
|
Erdmann J: Cancer's big sleep: Senescence
may be potential target for cancer therapies. J Natl Cancer Inst.
97:89–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cristofalo VJ and Pignolo RJ: Molecular
markers of senescence in fibroblast-like cultures. Exp Gerontol.
31:111–123. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Herbig U, Jobling WA, Chen BP, Chen DJ and
Sedivy JM: Telomere shortening triggers seneseence of human ceils
through a pathway involving ATM, p53, and p21(CIPI), but not
p16(INK4a). Mol Cell. 14:501–513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Serrano M, Lin AW, McCurrach ME, Beach D
and Lowe SW: Oncogenic ras provokes premature cell senescence
associated with accumulation of p53 and p16INK4a. Cell. 88:593–602.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sharpless NE and DePinho RA: Cancer: Crime
and punishment. Nature. 436:636–637. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Collado M, Gil J, Efeyan A, Guerra C,
Schuhmacher AJ, Barradas M, Benguría A, Zaballos A, Flores JM,
Barbacid M, et al: Tumour biology: Senescence in premalignant
turnouts. Nature. 436:6422005. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elmore LW, Rehder CW, Di X, McChesney PA,
Jackson-Cook CK, Gewirtz DA and Holt SE: Adriamycin-induced
senescence in breast tumor cells involves functional p53 and
telomere dysfunction. J Biol Chem. 277:35509–35515. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fujiwara-Igarashi A, Goto-Koshino Y,
Mochizuki H, Sato M, Fujino Y, Ohno K and Tsujimoto H: Inhibition
of p16 tumor suppressor gene expression via promoter
hypermethylation in canine lymphoid tumor cells. Res Vet Sci.
97:60–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kamesaki S, Kamesaki H, Jorgensen TJ,
Tanizawa A, Pommier Y and Cossman J: Bcl-2 protein inhibits
etoposide-induced apoptosis through its effects on events
subsequent to topoisomerase II-induced DNA strand breaks and their
repair. Cancer Res. 53:4251–4256. 1993.PubMed/NCBI
|